A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis
Abstract
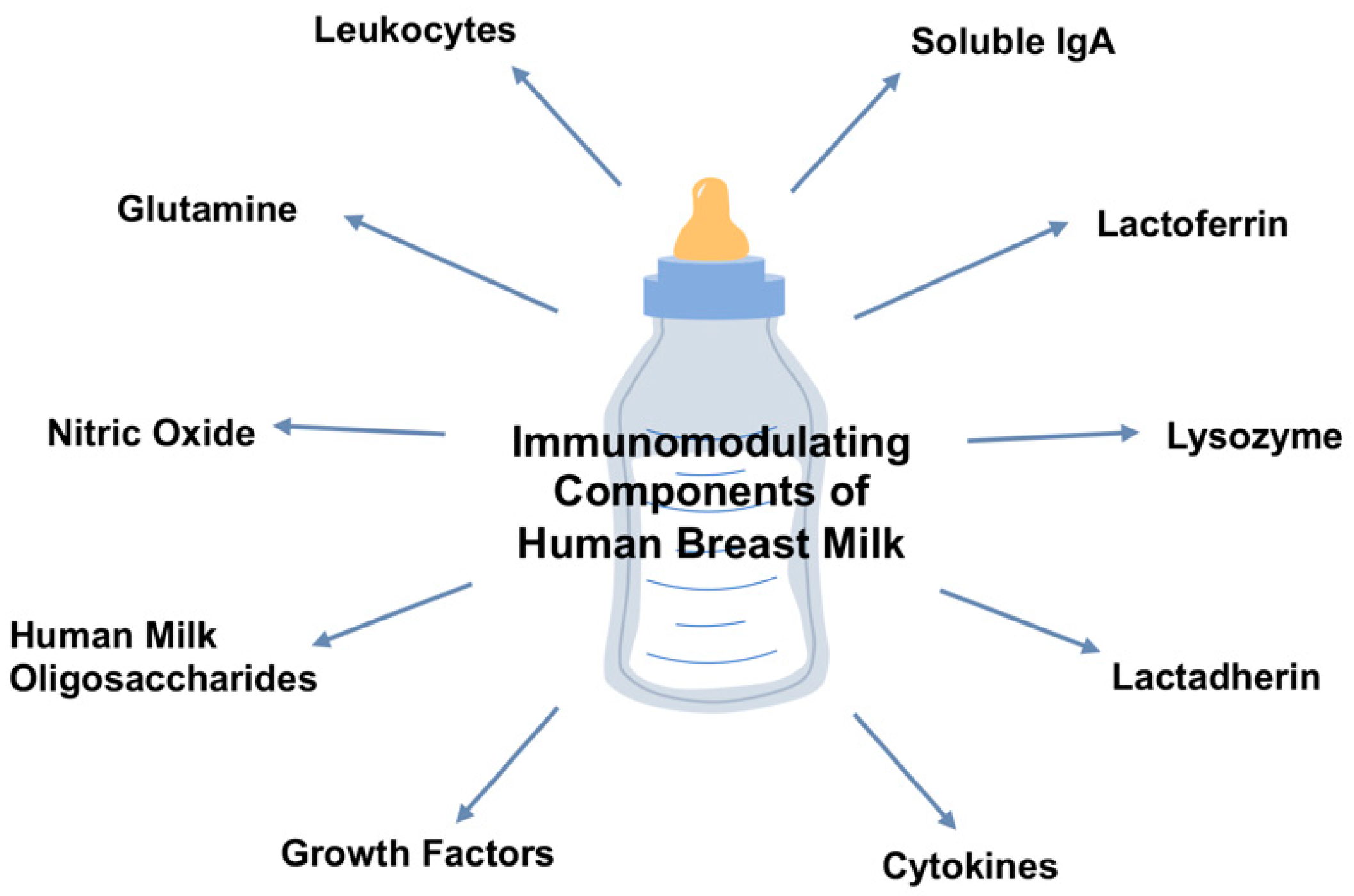
:1. Introduction
2. Breast Milk and the Host-Microbial Relationship
2.1. Maternal Soluble IgA
2.2. Lactoferrin
2.3. Lysozyme
2.4. Lactadherin
2.5. Epidermal Growth Factor
2.6. Heparin-Binding Epidermal Growth Factor
2.7. Transforming Growth Factor-β2
2.8. Prebiotics and Oligosaccharides
2.9. Glutamine
3. Breast Milk and Immune Homeostasis
3.1. Cellular Mechanisms
3.2. Cytokines
3.3. Nitric Oxide
4. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.W.; Stoll, B.J. Necrotising enterocolitis. Lancet 2006, 368, 1271–1283. [Google Scholar] [CrossRef]
- Lucas, A.; Cole, T.J. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
- Schanler, R.J. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005, 116, 400–406. [Google Scholar] [CrossRef]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567.e1. [Google Scholar] [CrossRef] [Green Version]
- Trend, S.; Strunk, T.; Lloyd, M.L.; Kok, C.H.; Metcalfe, J.; Geddes, D.T.; Lai, C.T.; Richmond, P.; Doherty, D.A.; Simmer, K.; et al. Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum. Br. J. Nutr. 2016, 115, 1178–1193. [Google Scholar] [CrossRef] [Green Version]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.C.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3074–3079. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.C.; Chen, C.H.; Lin, M.C.; Tsai, C.R.; Liang, J.T.; Wang, T.M. Changes in preterm breast milk nutrient content in the first month. Pediatr. Neonatol. 2014, 55, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.; Petrova, A. Biologically active breast milk proteins in association with very preterm delivery and stage of lactation. J. Perinatol. 2011, 31, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.C.; Kaetzel, C.S. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens 2014, 3, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Hassiotou, F.; Hepworth, A.R.; Metzger, P.; Tat Lai, C.; Trengove, N.; Hartmann, P.E.; Filgueira, L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl. Immunol. 2013, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of breast milk. Rev. Assoc. Med. Bras. 2016, 62, 584–593. [Google Scholar] [CrossRef]
- Togawa, J.I.; Nagase, H.; Tanaka, K.; Inamori, M.; Nakajima, A.; Ueno, N.; Saito, T.; Sekihara, H. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J. Gastroenterol. Hepatol. 2002, 17, 1291–1298. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 2017, CD007137. [Google Scholar] [CrossRef]
- He, Y.; Cao, L.; Yu, J. Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants. Medicine (Baltimore) 2018, 97, e11976. [Google Scholar] [CrossRef]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral lactoferrin to prevent infection for very preterm infants: The ELFIN RCT. Health Technol. Assess. 2018, 22, 1–60. [Google Scholar] [CrossRef]
- Mara, M.A.; Good, M.; Weitkamp, J.-H. Innate and adaptive immunity in necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 394–399. [Google Scholar] [CrossRef]
- McElroy, S.J.; Prince, L.S.; Weitkamp, J.-H.; Reese, J.; Slaughter, J.C.; Polk, D.B. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: A potential role in neonatal necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G656–G666. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sherman, M.P.; Prince, L.S.; Bader, D.; Weitkamp, J.-H.; Slaughter, J.C.; McElroy, S.J. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis. Model. Mech. 2012, 5, 522–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lueschow, S.R.; Stumphy, J.; Gong, H.; Kern, S.L.; Elgin, T.G.; Underwood, M.A.; Kalanetra, K.M.; Mills, D.A.; Wong, M.H.; Meyerholz, D.K.; et al. Loss of murine Paneth cell function alters the immature intestinal microbiome and mimics changes seen in neonatal necrotizing enterocolitis. PLoS ONE 2018, 13, e0204967. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.M.; Ishihara, S.; Mishima, Y.; Oshima, N.; Moriyama, I.; Yuki, T.; Kadowaki, Y.; Rumi, M.A.K.; Amano, Y.; Kinoshita, Y. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J. Immunol. 2009, 182, 7222–7232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Lei, Y.; He, X.; Liu, D.; He, Z. Role of lactadherin in intestinal barrier integrity in experimental neonatal necrotizing enterocolitis. J. Cell. Biochem. 2019, 120, 19509–19517. [Google Scholar] [CrossRef]
- Nair, R.R.; Warner, B.B.; Warner, B.W. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 107–113. [Google Scholar] [CrossRef]
- Warner, B.B.; Ryan, A.L.; Seeger, K.; Leonard, A.C.; Erwin, C.R.; Warner, B.W. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J. Pediatr. 2007, 150, 358–363. [Google Scholar] [CrossRef]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am. J. Physiol. Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef]
- Knott, A.W.; Juno, R.J.; Jarboe, M.D.; Zhang, Y.; Profitt, S.A.; Thoerner, J.C.; Erwin, C.R.; Warner, B.W. EGF receptor signaling affects bcl-2 family gene expression and apoptosis after massive small bowel resection. J. Pediatr. Surg. 2003, 38, 875–880. [Google Scholar] [CrossRef]
- Clark, J.A.; Lane, R.H.; Maclennan, N.K.; Holubec, H.; Dvorakova, K.; Halpern, M.D.; Williams, C.S.; Payne, C.M.; Dvorak, B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G755–G762. [Google Scholar] [CrossRef] [Green Version]
- Michalsky, M.P.; Lara-Marquez, M.; Chun, L.; Besner, G.E. Heparin-binding EGF-like growth factor is present in human amniotic fluid and breast milk. J. Pediatr. Surg. 2002, 37, 1–6. [Google Scholar] [CrossRef]
- Yang, J.; Su, Y.; Zhou, Y.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) therapy for intestinal injury: Application and future prospects. Pathophysiology 2014, 21, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; El-Assal, O.N.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin. Pediatr. Surg. 2005, 14, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, B.; Khailova, L.; Clark, J.A.; Hosseini, D.M.; Arganbright, K.M.; Reynolds, C.A.; Halpern, M.D. Comparison of epidermal growth factor and heparin-binding epidermal growth factor-like growth factor for prevention of experimental necrotizing enterocolitis. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sitarik, A.R.; Bobbitt, K.R.; Havstad, S.L.; Fujimura, K.E.; Levin, A.M.; Zoratti, E.M.; Kim, H.; Woodcroft, K.J.; Wegienka, G.; Ownby, D.R.; et al. Breast milk transforming growth factor β is associated with neonatal gut microbial composition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e60–e67. [Google Scholar] [CrossRef]
- Namachivayam, K.; Coffing, H.P.; Sankaranarayanan, N.V.; Jin, Y.; MohanKumar, K.; Frost, B.L.; Blanco, C.L.; Patel, A.L.; Meier, P.P.; Garzon, S.A.; et al. Transforming growth factor-β2 is sequestered in preterm human milk by chondroitin sulfate proteoglycans. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G171–G180. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, A.; Kelly, D.R.; Nicola, T.; Ambalavanan, N.; Jain, S.K.; Murphy-Ullrich, J.; Athar, M.; Shimamura, M.; Bhandari, V.; Aprahamian, C.; et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011, 140, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. Human milk oligosaccharides in the prevention of necrotizing enterocolitis: A journey from in vitro and in vivo models to mother-infant cohort studies. Front. Pediatr. 2018, 6, 385. [Google Scholar] [CrossRef]
- Moukarzel, S.; Bode, L. Human milk oligosaccharides and the preterm infant: A journey in sickness and in health. Clin. Perinatol. 2017, 44, 193–207. [Google Scholar] [CrossRef]
- Holscher, H.D.; Bode, L.; Tappenden, K.A. Human milk oligosaccharides influence intestinal epithelial cell maturation in vitro. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 296–301. [Google Scholar] [CrossRef]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef]
- Rudloff, S.; Kuntz, S.; Ostenfeldt Rasmussen, S.; Roggenbuck, M.; Sprenger, N.; Kunz, C.; Sangild, P.T.; Brandt Bering, S. Metabolism of milk oligosaccharides in preterm pigs sensitive to necrotizing enterocolitis. Front. Nutr. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.O.; Martin, L.; Østergaard, M.V.; Rudloff, S.; Roggenbuck, M.; Nguyen, D.N.; Sangild, P.T.; Bering, S.B. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J. Nutr. Biochem. 2017, 40, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Good, M.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Lu, P.; Fulton, W.B.; Martin, L.Y.; Prindle, T.; Nino, D.F.; Zhou, Q.; et al. The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 2016, 116, 1175–1187. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Carratù, B.; Boniglia, C.; Lammardo, A.M.; Riva, E.; Sanzini, E. Free glutamine and glutamic acid increase in human milk through a three-month lactation period. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 508–512. [Google Scholar] [CrossRef]
- Baldeón, M.E.; Zertuche, F.; Flores, N.; Fornasini, M. Free amino acid content in human milk is associated with infant gender and weight gain during the first four months of lactation. Nutrients 2019, 11, 2239. [Google Scholar] [CrossRef] [Green Version]
- Larnkjær, A.; Bruun, S.; Pedersen, D.; Zachariassen, G.; Barkholt, V.; Agostoni, C.; Mlgaard, C.; Husby, S.; Michaelsen, K.F. Free amino acids in human milk and associations with maternal anthropometry and infant growth. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Kim, H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Li, W.; Wang, W.; Liu, C.; Han, F.; et al. Glutamine and intestinal barrier function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef]
- Becker, R.M.; Wu, G.; Galanko, J.A.; Chen, W.; Maynor, A.R.; Bose, C.L.; Rhoads, J.M. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J. Pediatr. 2000, 137, 785–793. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Zheng, X.-H.; Rong, X.; Huang, L.-G. Glutamine downregulates TLR-2 and TLR-4 expression and protects intestinal tract in preterm neonatal rats with necrotizing enterocolitis. J. Pediatr. Surg. 2014, 49, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Neal, M.D.; Siggers, R.; Sho, S.; Ma, C.; Branca, M.F.; Prindle, T.; Russo, A.M.; Afrazi, A.; Good, M.; et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012, 143, 708–718.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, M.; Sodhi, C.P.; Egan, C.E.; Afrazi, A.; Jia, H.; Yamaguchi, Y.; Lu, P.; Branca, M.F.; Ma, C.; Prindle, T.; et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015, 8, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Shi, X.-H.; Richardson, W.M.; Grant, Z.S.; Shapiro, R.A.; Prindle, T.; Branca, M.; Russo, A.; Gribar, S.C.; Ma, C.; et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 2010, 138, 185–196. [Google Scholar] [CrossRef] [Green Version]
- El-Shimi, M.S.; Awad, H.A.; Abdelwahed, M.A.; Mohamed, M.H.; Khafagy, S.M.; Saleh, G. Enteral L-arginine and glutamine supplementation for prevention of NEC in preterm neonates. Int. J. Pediatr. 2015, 2015, 856091. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; Moe-Byrne, T.; McGuire, W. Glutamine supplementation for young infants with severe gastrointestinal disease. Cochrane Database Syst. Rev. 2007, CD005947. [Google Scholar] [CrossRef] [Green Version]
- Moe-Byrne, T.; Brown, J.V.E.; McGuire, W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2016, 4, CD001457. [Google Scholar]
- Zhou, L.; Yoshimura, Y.; Huang, Y.; Suzuki, R.; Yokoyama, M.; Okabe, M.; Shimamura, M. Two independent pathways of maternal cell transmission to offspring: Through placenta during pregnancy and by breast-feeding after birth. Immunology 2000, 101, 570–580. [Google Scholar] [CrossRef]
- Lewis, E.D.; Richard, C.; Larsen, B.M.; Field, C.J. The importance of human milk for immunity in preterm infants. Clin. Perinatol. 2017, 44, 23–47. [Google Scholar] [CrossRef]
- Trend, S.; de Jong, E.; Lloyd, M.L.; Kok, C.H.; Richmond, P.; Doherty, D.A.; Simmer, K.; Kakulas, F.; Strunk, T.; Currie, A. Leukocyte populations in human preterm and term breast milk identified by multicolour flow cytometry. PLoS ONE 2015, 10, e0135580. [Google Scholar] [CrossRef] [Green Version]
- Cabinian, A.; Sinsimer, D.; Tang, M.; Zumba, O.; Mehta, H.; Toma, A.; Sant’Angelo, D.; Laouar, Y.; Laouar, A. Transfer of maternal immune cells by breastfeeding: Maternal cytotoxic T lymphocytes present in breast milk localize in the peyer’s patches of the nursed infant. PLoS ONE 2016, 11, e0156762. [Google Scholar] [CrossRef] [PubMed]
- Riskin, A.; Almog, M.; Peri, R.; Halasz, K.; Srugo, I.; Kessel, A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatr. Res. 2012, 71, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustundag, B.; Yilmaz, E.; Dogan, Y.; Akarsu, S.; Canatan, H.; Halifeoglu, I.; Cikim, G.; Aygun, A.D. Levels of cytokines (IL-1beta, IL-2, IL-6, IL-8, TNF-alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediators Inflamm. 2005, 2005, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minekawa, R.; Takeda, T.; Sakata, M.; Hayashi, M.; Isobe, A.; Yamamoto, T.; Tasaka, K.; Murata, Y. Human breast milk suppresses the transcriptional regulation of IL-1beta-induced NF-kappaB signaling in human intestinal cells. Am. J. Physiol. Cell Physiol. 2004, 287, C1404–C1411. [Google Scholar] [CrossRef] [Green Version]
- Bryan, D.-L.; Forsyth, K.D.; Gibson, R.A.; Hawkes, J.S. Interleukin-2 in human milk: A potential modulator of lymphocyte development in the breastfed infant. Cytokine 2006, 33, 289–293. [Google Scholar] [CrossRef]
- Hassan, J.; Reen, D.J. Reduced primary antigen-specific T-cell precursor frequencies in neonates is associated with deficient interleukin-2 production. Immunology 1996, 87, 604–608. [Google Scholar] [CrossRef]
- Saito, S.; Maruyama, M.; Kato, Y.; Moriyama, I.; Ichijo, M. Detection of IL-6 in human milk and its involvement in IgA production. J. Reprod. Immunol. 1991, 20, 267–276. [Google Scholar] [CrossRef]
- Rudloff, H.E.; Schmalstieg, F.C.; Palkowetz, K.H.; Paszkiewicz, E.J.; Goldman, A.S. Interleukin-6 in human milk. J. Reprod. Immunol. 1993, 23, 13–20. [Google Scholar] [CrossRef]
- Maheshwari, A.; Lu, W.; Lacson, A.; Barleycorn, A.A.; Nolan, S.; Christensen, R.D.; Calhoun, D.A. Effects of interleukin-8 on the developing human intestine. Cytokine 2002, 20, 256–267. [Google Scholar] [CrossRef]
- Polat, A.; Tunc, T.; Erdem, G.; Yerebasmaz, N.; Tas, A.; Beken, S.; Basbozkurt, G.; Saldir, M.; Zenciroglu, A.; Yaman, H. Interleukin-8 and its receptors in human milk from mothers of full-term and premature infants. Breastfeed. Med. 2016, 11, 247–251. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.; Chheda, S.; Mei, F.; Palkowetz, K.H.; Rudloff, H.E.; Schmalstieg, F.C.; Rassin, D.K.; Goldman, A.S. Interleukin-10 in human milk. Pediatr. Res. 1995, 37, 444–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [PubMed]
- Jinquan, T.; Larsen, C.G.; Gesser, B.; Matsushima, K.; Thestrup-Pedersen, K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J. Immunol. 1993, 151, 4545–4551. [Google Scholar] [PubMed]
- Fluckiger, A.C.; Garrone, P.; Durand, I.; Galizzi, J.P.; Banchereau, J. Interleukin 10 (IL-10) upregulates functional high affinity IL-2 receptors on normal and leukemic B lymphocytes. J. Exp. Med. 1993, 178, 1473–1481. [Google Scholar] [CrossRef] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Beaurepaire, C.; Smyth, D.; McKay, D.M. Interferon-gamma regulation of intestinal epithelial permeability. J. Interferon Cytokine Res. 2009, 29, 133–144. [Google Scholar] [CrossRef]
- Hui, L.; Dai, Y.; Guo, Z.; Zhang, J.; Zheng, F.; Bian, X.; Wu, Z.; Jiang, Q.; Guo, M.; Ma, K.; et al. Immunoregulation effects of different γδT cells and toll-like receptor signaling pathways in neonatal necrotizing enterocolitis. Medicine (Baltimore). 2017, 96, e6077. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; da Salles, É.S.L.; Bhatia, J.; Hale, V.L.; Baban, B. Innate immunity of neonates and infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Buescher, E.S.; Malinowska, I. Soluble receptors and cytokine antagonists in human milk. Pediatr. Res. 1996, 40, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, A. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, N.K.; Guner, Y.S.; Hunter, C.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nankervis, C.A.; Giannone, P.J.; Reber, K.M. The neonatal intestinal vasculature: Contributing factors to necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Akçay, F.; Aksoy, H.; Memişoǧullari, R. Effect of breast-feeding on concentration of nitric oxide in breast milk. Ann. Clin. Biochem. 2002, 39, 68–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, H.; Watkins, S.; Reblock, K.; Rowe, M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J. Pediatr. Surg. 1997, 32, 275–282. [Google Scholar] [CrossRef]
- Yazji, I.; Sodhi, C.P.; Lee, E.K.; Good, M.; Egan, C.E.; Afrazi, A.; Neal, M.D.; Jia, H.; Lin, J.; Ma, C.; et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 9451–9456. [Google Scholar] [CrossRef] [Green Version]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [Green Version]
- Gay, M.C.L.; Koleva, P.T.; Slupsky, C.M.; du Toit, E.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; Campbell, D.E.; Prescott, S.L.; et al. Worldwide variation in human milk metabolome: Indicators of breast physiology and maternal lifestyle? Nutrients 2018, 10, 1151. [Google Scholar] [CrossRef] [Green Version]
| Cytokine | Composition in Human Milk and Significance | References |
|---|---|---|
| Interleukin (IL)-1 |
| [53,64] |
| IL-2 |
| [63,65,66] |
| IL-6 |
| [63,67,68] |
| IL-8 |
| [63,69,70] |
| IL-10 |
| [71,72,73,74,75] |
| IFN-γ |
| [76,77,78,79] |
| TNF-α |
| [63,80,81] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolan, L.S.; Parks, O.B.; Good, M. A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis. Nutrients 2020, 12, 14. https://doi.org/10.3390/nu12010014
Nolan LS, Parks OB, Good M. A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis. Nutrients. 2020; 12(1):14. https://doi.org/10.3390/nu12010014
Chicago/Turabian StyleNolan, Lila S., Olivia B. Parks, and Misty Good. 2020. "A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis" Nutrients 12, no. 1: 14. https://doi.org/10.3390/nu12010014
APA StyleNolan, L. S., Parks, O. B., & Good, M. (2020). A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis. Nutrients, 12(1), 14. https://doi.org/10.3390/nu12010014





