Antinociceptive and Anti-Inflammatory Effects of Nypa fruticans Wurmb by Suppressing TRPV1 in the Sciatic Neuropathies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animal
2.2. Preparation of the Extract
2.3. Sciatic Nerve Crush Injury Model
2.4. Experimental Group and Medication
2.5. Heat Hyperalgegia Test
2.6. Protein Quantification (Western Blotting)
2.7. Analysis of Inflammatory Markers (Cytokine Analysis)
2.8. Statistical Analysis
3. Results
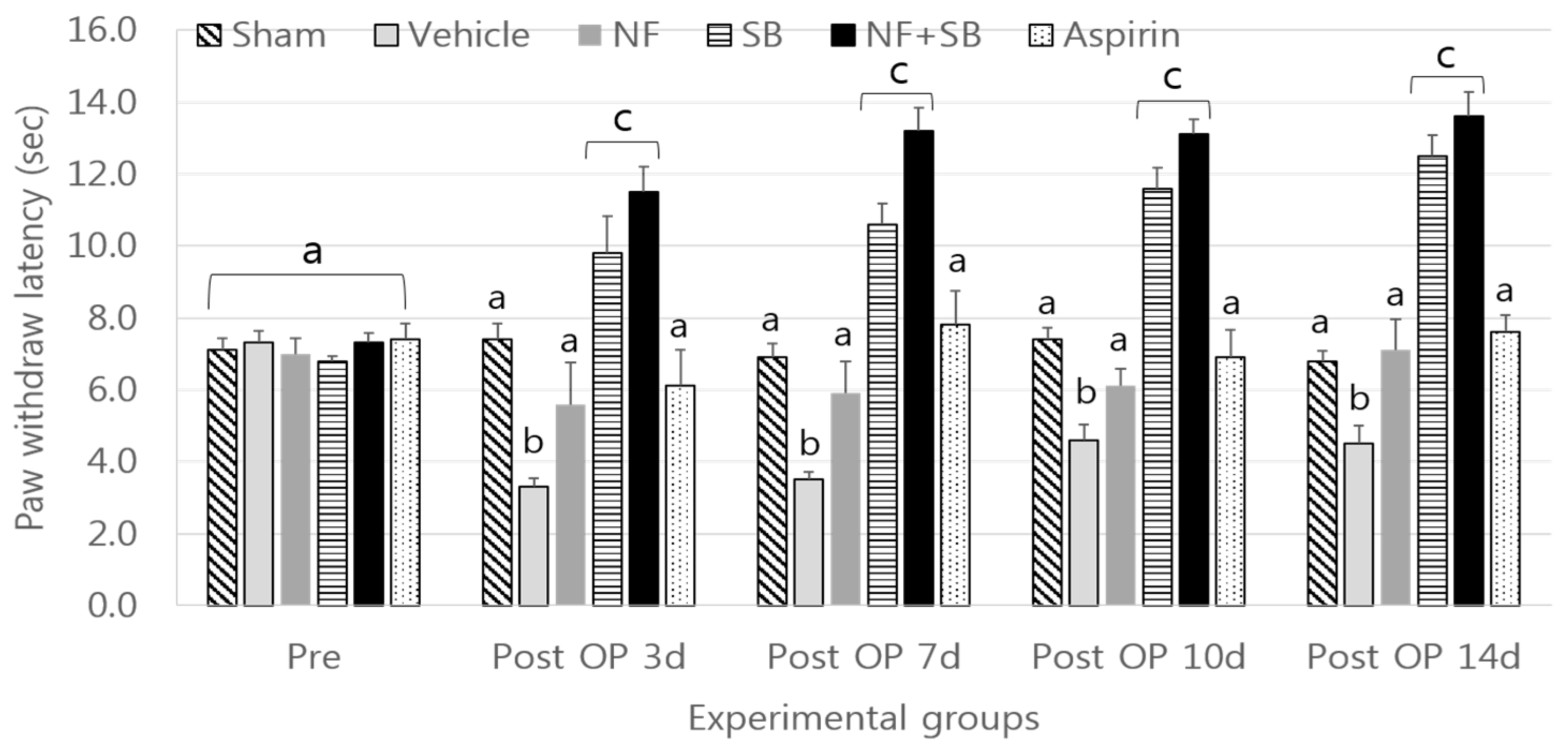
3.1. Heat Hyperalgegia Test
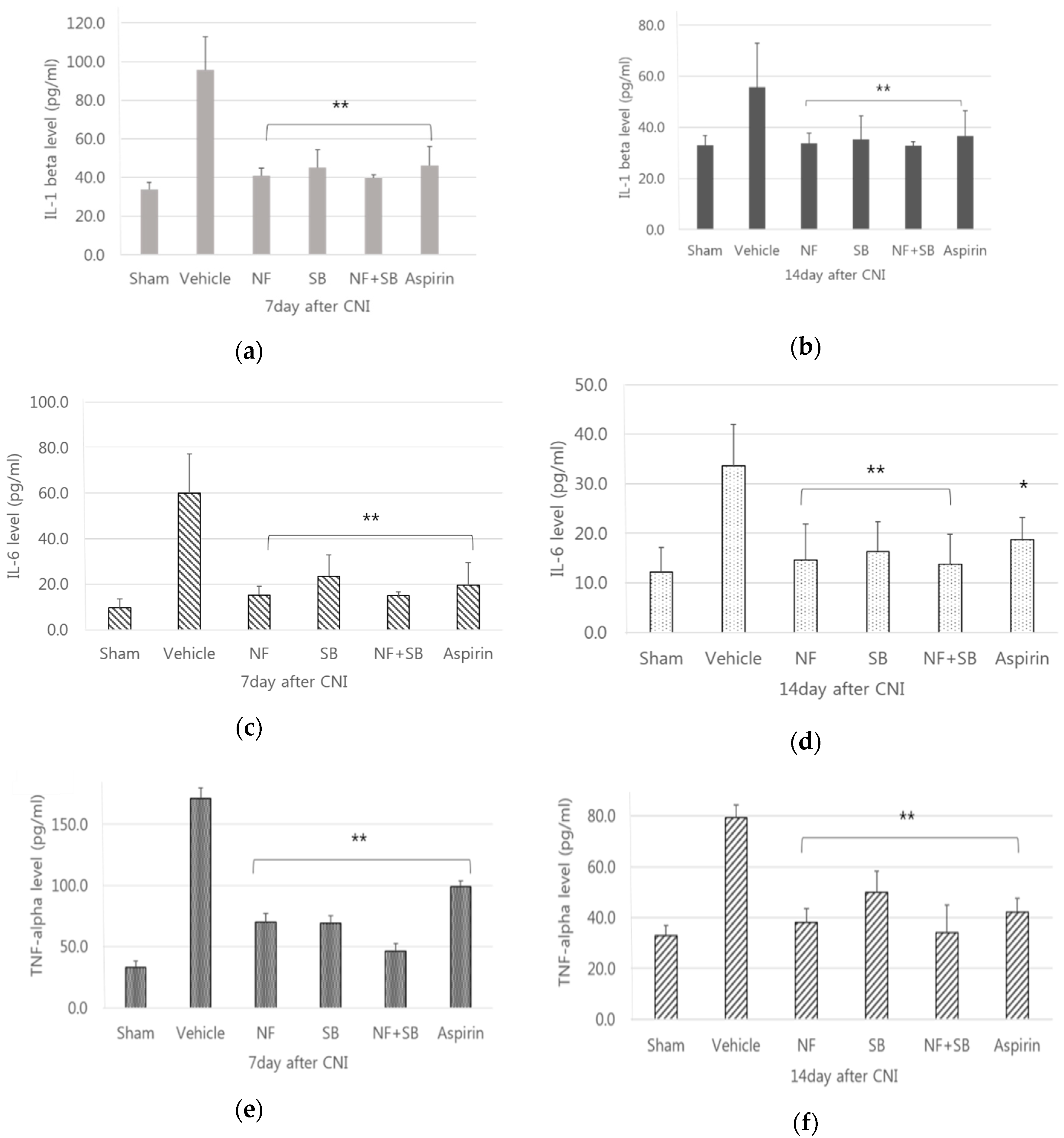
3.2. Analysis of Inflammatory Markers
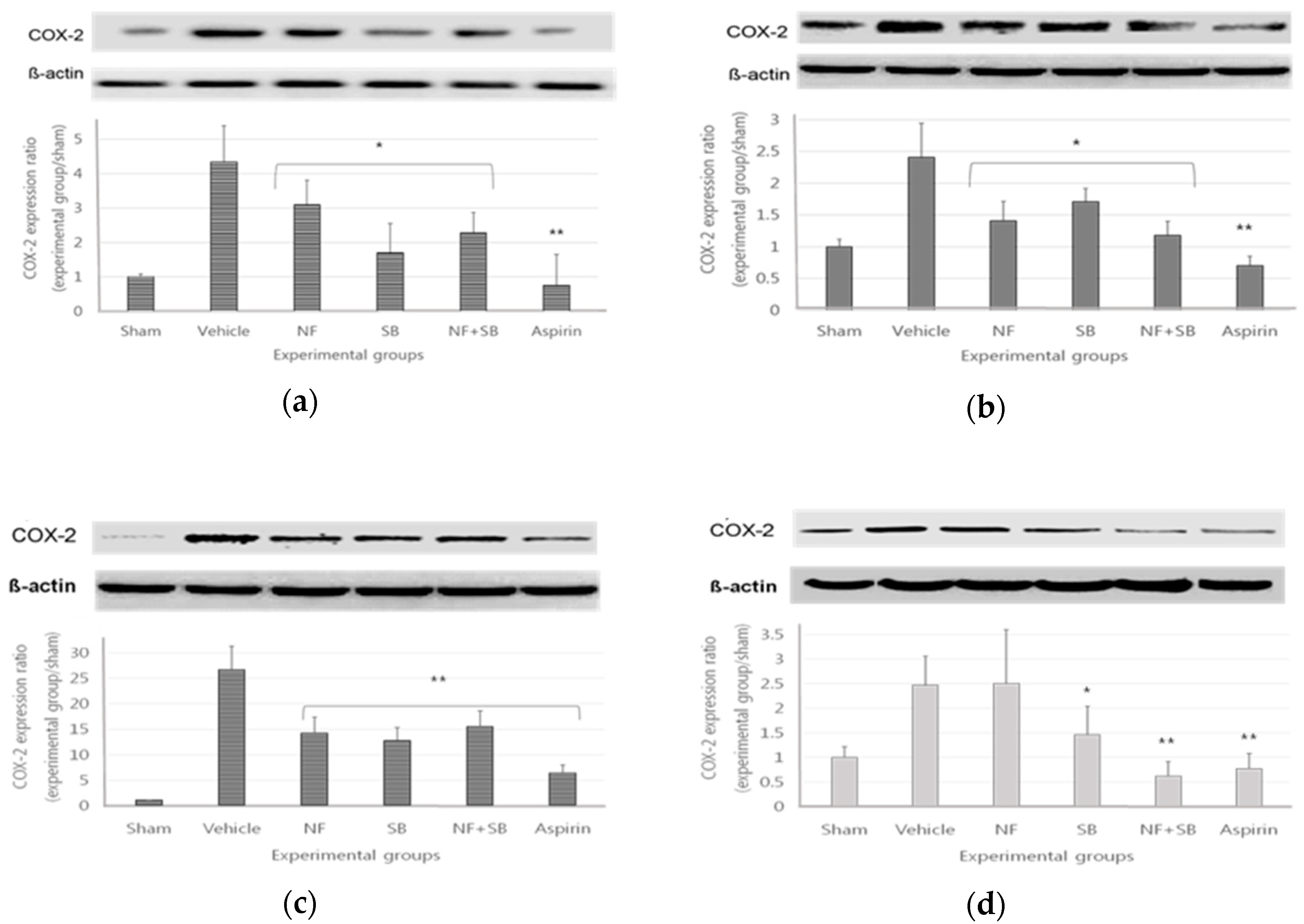
3.3. The COX2 Expression in Sciatic Crush Injury Models
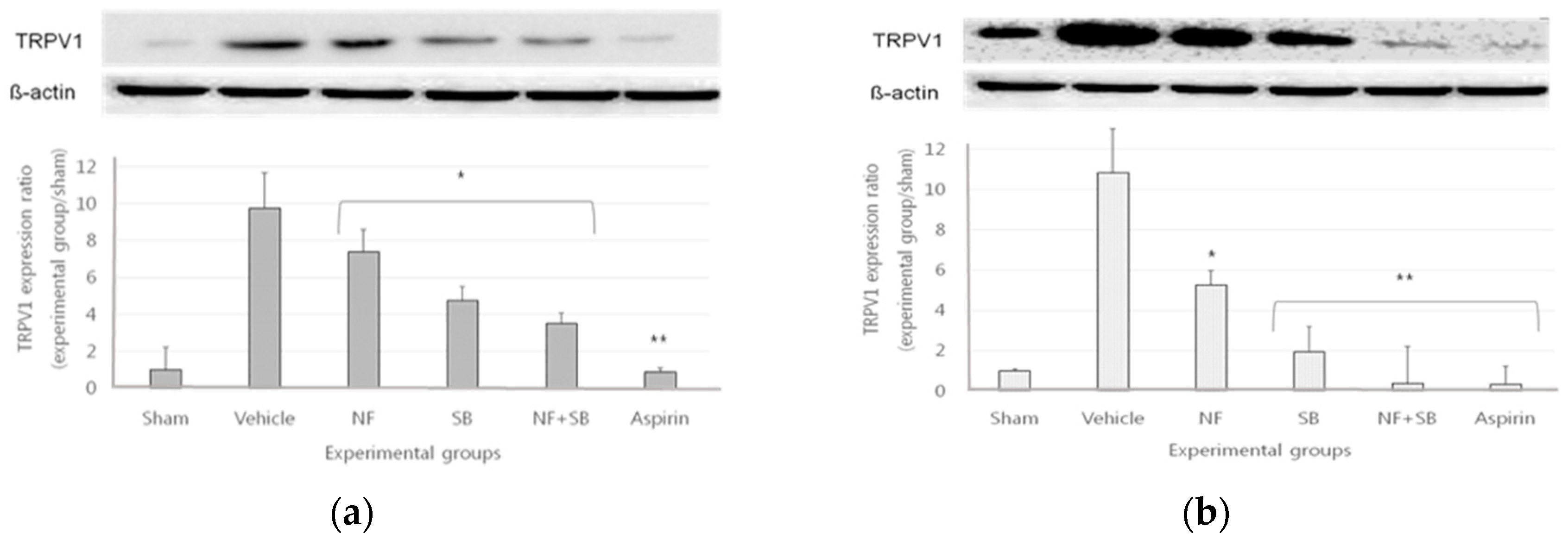
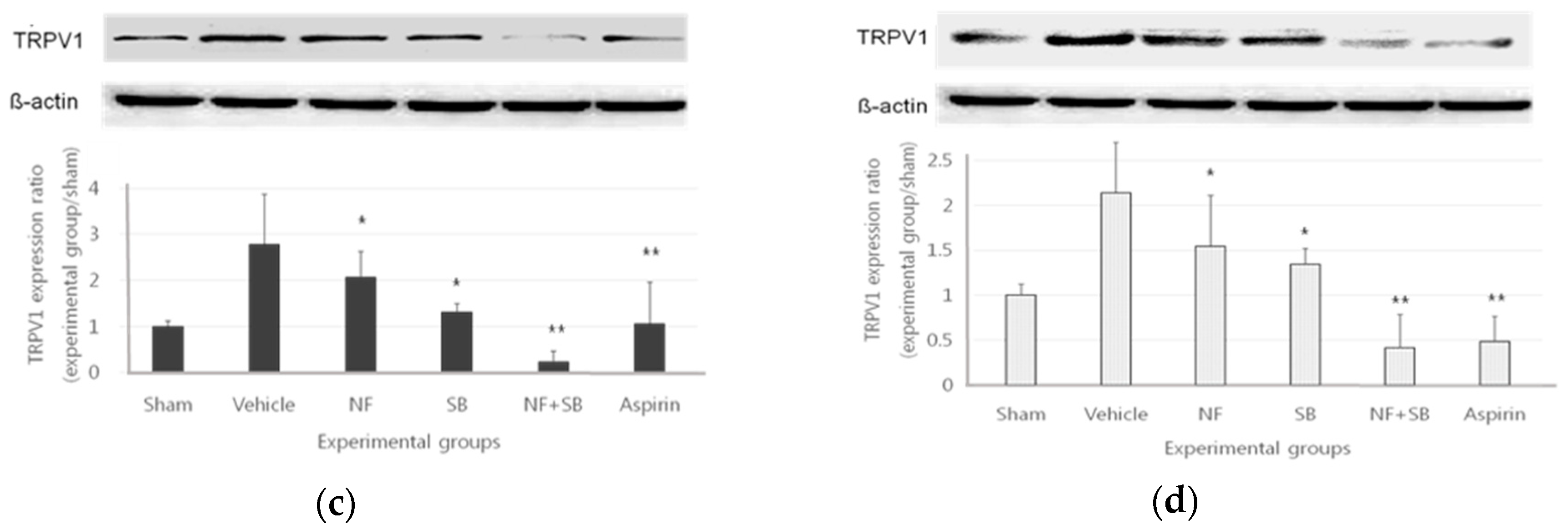
3.4. The TRPV1 Expression in Sciatic Crush Injury Models
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Guclu, B.; Kazanci, B.; Cakir, M.; Coban, M.K. Neuroprotective effects of agmatine in experimental peripheral nerve injury in rats: A prospective randomized and placebo-controlled trial. Turk. Neurosurg. 2014, 24, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, M.F.; Parlakpınar, H.; Ceylan, M.F.; Ediz, L.; Şamdancı, E.; Kekilli, E.; Sağır, M. The effect of sildenafil on recuperation from sciatic nerve injury in rats. Balk. Med. J. 2016, 33, 204. [Google Scholar] [CrossRef] [PubMed]
- Somay, H.; Emon, S.T.; Uslu, S.; Orakdogen, M.; Meric, Z.C.; Ince, U.; Hakan, T. The Histological Effects of Ozone Therapy on Sciatic Nerve Crush Injury in Rats. World Neurosurg. 2017, 105, 702–708. [Google Scholar] [CrossRef] [PubMed]
- White, F.A.; Jung, H.; Miller, R.J. Chemokines and the pathophysiology of neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 20151–20158. [Google Scholar] [CrossRef] [Green Version]
- Camara-Lemarroy, C.R.; Guzman-de la Garza, F.J.; Fernandez-Garza, N.E. Molecular Inflammatory Mediators in Peripheral Nerve Degeneration and Regeneration. Neuroimmunomodulation 2010, 17, 314–324. [Google Scholar] [CrossRef]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef]
- Carlton, S.M.; Coggeshall, R.E. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: A possible mechanism for peripheral sensitization. Neurosci. Lett. 2001, 310, 53–56. [Google Scholar] [CrossRef]
- Ji, R.-R.; Samad, T.A.; Jin, S.-X.; Schmoll, R.; Woolf, C.J. p38 MAPK Activation by NGF in Primary Sensory Neurons after Inflammation Increases TRPV1 Levels and Maintains Heat Hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Funakoshi, K.; Nakano, M.; Atobe, Y.; Goris, R.C.; Kadota, T.; Yazama, F. Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res. 2006, 323, 27–41. [Google Scholar] [CrossRef]
- Engel, M.A.; Khalil, M.; Mueller-Tribbensee, S.M.; Becker, C.; Neuhuber, W.L.; Neurath, M.F.; Reeh, P.W. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J. Gastroenterol. 2012, 47, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Masuko, S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006, 1085, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-L.; Peng, Y.-Y.; Peng, B.-W. Modulation of neuroinflammation: Role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain Behav. Immunity 2017, 64, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Emel, E.; Ergün, S.S.; Kotan, D.; Gürsoy, E.B.; Parman, Y.; Zengin, A.; Nurten, A. Effects of insulin-like growth factor–I and platelet-rich plasma on sciatic nerve crush injury in a rat model. J. Neurosurg. 2011, 114, 522–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimori, S.; Gudis, K.; Sakamoto, C. A Review of Anti-Inflammatory Drug-Induced Gastrointestinal Injury: Focus on Prevention of Small Intestinal Injury. Pharmaceuticals 2010, 3, 1187–1201. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.; Islam, M. Utilization of Mangrove Forest Plant: Nipa Palm (Nypa fruticans Wurmb.). Am. J. Agric. For. 2015, 3, 156–160. [Google Scholar]
- Prasad, N.; Yang, B.; Kong, K.W.; Khoo, H.E.; Sun, J.; Azlan, A.; Ismail, A.; Romli, Z.B. Phytochemicals and Antioxidant Capacity from Nypa fruticans Wurmb. Fruit. Evid. Based Complement. Altern. Med. 2013, 2013, 154606. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Sadeak, I.; Bachar, S.C.; Hossain, T.; Jahan, N.; Chowdhury, M.H.; Jahan, R.; Nasrin, D.; Rahman, M.; Rahman, S. Brine shrimp toxicity study of different Bangladeshi medicinal plants. Adv. Nat. Appl. Sci. 2010, 4, 163–174. [Google Scholar]
- Bandaranayake, W. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133–148. [Google Scholar] [CrossRef]
- Kalender, A.M.; Dogan, A.; Bakan, V.; Yildiz, H.; Gokalp, M.A.; Kalender, M. Effect of Zofenopril on regeneration of sciatic nerve crush injury in a rat model. J. Brachial Plexus Peripher. Nerve Injury 2009, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, A.; Németh, J.; Szabó, Á.; McDougall, J.J.; Zhang, C.; Elekes, K.; Pintér, E.; Szolcsányi, J.; Helyes, Z. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci. Lett. 2005, 385, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Hara, T.; Imai, A.; Sakakibara, A. Differential involvement of TRPV1 receptors at the central and peripheral nerves in CFA-induced mechanical and thermal hyperalgesia. J. Pharm. Pharmacol. 2007, 59, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Y.; Winston, J.H.; Shenoy, M.; Yin, H.; Pendyala, S.; Pasricha, P.J. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology 2007, 133, 1282–1292. [Google Scholar] [CrossRef]
- Chen, Y.; Geis, C.; Sommer, C. Activation of TRPV1 contributes to morphine tolerance: Involvement of the mitogen-activated protein kinase signaling pathway. J. Neurosci. 2008, 28, 5836–5845. [Google Scholar] [CrossRef]
- Altman, R.D. A rationale for combining acetaminophen and NSAIDs for mild-to-moderate pain. Clin. Exp. Rheumatol. 2004, 22, 110–117. [Google Scholar]
- Blondell, R.D.; Azadfard, M.; Wisniewski, A.M. Pharmacologic therapy for acute pain. Am. Fam. Phys. 2013, 87, 766–772. [Google Scholar]
- Voelker, M.; Schachtel, B.P.; Cooper, S.A.; Gatoulis, S.C. Efficacy of disintegrating aspirin in two different models for acute mild-to-moderate pain: Sore throat pain and dental pain. Inflammopharmacology 2016, 24, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Saeed, S.; Gilani, A.; Majoo, R.; Shah, B. Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacol. Res. 1997, 36, 1–7. [Google Scholar] [CrossRef]
- Taha, A.; Angerson, W.; Knill-Jones, R.; Blatchford, O. Upper gastrointestinal haemorrhage associated with low-dose aspirin and anti-thrombotic drugs–A 6-year analysis and comparison with non-steroidal anti-inflammatory drugs. Aliment. Pharmacol. Ther. 2005, 22, 285–289. [Google Scholar] [CrossRef]
- Reilly, C.A.; Taylor, J.L.; Lanza, D.L.; Carr, B.A.; Crouch, D.J.; Yost, G.S. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol. Sci. 2003, 73, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, K.M.; Urban, L.; Medhurst, S.J.; Patel, S.; Panesar, M.; Fox, A.J.; McIntyre, P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003, 304, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, F.; Eslamizade, M.J.; Ghadiri, T.; Modarres Mousavi, S.M.; Hadjighassem, M.; Gorji, A. Effects of TRPV1 on the hippocampal synaptic plasticity in the epileptic rat brain. Synapse 2015, 69, 375–383. [Google Scholar] [CrossRef]
- Takahashi, M.; Kawaguchi, M.; Shimada, K.; Konishi, N.; Furuya, H.; Nakashima, T. Cyclooxygenase-2 expression in Schwann cells and macrophages in the sciatic nerve after single spinal nerve injury in rats. Neurosci. Lett. 2004, 363, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Valente, P.; Ferrer-Montiel, A.; Qin, F.; Szallasi, A. Complex Regulation of TRPV1 and Related Thermo-TRPs: Implications for Therapeutic Intervention. In Transient Receptor Potential Channels; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 491–515. [Google Scholar] [CrossRef]
- Carrasco, C.; Naziroglu, M.; Rodriguez, A.B.; Pariente, J.A. Neuropathic Pain: Delving into the Oxidative Origin and the Possible Implication of Transient Receptor Potential Channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Keeble, J.E.; Bodkin, J.V.; Liang, L.; Wodarski, R.; Davies, M.; Fernandes, E.S.; Coelho Cde, F.; Russell, F.; Graepel, R.; Muscara, M.N.; et al. Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain 2009, 141, 135–142. [Google Scholar] [CrossRef]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhe, V.; Plee-Gautier, E.; Carre, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [Green Version]
- Oehler, B.; Kistner, K.; Martin, C.; Schiller, J.; Mayer, R.; Mohammadi, M.; Sauer, R.S.; Filipovic, M.R.; Nieto, F.R.; Kloka, J.; et al. Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation. Sci. Rep. 2017, 7, 5447. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, S.; Filali, M.; Zhang, J.; Kerr, B.J.; Rivest, S.; Soulet, D.; Iwakura, Y.; de Rivero Vaccari, J.P.; Keane, R.W.; Lacroix, S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: Implications for neuropathic pain. J. Neurosci. 2011, 31, 12533–12542. [Google Scholar] [CrossRef]
- Paul, A.; Cuenda, A.; Bryant, C.E.; Murray, J.; Chilvers, E.R.; Cohen, P.; Gould, G.W.; Plevin, R. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell. Signal. 1999, 11, 491–497. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Franchi, S.; Moretti, S.; Castelli, M.; Procacci, P.; Magnaghi, V.; Panerai, A.E. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J. Neuroimmune Pharmacol. 2013, 8, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration. Glia 2015, 63, 1870–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.; Kendall, D.A. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-X.; Yu, F.; Sanchez, R.M.; Liu, Y.-Q.; Min, J.-W.; Hu, J.-J.; Bsoul, N.B.; Han, S.; Yin, J.; Liu, W.-H. TRPV1 promotes repetitive febrile seizures by pro-inflammatory cytokines in immature brain. Brain Behav. Immunity 2015, 48, 68–77. [Google Scholar] [CrossRef]
- Talbot, S.; Foster, S.L.; Woolf, C.J. Neuroimmunity: Physiology and pathology. Ann. Rev. Immunol. 2016, 34, 421–447. [Google Scholar] [CrossRef]
- Nilius, B.; Flockerzi, V. Mammalian transient receptor potential (TRP) cation channels. Handbook of Experimental Pharmacology. Springer 2014, 222, 207–245. [Google Scholar]
- Laing, R.J.; Dhaka, A. ThermoTRPs and pain. Neuroscientist 2016, 22, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Lee, S.T.; Wu, W.T.; Fu, W.M.; Ho, F.M.; Lin, W.W. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br. J. Pharmacol. 2003, 140, 1077–1087. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.H.; Tavitian, B.; Consortium, I. Noninvasive molecular imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 2012, 32, 1393–1415. [Google Scholar] [CrossRef] [Green Version]
- Legido, A.; Katsetos, C.D. Experimental Studies in Epilepsy: Immunologic and Inflammatory Mechanisms. Semin. Pediatr. Neurol. 2014, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Reza, H.; Haq, W.M.; Das, A.K.; Rahman, S.; Jahan, R.; Rahmatullah, M. Anti-hyperglycemic and antinociceptive activity of methanol leaf and stem extract of Nypa fruticans Wurmb. Pak. J. Pharm. Sci. 2011, 24, 485–488. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.-s.; Hyun, K.-Y. Antinociceptive and Anti-Inflammatory Effects of Nypa fruticans Wurmb by Suppressing TRPV1 in the Sciatic Neuropathies. Nutrients 2020, 12, 135. https://doi.org/10.3390/nu12010135
Kang M-s, Hyun K-Y. Antinociceptive and Anti-Inflammatory Effects of Nypa fruticans Wurmb by Suppressing TRPV1 in the Sciatic Neuropathies. Nutrients. 2020; 12(1):135. https://doi.org/10.3390/nu12010135
Chicago/Turabian StyleKang, Mi-sun, and Kyung-Yae Hyun. 2020. "Antinociceptive and Anti-Inflammatory Effects of Nypa fruticans Wurmb by Suppressing TRPV1 in the Sciatic Neuropathies" Nutrients 12, no. 1: 135. https://doi.org/10.3390/nu12010135



