Food-Intake Normalization of Dysregulated Fatty Acids in Women with Anorexia Nervosa
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Participants and Study Flow
2.2. Fatty Acid Analysis
2.3. Enzyme Activity Indices
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics and Phenotypes
3.2. Fatty Acid Profile
3.3. Fasting and Postprandial Concentrations of Individual Fatty Acids
3.4. Fatty Acid Correlations with Body Mass Index and Psychiatric Phenotypes
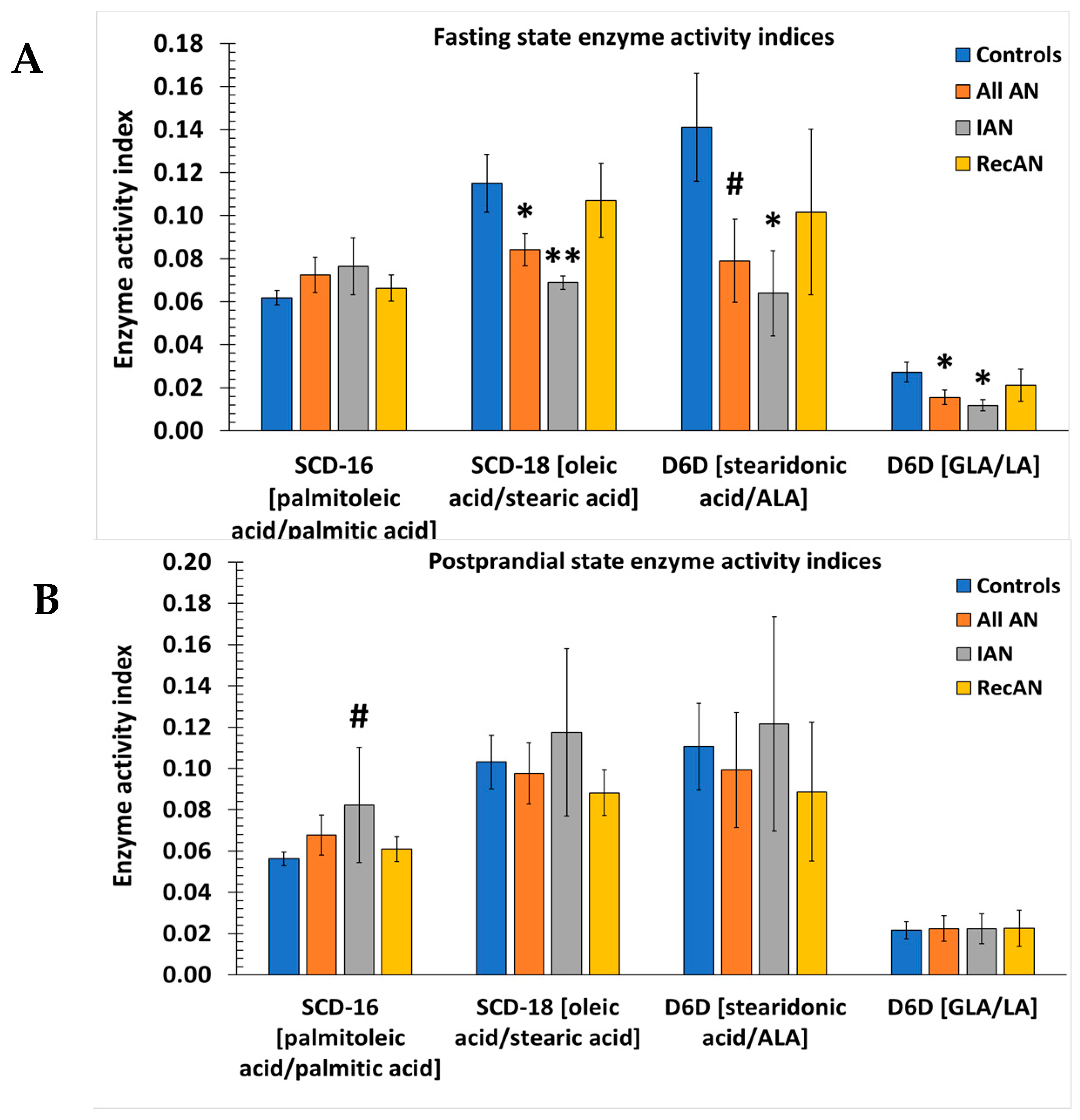
3.5. Desaturase and Elongase Indices
3.6. n-6 to n-3 Fatty Acid Ratios
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Feeding and eating disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Moskowitz, L.; Weiselberg, E. Anorexia Nervosa/Atypical Anorexia Nervosa. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Smink, F.R.; van Hoeken, D.; Hoek, H.W. Epidemiology, course, and outcome of eating disorders. Curr. Opin. Psychiatry 2013, 26, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Hoek, H.W. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr. Opin. Psychiatry 2006, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Halmi, K.A. Perplexities of treatment resistance in eating disorders. BMC Psychiatry 2013, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.H.; Schaumberg, K.; Munn-Chernoff, M.A. Genetics of Anorexia Nervosa. Curr. Psychiatry Rep. 2017, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Berends, T.; van Meijel, B.; Nugteren, W.; Deen, M.; Danner, U.N.; Hoek, H.W.; van Elburg, A.A. Rate, timing and predictors of relapse in patients with anorexia nervosa following a relapse prevention program: A cohort study. BMC Psychiatry 2016, 16, 316. [Google Scholar] [CrossRef] [PubMed]
- Strober, M.; Freeman, R.; Morrell, W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int. J. Eat. Disord. 1997, 22, 339–360. [Google Scholar] [CrossRef]
- Eckert, E.D.; Halmi, K.A.; Marchi, P.; Grove, W.; Crosby, R. Ten-year follow-up of anorexia nervosa: Clinical course and outcome. Psychol. Med. 1995, 25, 143–156. [Google Scholar] [CrossRef]
- Keel, P.K.; Dorer, D.J.; Franko, D.L.; Jackson, S.C.; Herzog, D.B. Postremission predictors of relapse in women with eating disorders. Am. J. Psychiatry 2005, 162, 2263–2268. [Google Scholar] [CrossRef]
- Walsh, B.T. The enigmatic persistence of anorexia nervosa. Am. J. Psychiatry 2013, 170, 477–484. [Google Scholar] [CrossRef]
- Drewnowski, A.; Pierce, B.; Halmi, K.A. Fat aversion in eating disorders. Appetite 1988, 10, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Simon, Y.; Bellisle, F.; Monneuse, M.O.; Samuel-Lajeunesse, B.; Drewnowski, A. Taste responsiveness in anorexia nervosa. Br. J. Psychiatry 1993, 162, 244–246. [Google Scholar] [CrossRef]
- Jauregui Lobera, I.; Bolanos Rios, P. Choice of diet in patients with anorexia nervosa. Nutr. Hosp. 2009, 24, 682–687. [Google Scholar] [PubMed]
- Schebendach, J.E.; Mayer, L.E.; Devlin, M.J.; Attia, E.; Contento, I.R.; Wolf, R.L.; Walsh, B.T. Food choice and diet variety in weight-restored patients with anorexia nervosa. J. Am. Diet. Assoc. 2011, 111, 732–736. [Google Scholar] [CrossRef]
- Naisberg, Y.; Modai, I.; Weizman, A. Metabolic bioenergy homeostatic disruption: A cause of anorexia nervosa. Med. Hypotheses 2001, 56, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Nunes, E.A.; Rafacho, A. Implications of Palmitoleic Acid (Palmitoleate) On Glucose Homeostasis, Insulin Resistance and Diabetes. Curr. Drug Targets 2017, 18, 619–628. [Google Scholar] [CrossRef]
- Nestel, P.; Clifton, P.; Noakes, M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J. Lipid Res. 1994, 35, 656–662. [Google Scholar] [PubMed]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. 2011, 155, 117–139. [Google Scholar] [CrossRef]
- Lee, H.; Park, W.J. Unsaturated fatty acids, desaturases, and human health. J. Med. Food. 2014, 17, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Procter, N.; Gordon, A.; Bogomolova, S.; O’Dea, K.; Strachan, J.; Ballestrin, M.; et al. People with schizophrenia and depression have a low omega-3 index. Prostaglandins Leukot. Essent. Fat. Acids 2016, 110, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thesing, C.S.; Bot, M.; Milaneschi, Y.; Giltay, E.J.; Penninx, B. Omega-3 and omega-6 fatty acid levels in depressive and anxiety disorders. Psychoneuroendocrinology 2018, 87, 53–62. [Google Scholar] [CrossRef]
- Shih, P.B.; Yang, J.; Morisseau, C.; German, J.B.; Zeeland, A.A.; Armando, A.M.; Quehenberger, O.; Bergen, A.W.; Bergen, P.; Berrettini, W.; et al. Dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol. Psychiatry 2016, 21, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, V.; Slobodianik, N.; Pallaro, A.; Bello, M.; Geissler, C. Risk factors for cardiovascular disease in patients with anorexia nervosa. Int. J. Psychiatr. Nurs. Res. 2007, 13, 1531–1545. [Google Scholar]
- Holman, R.T.; E Adams, C.; A Nelson, R.; Grater, S.J.; A Jaskiewicz, J.; Johnson, S.B.; Erdman, J.W. Patients with anorexia nervosa demonstrate deficiencies of selected essential fatty acids, compensatory changes in nonessential fatty acids and decreased fluidity of plasma lipids. J. Nutr. 1995, 125, 901–907. [Google Scholar]
- Swenne, I.; Rosling, A. Omega-3 essential fatty acid status is improved during nutritional rehabilitation of adolescent girls with eating disorders and weight loss. Acta Paediatr. 2012, 101, 858–861. [Google Scholar] [CrossRef]
- Langan, S.M.; Farrell, P.M. Vitamin E, vitamin A and essential fatty acid status of patients hospitalized for anorexia nervosa. Am. J. Clin. Nutr. 1985, 41, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim. Biophys. Acta 2011, 1811, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Drag, J.; Gozdzialska, A.; Knapik-Czajka, M.; Gawedzka, A.; Gawlik, K.; Jaskiewicz, J. Effect of high carbohydrate diet on elongase and desaturase activity and accompanying gene expression in rat’s liver. Genes Nutr. 2017, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Marklund, M.; Morris, A.P.; Mahajan, A.; Ingelsson, E.; Lindgren, C.M.; Lind, L.; Risérus, U. Genome-Wide Association Studies of Estimated Fatty Acid Desaturase Activity in Serum and Adipose Tissue in Elderly Individuals: Associations with Insulin Sensitivity. Nutrients 2018, 10, 1791. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.C.; Su, H.M.; Yao, T.C.; Kuo, M.L.; Lai, M.W.; Tsai, M.H.; Huang, J.L. Alternation of plasma fatty acids composition and desaturase activities in children with liver steatosis. PLoS ONE 2017, 12, e0182277. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vicario, C.; Gonzalez-Periz, A.; Rius, B.; Moran-Salvador, E.; Garcia-Alonso, V.; Lozano, J.J.; Bataller, R.; Cofan, M.; Kang, J.X.; Arroyo, V.; et al. Molecular interplay between Delta5/Delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 2014, 63, 344–355. [Google Scholar] [CrossRef]
- Cormier, H.; Rudkowska, I.; Lemieux, S.; Couture, P.; Julien, P.; Vohl, M.C. Effects of FADS and ELOVL polymorphisms on indexes of desaturase and elongase activities: Results from a pre-post fish oil supplementation. Genes Nutr. 2014, 9, 437. [Google Scholar] [CrossRef]
- Duncan, L.; Yilmaz, Z.; Gaspar, H.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Thornton, L.; Hinney, A.; et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef]
- Watson, H.J.; Initiative, A.N.G.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, A.; Hübel, C.; Stahl, D.; Stadler, M.; Ismail, K.; Breen, G.; Treasure, J.; Kan, C. The metabolic underpinning of eating disorders: A systematic review and meta-analysis of insulin sensitivity. Mol. Cell. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Goldberg, I.J. Lipoprotein lipase and lipolysis: Central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 1996, 37, 693–707. [Google Scholar] [PubMed]
- Weinstock, P.H.; Levak-Frank, S.; Hudgins, L.C.; Radner, H.; Friedman, J.M.; Zechner, R.; Breslow, J.L. Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proc. Natl. Acad. Sci. USA 1997, 94, 10261–10266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fielding, B.A.; Frayn, K.N. Lipoprotein lipase and the disposition of dietary fatty acids. Br. J. Nutr. 1998, 80, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS—Lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [PubMed]
- de Lorgeril, M.; Salen, P. Alpha-linolenic acid and coronary heart disease. Nutr. Metab. Cardiovasc Dis. 2004, 14, 162–169. [Google Scholar] [CrossRef]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. Alpha-linolenic acid: An omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? BioMed. Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, N. The nutraceutical potential of omega-3 alpha-linolenic acid in reducing the consequences of stroke. Biochimie 2016, 120, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Satogami, K.; Tseng, P.T.; Su, K.P.; Takahashi, S.; Ukai, S.; Li, D.J.; Chen, T.Y.; Lin, P.Y.; Chen, Y.W.; Matsuoka, Y.J. Relationship between polyunsaturated fatty acid and eating disorders: Systematic review and meta-analysis. Prostaglandins Leukot. Essent. Fat. Acids 2019, 142, 11–19. [Google Scholar] [CrossRef]
- Nonaka, Y.; Takagi, T.; Inai, M.; Nishimura, S.; Urashima, S.; Honda, K.; Aoyama, T.; Terada, S. Lauric Acid Stimulates Ketone Body Production in the KT-5 Astrocyte Cell Line. J. Oleo Sci. 2016, 65, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.T.; Chen, J.W.; Rathod, J.; Jiang, Y.Z.; Tsai, P.J.; Hung, Y.P.; Ko, W.C.; Sabja, D.P.; Huang, I.H. Lauric Acid Is an Inhibitor of Clostridium difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model. Front. Microbiol. 2017, 8, 2635. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Tiwari, V.; Singh, M.; Yadav, R.K.; Roy, S.; Devi, U.; Gautam, S.; Rawat, J.K.; Ansari, M.N.; Saeedan, A.S.; et al. Comparative efficacy of alpha-linolenic acid and gamma-linolenic acid to attenuate valproic acid-induced autism-like features. J. Physiol. Biochem. 2017, 73, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, G.; Almeida, A.; Ravasco, P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition 2015, 31, 549–555. [Google Scholar] [CrossRef]
- Solís-Martínez, O.; Plasa-Carvalho, V.; Phillips-Sixtos, G.; Trujillo-Cabrera, Y.; Hernández-Cuellar, A.; Queipo-García, G.E.; Meaney-Mendiolea, E.; Ceballos-Reyes, G.M.; Fuchs-Tarlovsky, V. Effect of Eicosapentaenoic Acid on Body Composition and Inflammation Markers in Patients with Head and Neck Squamous Cell Cancer from a Public Hospital in Mexico. Nutr. Cancer 2018, 70, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H.; Von Meyenfeldt, M.F.; Moses, A.G.W.; Van Geenen, R.; Roy, A.; Gouma, D.J.; Giacosa, A.; Van Gossum, A.; Bauer, J.; Barber, M.D.; et al. Effect of a protein and energy dense n-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: A randomised double blind trial. Gut 2003, 52, 1479–1486. [Google Scholar] [CrossRef]
- Sarris, J.; Mischoulon, D.; Schweitzer, I. Omega-3 for bipolar disorder: Meta-analyses of use in mania and bipolar depression. J. Clin. Psychiatry 2012, 73, 81–86. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Berger, G. Eicosapentaenoic acid interventions in schizophrenia: Meta-analysis of randomized, placebo-controlled studies. J. Clin. Psychopharmacol. 2012, 32, 179–185. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schafer, M.R.; Papageorgiou, K.; Klier, C.M.; Cotton, S.M.; Harrigan, S.M.; Mackinnon, A.; McGorry, P.D.; Berger, G.E. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch. Gen. Psychiatry 2010, 67, 146–154. [Google Scholar] [CrossRef]
- Ayton, A.K.; Azaz, A.; Horrobin, D.F. A pilot open case series of ethyl-EPA supplementation in the treatment of anorexia nervosa. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Ayton, A.K.; Azaz, A.; Horrobin, D.F. Rapid improvement of severe anorexia nervosa during treatment with ethyl-eicosapentaenoate and micronutrients. Eur. Psychiatry 2004, 19, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Caspar-Bauguil, S.; Montastier, E.; Galinon, F.; Frisch-Benarous, D.; Salvayre, R.; Ritz, P. Anorexia nervosa patients display a deficit in membrane long chain poly-unsaturated fatty acids. Clin. Nutr. 2012, 31, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.M.; Smesny, S.; Kim, S.W.; Davey, C.G.; Rice, S.; Sarnyai, Z.; Schlögelhofer, M.; Schäfer, M.R.; Berk, M.; McGorry, P.D.; et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: A 7-year longitudinal study. Transl. Psychiatry 2017, 7, e1220. [Google Scholar] [CrossRef] [PubMed]
- LaChance, L.; McKenzie, K.; Taylor, V.H.; Vigod, S.N. Omega-6 to Omega-3 Fatty Acid Ratio in Patients with ADHD: A Meta-Analysis. J. Can. Acad. Child Adolesc. Psychiatry 2016, 25, 87–96. [Google Scholar] [PubMed]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Swenne, I.; Rosling, A.; Tengblad, S.; Vessby, B. Omega-3 polyunsaturated essential fatty acids are associated with depression in adolescents with eating disorders and weight loss. Acta Paediatr. 2011, 100, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Villamor, E.; Mora-Plazas, M.; Marin, C.; Baylin, A. Alpha-linolenic acid (ALA) is inversely related to development of adiposity in school-age children. Eur. J. Clin. Nutr. 2015, 69, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Conrad, Z.; Johnson, L.K.; Picklo, M.J.; Jahns, L. Relationship of the Reported Intakes of Fat and Fatty Acids to Body Weight in US Adults. Nutrients 2017, 9, 438. [Google Scholar] [CrossRef]
- Logan, A.C. Omega-3 fatty acids and major depression: A primer for the mental health professional. Lipids Health Dis. 2004, 3, 25. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Vinknes, K.J.; Elshorbagy, A.K.; Nurk, E.; Drevon, C.A.; Gjesdal, C.G.; Tell, G.S.; Nygård, O.; Vollset, S.E.; Refsum, H. Plasma stearoyl-CoA desaturase indices: Association with lifestyle, diet, and body composition. Obesity 2013, 21, E294–E302. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Miyazaki, M.; Socci, N.D.; Hagge-Greenberg, A.; Liedtke, W.; Soukas, A.A.; Sharma, R.; Hudgins, L.C.; Ntambi, J.M.; Friedman, J.M. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 2002, 297, 240–243. [Google Scholar] [CrossRef]
- Zak, A.; Vecka, M.; Tvrzicka, E.; Hruby, M.; Novak, F.; Papezova, H.; Lubanda, H.; Vesela, L.; Stankova, B. Composition of plasma fatty acids and non-cholesterol sterols in anorexia nervosa. Physiol. Res. 2005, 54, 443–451. [Google Scholar] [PubMed]
- Swenne, I.; Rosling, A.; Tengblad, S.; Vessby, B. Essential fatty acid status in teenage girls with eating disorders and weight loss. Acta Paediatr. 2011, 100, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Sartori, F.; Guarini, P.; Olivieri, O.; Martinelli, N. Delta-5 and delta-6 desaturases: Crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv. Exp. Med. Biol. 2014, 824, 61–81. [Google Scholar] [PubMed]
- Dubois, A.; Gross, H.A.; Richter, J.E.; Ebert, M.H. Effect of bethanechol on gastric functions in primary anorexia nervosa. Dig. Dis. Sci. 1981, 26, 598–600. [Google Scholar] [CrossRef]
- Holt, S.; Ford, M.J.; Grant, S.; Heading, R.C. Abnormal gastric emptying in primary anorexia nervosa. Br. J. Psychiatry 1981, 139, 550–552. [Google Scholar] [CrossRef] [PubMed]
- McCallum, R.W.; Grill, B.B.; Lange, R.; Planky, M.; Glass, E.E.; Greenfeld, D.G. Definition of a gastric emptying abnormality in patients with anorexia nervosa. Dig. Dis. Sci. 1985, 30, 713–722. [Google Scholar] [CrossRef]
- Stacher, G.; Kiss, A.; Wiesnagrotzki, S.; Bergmann, H.; Hobart, J.; Schneider, C. Oesophageal and gastric motility disorders in patients categorised as having primary anorexia nervosa. Gut 1986, 27, 1120–1126. [Google Scholar] [CrossRef]
- Rigaud, D.; Bedig, G.; Merrouche, M.; Vulpillat, M.; Bonfils, S.; Apfelbaum, M. Delayed gastric emptying in anorexia nervosa is improved by completion of a renutrition program. Dig. Dis. Sci. 1988, 33, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Benini, L.; Todesco, T.; Dalle Grave, R.; Deiorio, F.; Salandini, L.; Vantini, I. Gastric emptying in patients with restricting and binge/purging subtypes of anorexia nervosa. Am. J. Gastroenterol. 2004, 99, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Okada, T.; Iida, M.; Tamai, H.; Kobayashi, N.; Nakagawa, T.; Fujishima, M. Small bowel transit time measured by hydrogen breath test in patients with anorexia nervosa. Dig. Dis. Sci. 1990, 35, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Chiarioni, G.; Bassotti, G.; Monsignori, A.; Menegotti, M.; Salandini, L.; Di Matteo, G.; Vantini, I.; Whitehead, W.E. Anorectal dysfunction in constipated women with anorexia nervosa. Mayo Clin. Proc. 2000, 75, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.; Stolte-Dijkstra, I.; van Rheenen, P.; Smith, R.G.; Scheers, T.; Walia, J.S. Clinical spectrum of KIAA2022 pathogenic variants in males: Case report of two boys with KIAA2022 pathogenic variants and review of the literature. Am. J. Med. Genet. A 2018, 176, 1455–1462. [Google Scholar] [CrossRef]
- Muller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.; Stolte-Dijkstra, I.; Van Rheenen, P.; Smith, R.G.; Scheers, T.; Walia, J.S. Intestinal Dysmotility Syndromes following Systemic Infection by Flaviviruses. Cell 2018, 175, 1198–1212. [Google Scholar]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363, eaat9931. [Google Scholar] [CrossRef]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef]



| Characteristic | All AN (N = 50) | IAN (N = 30) | RecAN (N = 20) | Controls (N = 47) | Statistics | ||
|---|---|---|---|---|---|---|---|
| All AN to Controls | IAN to Controls | RecAN to Controls | |||||
| Age, year | 30.1 ± 10.2 | 29.6 ± 9.0 | 31.1 ± 12.0 | 30.5 ± 9.3 | 0.710 | 0.68 | 0.88 |
| BMI, kg/m2 | 18.9 ± 3.8 | 16.7 ± 2.4 | 22.3 ± 2.8 | 22.9 ± 3.5 | <0.001 ** | <0.001 ** | 0.5 |
| BDI | 21.8 ± 16.4 | 28.0 ± 16.7 | 12.6 ± 10.9 | 3.9 ± 7.4 | <0.001 ** | <0.001 ** | <0.001 ** |
| Fasting BAI | 21.5 ± 13.7 | 26.6 ± 13.1 | 14.4 ± 11.6 | 4.3 ± 5.8 | <0.001 ** | <0.001 ** | <0.001 ** |
| Change in BAI | −3.4 ± 7.5 | −5.1 ± 10.9 | −2.3 ± 4.5 | −1.3 ± 3.3 | 0.013 * | 0.109 | 0.025 * |
| Fatty Acids (All Subjects) | BMI | Fasting BDI | Fasting BAI | Change in BAI | ||||
|---|---|---|---|---|---|---|---|---|
| rs | p-Value | rs | p-Value | rs | p-Value | rs | p-Value | |
| Lauric acid (12:0 saturated) | −0.28 | 0.005 | 0.15 | 0.150 | 0.10 | 0.350 | 0.02 | 0.850 |
| Alpha-linoleic acid (ALA) (18:3 n-3) | −0.46 | <0.001 | 0.31 | 0.002 | 0.28 | 0.006 | −0.04 | 0.744 |
| Eicosapentaenoic acid (EPA) (20:5 n-3) | −0.29 | 0.004 | 0.28 | 0.005 | 0.26 | 0.010 | −0.12 | 0.312 |
| Docosapentaenoic acid (DPA) (22:5 n-3) | −0.39 | <0.001 | 0.31 | 0.002 | 0.24 | 0.019 | 0.03 | 0.785 |
| Fatty Acids (AN Group) | ||||||||
| Lauric acid (12:0 saturated) | −0.27 | 0.062 | 0.17 | 0.251 | 0.04 | 0.765 | −0.15 | 0.446 |
| Alpha-linoleic acid (ALA) (18:3 n-3) | −0.33 | 0.019 | −0.03 | 0.832 | −0.09 | 0.534 | 0.08 | 0.684 |
| Eicosapentaenoic acid (EPA) (20:5 n-3) | −0.11 | 0.441 | −0.02 | 0.900 | −0.14 | 0.318 | 0.03 | 0.876 |
| Docosapentaenoic acid (DPA) (22:5 n-3) | −0.30 | 0.032 | 0.27 | 0.060 | 0.05 | 0.727 | 0.05 | 0.782 |
| Fatty Acids (Control Group) | ||||||||
| Lauric acid (12:0 saturated) | −0.15 | 0.318 | −0.11 | 0.474 | −0.15 | 0.326 | 0.14 | 0.347 |
| Alpha-linoleic acid (ALA) (18:3 n-3) | −0.23 | 0.116 | <0.01 | 0.980 | −0.03 | 0.840 | 0.10 | 0.493 |
| Eicosapentaenoic acid (EPA) (20:5 n-3) | −0.20 | 0.175 | 0.21 | 0.162 | 0.26 | 0.080 | −0.10 | 0.498 |
| Docosapentaenoic acid (DPA) (22:5 n-3) | −0.24 | 0.111 | −0.06 | 0.690 | −0.03 | 0.843 | 0.12 | 0.427 |
| Fatty Acids (All Subjects) | BMI | Fasting BDI | Postprandial BAI | Change in BAI | ||||
|---|---|---|---|---|---|---|---|---|
| rs | p-Value | rs | p-Value | rs | p-Value | rs | p-Value | |
| Lauric acid (12:0 saturated) | −0.27 | 0.018 | 0.06 | 0.640 | 0.10 | 0.408 | 0.06 | 0.592 |
| Alpha-linoleic acid (ALA) (18:3 n-3) | −0.26 | 0.027 | 0.28 | 0.016 | 0.24 | 0.037 | −0.25 | 0.030 |
| Eicosapentaenoic acid (EPA) (20:5 n-3) | −0.15 | 0.191 | 0.25 | 0.033 | 0.28 | 0.017 | −0.09 | 0.435 |
| Docosapentaenoic acid (DPA) (22:5 n-3) | −0.17 | 0.198 | 0.15 | 0.198 | 0.16 | 0.183 | −0.10 | 0.393 |
| Fatty Acids (AN Group) | ||||||||
| Lauric acid (12:0 saturated) | −0.36 | 0.058 | 0.06 | 0.764 | <0.01 | 0.99 | 0.05 | 0.812 |
| Alpha-linoleic acid (ALA) (18:3 n-3) | −0.14 | 0.49 | 0.01 | 0.959 | −0.08 | 0.702 | −0.1 | 0.602 |
| Eicosapentaenoic acid (EPA) (20:5 n-3) | 0.12 | 0.544 | 0.09 | 0.648 | −0.04 | 0.854 | 0.01 | 0.979 |
| Docosapentaenoic acid (DPA) (22:5 n-3) | <0.01 | 0.982 | 0.14 | 0.48 | 0.03 | 0.866 | −0.16 | 0.403 |
| Fatty Acids (Control Group) | ||||||||
| Lauric acid (12:0 saturated) | −0.16 | 0.294 | −0.04 | 0.806 | 0.05 | 0.746 | 0.11 | 0.477 |
| Alpha-linoleic acid (ALA) (18:3 n-3) | −0.27 | 0.074 | 0.28 | 0.06 | 0.34 | 0.022 | −0.14 | 0.358 |
| Eicosapentaenoic acid (EPA) (20:5 n-3) | −0.22 | 0.135 | 0.23 | 0.119 | 0.33 | 0.026 | −0.02 | 0.875 |
| Docosapentaenoic acid (DPA) (22:5 n-3) | −0.21 | 0.157 | 0.09 | 0.545 | 0.15 | 0.332 | 0.01 | 0.941 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.; Dow, M.; Woodside, B.; German, J.B.; Quehenberger, O.; Shih, P.-a.B. Food-Intake Normalization of Dysregulated Fatty Acids in Women with Anorexia Nervosa. Nutrients 2019, 11, 2208. https://doi.org/10.3390/nu11092208
Nguyen N, Dow M, Woodside B, German JB, Quehenberger O, Shih P-aB. Food-Intake Normalization of Dysregulated Fatty Acids in Women with Anorexia Nervosa. Nutrients. 2019; 11(9):2208. https://doi.org/10.3390/nu11092208
Chicago/Turabian StyleNguyen, Nhien, Michelle Dow, Blake Woodside, J. Bruce German, Oswald Quehenberger, and Pei-an Betty Shih. 2019. "Food-Intake Normalization of Dysregulated Fatty Acids in Women with Anorexia Nervosa" Nutrients 11, no. 9: 2208. https://doi.org/10.3390/nu11092208
APA StyleNguyen, N., Dow, M., Woodside, B., German, J. B., Quehenberger, O., & Shih, P.-a. B. (2019). Food-Intake Normalization of Dysregulated Fatty Acids in Women with Anorexia Nervosa. Nutrients, 11(9), 2208. https://doi.org/10.3390/nu11092208





