The Role of a Nutrition Support Team in the Management of Intestinal Failure Patients
Abstract
:1. Introduction
2. General Management of Intestinal Failure Patients
3. The Composition of a Nutrition Support Team
4. Improved Outcomes with Nutrition Support Teams
4.1. Complications in Adults Receiving Parenteral Nutrition
4.2. Complications in Children Receiving Parenteral Nutrition
4.3. Prescription of Parenteral Nutrition
4.4. Discussion
5. Experience of Two Dutch Intestinal Failure Nutrition Support Teams
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fleming, C.R. Intestinal failure. Nutr. Surg. Patient Clin. Surg. Int. 1981, 2, 219–235. [Google Scholar]
- Grainger, J.T.; Maeda, Y.; Donnelly, S.C.; Vaizey, C.J. Assessment and management of patients with intestinal failure: A multidisciplinary approach. Clin. Exp. Gastroenterol. 2018, 11, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. Espen guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Pelaez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. Espen endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef]
- Goulet, O.; Olieman, J.; Ksiazyk, J.; Spolidoro, J.; Tibboe, D.; Kohler, H.; Yagci, R.V.; Falconer, J.; Grimble, G.; Beattie, R.M. Neonatal short bowel syndrome as a model of intestinal failure: Physiological background for enteral feeding. Clin. Nutr. 2013, 32, 162–171. [Google Scholar] [CrossRef]
- Gales, B.J.; Riley, D.G. Improved total parenteral nutrition therapy management by a nutritional support team. Hosp. Pharm. 1994, 29, 469–470, 473–475. [Google Scholar]
- Ochoa, J.B.; Magnuson, B.; Swintowsky, M.; Loan, T.; Boulanger, B.; McClain, C.; Kearney, P. Long-term reduction in the cost of nutritional intervention achieved by a nutrition support service. Nutr. Clin. Pract. 2000, 15, 174–180. [Google Scholar] [CrossRef]
- Sacks, G.S. The shrinking of formalized nutrition education in health professions curricula and postgraduate training. JPEN J. Parenter. Enter. Nutr. 2017, 41, 217–225. [Google Scholar] [CrossRef]
- Cuerda, C.; Muscaritoli, M.; Donini, L.M.; Baque, P.; Barazzoni, R.; Gaudio, E.; Jezek, D.; Krznaric, Z.; Pirlich, M.; Schetgen, M.; et al. Nutrition education in medical schools (nems). An espen position paper. Clin. Nutr. 2019, 38, 969–974. [Google Scholar] [CrossRef]
- Devries, S.; Willett, W.; Bonow, R.O. Nutrition education in medical school, residency training, and practice. JAMA 2019, 321, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Van Puffelen, E.; Hulst, J.M.; Vanhorebeek, I.; Dulfer, K.; Van den Berghe, G.; Joosten, K.F.M.; Verbruggen, S. Effect of late versus early initiation of parenteral nutrition on weight deterioration during picu stay: Secondary analysis of the pepanic randomised controlled trial. Clin. Nutr. 2019, 39, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Bellingan, G.; Leonard, R.; Mythen, M.G.; Rowan, K.M.; et al. Trial of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, S. Short-bowel syndrome. In Textbook of Pediatric Gastroenterology and Nutrition; Springer Publishing International: New York, NY, USA, 2016; pp. 495–503. [Google Scholar]
- Baker, M.L.; Williams, R.N.; Nightingale, J.M. Causes and management of a high-output stoma. Colorectal Dis. 2011, 13, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Neelis, E.; Kouwenhoven, S.; Olieman, J.; Tabbers, M.; Jonkers, C.; Wells, J.; Fewtrell, M.; Wijnen, R.; Rings, E.; de Koning, B.; et al. Body composition using air displacement plethysmography in children with intestinal failure receiving long-term home parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Skallerup, A.; Nygaard, L.; Olesen, S.S.; Kohler, M.; Vinter-Jensen, L.; Rasmussen, H.H. The prevalence of sarcopenia is markedly increased in patients with intestinal failure and associates with several risk factors. Clin. Nutr. 2018, 37, 2029–2035. [Google Scholar] [CrossRef]
- Neelis, E.; Olieman, J.; Rizopoulos, D.; Wijnen, R.; Rings, E.; de Koning, B.; Hulst, J. Growth, body composition, and micronutrient abnormalities during and after weaning off home parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, e95–e100. [Google Scholar] [CrossRef] [Green Version]
- Ubesie, A.C.; Kocoshis, S.A.; Mezoff, A.G.; Henderson, C.J.; Helmrath, M.A.; Cole, C.R. Multiple micronutrient deficiencies among patients with intestinal failure during and after transition to enteral nutrition. J. Pediatr. 2013, 163, 1692–1696. [Google Scholar] [CrossRef] [Green Version]
- Culkin, A. Nutrition support in intestinal failure. In Advanced Nutrition and Dietetics in Nutrition Support; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 302–312. [Google Scholar]
- Hill, S.; Ksiazyk, J.; Prell, C.; Tabbers, M.; The ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. Espghan/espen/espr/cspen guidelines on pediatric parenteral nutrition: Home parenteral nutrition. Clin. Nutr. 2018, 37, 2401–2408. [Google Scholar] [CrossRef] [Green Version]
- Zemrani, B.; Bines, J.E. Monitoring of long-term parenteral nutrition in children with intestinal failure. JGH Open 2019, 3, 163–172. [Google Scholar] [CrossRef]
- Philip, N.A.; Ahmed, N.; Pitchumoni, C.S. Spectrum of drug-induced chronic diarrhea. J. Clin. Gastroenterol. 2017, 51, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.H.; Duggan, C.; Teitelbaum, D.H.; Wales, P.W.; Balint, J.; Venick, R.; Rhee, S.; Sudan, D.; Mercer, D.; Martinez, J.A.; et al. Natural history of pediatric intestinal failure: Initial report from the Pediatric Intestinal Failure Consortium. J. Pediatr. 2012, 161, 723–728.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Gastroenterological, A. American gastroenterological association medical position statement: Short bowel syndrome and intestinal transplantation. Gastroenterology 2003, 124, 1105–1110. [Google Scholar]
- Bielawska, B.; Allard, J.P. Parenteral nutrition and intestinal failure. Nutrients 2017, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, P.B. Spectrum of short bowel syndrome in adults: Intestinal insufficiency to intestinal failure. JPEN J. Parenter. Enter. Nutr. 2014, 38, 8S–13S. [Google Scholar] [CrossRef] [Green Version]
- Howard, L.; Ashley, C. Management of complications in patients receiving home parenteral nutrition. Gastroenterology 2003, 124, 1651–1661. [Google Scholar] [CrossRef]
- Beath, S.V.; Gowen, H.; Puntis, J.W. Trends in paediatric home parenteral nutrition and implications for service development. Clin. Nutr. 2011, 30, 499–502. [Google Scholar] [CrossRef]
- Pironi, L.; Goulet, O.; Buchman, A.; Messing, B.; Gabe, S.; Candusso, M.; Bond, G.; Gupte, G.; Pertkiewicz, M.; Steiger, E.; et al. Outcome on home parenteral nutrition for benign intestinal failure: A review of the literature and benchmarking with the european prospective survey of espen. Clin. Nutr. 2012, 31, 831–845. [Google Scholar] [CrossRef]
- Duggan, C.P.; Jaksic, T. Pediatric intestinal failure. N. Engl. J. Med. 2017, 377, 666–675. [Google Scholar] [CrossRef]
- Nightingale, J. Nutrition support teams: How they work, are set up and maintained. Frontline Gastroenterol. 2010, 1, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.P. The uses and limitations of nutritional support the arvid wretlind lecture given at the 14th espen congress in vienna, 1992. Clin. Nutr. 1992, 11, 319–330. [Google Scholar] [CrossRef]
- Minard, G. The history of surgically placed feeding tubes. Nutr. Clin. Pract. 2006, 21, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Dudrick, S.J.; Wilmore, D.W.; Vars, H.M.; Rhoads, J.E. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery 1968, 64, 134–142. [Google Scholar] [PubMed]
- DeJong, P.C.; Von Meyenfeldt, M.R.; Rouflart, M.; Wesdorp, R.I.; Soeters, P.B. Complications of central venous catheterization of the subclavian vein: The influence of a parenteral nutrition team. Acta Anaesthesiol. Scand. Suppl. 1985, 81, 48–52. [Google Scholar] [PubMed]
- Koehler, A.N.; Yaworski, J.A.; Gardner, M.; Kocoshis, S.; Reyes, J.; Barksdale, E.M., Jr. Coordinated interdisciplinary management of pediatric intestinal failure: A 2-year review. J. Pediatr. Surg. 2000, 35, 380–385. [Google Scholar] [CrossRef]
- Butterworth, C.E. The skeleton in the hospital closet. 1974. Nutrition 1994, 10, 435–441. [Google Scholar]
- King’s, F.; Lennard-Jones, J.E. A Positive Approach to Nutrition as Treatment; King’s Fund Centre London: London, UK, 1992. [Google Scholar]
- Reber, E.; Strahm, R.; Bally, L.; Schuetz, P.; Stanga, Z. Efficacy and efficiency of nutritional support teams. J. Clin. Med. 2019, 8, 1281. [Google Scholar] [CrossRef] [Green Version]
- Worthington, P.; Balint, J.; Bechtold, M.; Bingham, A.; Chan, L.N.; Durfee, S.; Jevenn, A.K.; Malone, A.; Mascarenhas, M.; Robinson, D.T.; et al. When is parenteral nutrition appropriate? JPEN J. Parenter. Enter. Nutr. 2017, 41, 324–377. [Google Scholar] [CrossRef]
- Dreesen, M.; Foulon, V.; Vanhaecht, K.; De Pourcq, L.; Hiele, M.; Willems, L. Guidelines recommendations on care of adult patients receiving home parenteral nutrition: A systematic review of global practices. Clin. Nutr. 2012, 31, 602–608. [Google Scholar] [CrossRef]
- Staun, M.; Pironi, L.; Bozzetti, F.; Baxter, J.; Forbes, A.; Joly, F.; Jeppesen, P.; Moreno, J.; Hebuterne, X.; Pertkiewicz, M.; et al. Espen guidelines on parenteral nutrition: Home parenteral nutrition (hpn) in adult patients. Clin. Nutr. 2009, 28, 467–479. [Google Scholar] [CrossRef]
- Gillanders, L.; Angstmann, K.; Ball, P.; Chapman-Kiddell, C.; Hardy, G.; Hope, J.; Smith, R.; Strauss, B.; Russell, D.; Australasian Society of Parenteral and Enteral Nutrition. Auspen clinical practice guideline for home parenteral nutrition patients in australia and new zealand. Nutrition 2008, 24, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- ASPEN Board of Directors; The Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J. Parenter. Enter. Nutr. 2002, 26, 1SA–138SA. [Google Scholar] [CrossRef] [Green Version]
- DeLegge, M.H.; Kelly, A.T. State of nutrition support teams. Nutr. Clin. Pract. 2013, 28, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Neelis, E.; de Koning, B.; van Winckel, M.; Tabbers, M.; Hill, S.; Hulst, J. Wide variation in organisation and clinical practice of paediatric intestinal failure teams: An international survey. Clin. Nutr. 2018, 37, 2271–2279. [Google Scholar] [CrossRef]
- Johnson, T.; Sexton, E. Managing children and adolescents on parenteral nutrition: Challenges for the nutritional support team. Proc. Nutr. Soc. 2006, 65, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Javid, P.J.; Wendel, D.; Horslen, S.P. Organization and outcomes of multidisciplinary intestinal failure teams. Semin. Pediatr. Surg. 2018, 27, 218–222. [Google Scholar] [CrossRef]
- Huisman-de Waal, G.; Schoonhoven, L.; Jansen, J.; Wanten, G.; van Achterberg, T. The impact of home parenteral nutrition on daily life—A review. Clin. Nutr. 2007, 26, 275–288. [Google Scholar] [CrossRef]
- Van Oers, H.A.; Haverman, L.; Olieman, J.F.; Neelis, E.G.; Jonkers-Schuitema, C.F.; Grootenhuis, M.A.; Tabbers, M.M. Health-related quality of life, anxiety, depression and distress of mothers and fathers of children on home parenteral nutrition. Clin. Nutr. 2019, 38, 1905–1912. [Google Scholar] [CrossRef]
- Nehme, A.E. Nutritional support of the hospitalized patient. The team concept. JAMA 1980, 243, 1906–1908. [Google Scholar] [CrossRef]
- Braun, K.; Utech, A.; Velez, M.E.; Walker, R. Parenteral nutrition electrolyte abnormalities and associated factors before and after nutrition support team initiation. JPEN J. Parenter. Enter. Nutr. 2018, 42, 387–392. [Google Scholar] [CrossRef]
- Chong, P.F.; Paraidathathu, T. Effects of a nutrition support team on clinical outcomes, metabolic complications and electrolyte abnormalities in patients receiving parenteral nutrition. Asia Pac. J. Clin. Nutr. 2013, 22, 548–556. [Google Scholar] [PubMed]
- Naylor, C.J.; Griffiths, R.D.; Fernandez, R.S. Does a multidisciplinary total parenteral nutrition team improve patient outcomes? A systematic review. JPEN J. Parenter. Enter. Nutr. 2004, 28, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, E.B.; Young, L.S.; Chertow, G.M.; Randall, S.; Clemons, T.; Jacobs, D.O.; Robinson, M.K. Metabolic and monetary costs of avoidable parenteral nutrition use. JPEN J. Parenter. Enter. Nutr. 1999, 23, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.J.; Schepers, G.; Gee, J.P.; Alberts, C.C.; Eckhauser, F.E.; Kirking, D.M. Consultative total parenteral nutrition teams: The effect on the incidence of total parenteral nutrition-related complications. JPEN J. Parenter. Enter. Nutr. 1984, 8, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.M.; Munyer, T.O.; Salem, R.B.; Yost, R.L. Parenteral nutrition utilization: Evaluation of an educational protocol and consult service. JPEN J. Parenter. Enter. Nutr. 1979, 3, 433–437. [Google Scholar] [CrossRef]
- Jacobs, D.O.; Melnik, G.; Forlaw, L.; Gebhardt, C.; Settle, R.G.; DiSipio, M.; Rombeau, J.L. Impact of a nutritional support service on va surgical patients. J. Am. Coll. Nutr. 1984, 3, 311–315. [Google Scholar] [CrossRef]
- Traeger, S.M.; Williams, G.B.; Milliren, G.; Young, D.S.; Fisher, M.; Haug, M.T., III. Total parenteral nutrition by a nutrition support team: Improved quality of care. JPEN J. Parenter. Enter. Nutr. 1986, 10, 408–412. [Google Scholar] [CrossRef]
- Faubion, W.C.; Wesley, J.R.; Khalidi, N.; Silva, J. Total parenteral nutrition catheter sepsis: Impact of the team approach. JPEN J. Parenter. Enter. Nutr. 1986, 10, 642–645. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Nightingale, J.M. Cost savings of an adult hospital nutrition support team. Nutrition 2005, 21, 1127–1133. [Google Scholar] [CrossRef]
- Szlagatys-Sidorkiewicz, A.; Borkowska, A.; Jankowska, A.; Sroka, M.; Zagierski, M.; Gosk, A.; Slominska-Fraczek, M.; Bogowski, G.; Plata-Nazar, K.; Sznurkowska, K.; et al. Reorganization of nutritional therapy can markedly reduce the rate of catheter-related blood stream infections in pediatric patients receiving parenteral nutrition—A 7-year prospective follow-up study. Nutr. Hosp. 2014, 31, 1116–1121. [Google Scholar]
- Diamond, I.R.; de Silva, N.; Pencharz, P.B.; Kim, J.H.; Wales, P.W.; Group for the Improvement of Intestinal Function and Treatment. Neonatal short bowel syndrome outcomes after the establishment of the first canadian multidisciplinary intestinal rehabilitation program: Preliminary experience. J. Pediatr. Surg. 2007, 42, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Furtado, S.; Ahmed, N.; Forget, S.; Sant’Anna, A. Outcomes of patients with intestinal failure after the development and implementation of a multidisciplinary team. Can. J. Gastroenterol. Hepatol. 2016, 2016, 9132134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modi, B.P.; Langer, M.; Ching, Y.A.; Valim, C.; Waterford, S.D.; Iglesias, J.; Duro, D.; Lo, C.; Jaksic, T.; Duggan, C. Improved survival in a multidisciplinary short bowel syndrome program. J. Pediatr. Surg. 2008, 43, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, R.A.; Welch, K.B.; Brown, P.I.; Teitelbaum, D.H. Survival outcomes of pediatric intestinal failure patients: Analysis of factors contributing to improved survival over the past two decades. J. Surg. Res. 2011, 170, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; de Silva, N.T.; Stanojevic, S.; Avitzur, Y.; Bayoumi, A.M.; Ungar, W.J.; Hoch, J.S.; Wales, P.W. Change of outcomes in pediatric intestinal failure: Use of time-series analysis to assess the evolution of an intestinal rehabilitation program. J. Am. Coll. Surg. 2016, 222, 1180–1188.e3. [Google Scholar] [CrossRef] [PubMed]
- Stanger, J.D.; Oliveira, C.; Blackmore, C.; Avitzur, Y.; Wales, P.W. The impact of multi-disciplinary intestinal rehabilitation programs on the outcome of pediatric patients with intestinal failure: A systematic review and meta-analysis. J. Pediatr. Surg. 2013, 48, 983–992. [Google Scholar] [CrossRef]
- Parent, B.; Shelton, M.; Nordlund, M.; Aarabi, S.; O’Keefe, G. Parenteral nutrition utilization after implementation of multidisciplinary nutrition support team oversight: A prospective cohort study. JPEN J. Parenter. Enter. Nutr. 2016, 40, 1151–1157. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, J.E.; Park, S.H.; Jin, H.K.; Jang, S.M.; Kim, S.A.; Rhie, S.J. Nutrition and clinical outcomes of nutrition support in multidisciplinary team for critically ill patients. Nutr. Clin. Pract. 2018, 33, 633–639. [Google Scholar] [CrossRef]
- Piquet, M.A.; Bertrand, P.C.; Roulet, M. Role of a nutrition support team in reducing the inappropriate use of parenteral nutrition. Clin. Nutr. 2004, 23, 437, author reply 438. [Google Scholar] [CrossRef]
- Sigalet, D.; Boctor, D.; Robertson, M.; Lam, V.; Brindle, M.; Sarkhosh, K.; Driedger, L.; Sajedi, M. Improved outcomes in paediatric intestinal failure with aggressive prevention of liver disease. Eur. J. Pediatr. Surg. 2009, 19, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Modi, B.P.; Jaksic, T. Pediatric intestinal failure and vascular access. Surg. Clin. N. Am. 2012, 92, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.J.; Cohran, V.; Raphael, B.P.; Sentongo, T.; Volpert, D.; Warner, B.W.; Goday, P.S.; Nutrition Committee of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Intestinal rehabilitation programs in the management of pediatric intestinal failure and short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 588–596. [Google Scholar] [CrossRef] [PubMed]

| Adults | Children | ||
|---|---|---|---|
| Cause | Underlying Diseases | Cause | Underlying Diseases |
| Short bowel syndrome (extensive bowel resection) |
| Short bowel syndrome (extensive bowel resection or congenital) |
|
| Intestinal motility disorder |
| Intestinal motility disorder |
|
| Congenital enteropathy |
| Congenital enteropathy |
|
| Intestinal fistula |
| ||
| Mechanical obstruction |
|
| Component | Description |
|---|---|
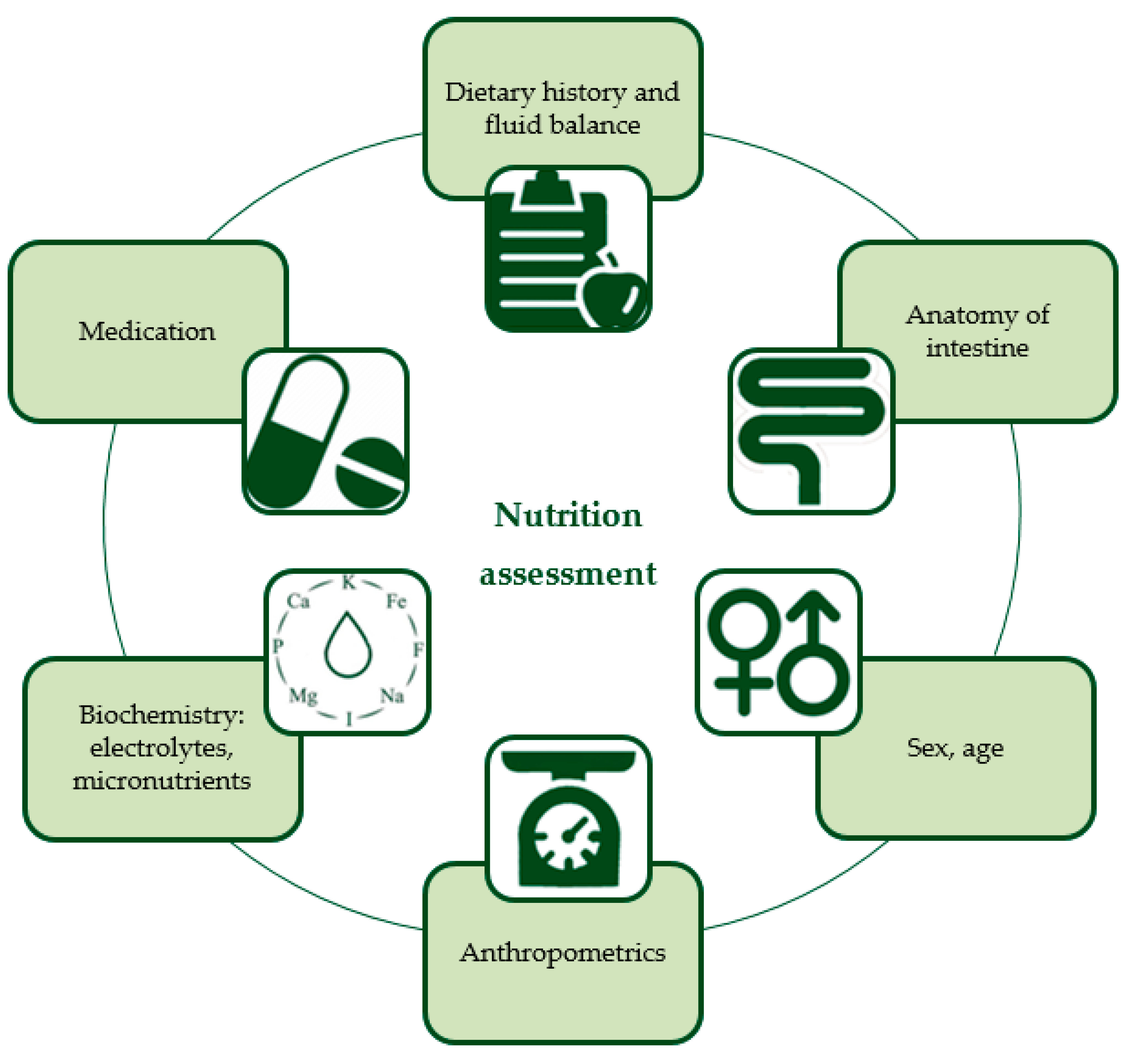
| Dietary history and fluid balance | Detailed information about previously tried diets including route, amount, and type of nutrition/formula with reasons for lack of success, and measurement of current fluid balance is required for designing a new individualized feeding regimen. |
| Anatomy of intestine | It is important to document the anatomy and function of the intestine or remaining intestine. Most nutrients are absorbed in the first part of the jejunum. In case of jejunum resection, the residual ileum is able to adapt and to partly take over the role of the jejunum in nutrient absorption. However, when the terminal ileum is resected, the reabsorption of vitamin B12 and bile salts cannot be replaced by jejunal cells. Resection of the ileocecal valve decreases intestinal transit time and supposedly predisposes to reflux of colonic content (including higher bacterial counts) back into the small intestine. Dysmotility and/or dilated loops cause intestinal stasis leading to SIBO, which negatively impacts the digestion and absorption of nutrients. [14]. High-output stomas may cause water, sodium, and magnesium depletion [15]. |
| Energy requirements, anthropometrics, sex and age | Energy requirements are preferably measured by indirect calorimetry. If this is not possible, these requirements should be calculated based on body weight, height, sex, and age, and adjusted accordingly by patient response (i.e., when not gaining weight as expected). To measure the effect of a nutritional intervention, anthropometrics should be monitored with growth charts in pediatric patients. Next to weight and height, it is also recommended to assess and monitor body composition (with for example air-displacement plethysmography) and muscle function (with for example handgrip strength). In a recent study in pediatric IF patients receiving long-term PN, Neelis et al. reported that these children had higher fat mass and lower fat-free mass (i.e., muscle, water, bone, and internal organs), compared with healthy peers [16]. In another study, involving adult IF patients, it was shown that 73% had sarcopenia (i.e., loss of muscle mass and function) [17]. |
| Biochemistry: electrolytes and micronutrients | Micronutrient deficiencies are common in IF patients [18,19]. Electrolytes such as sodium and magnesium may be low due to excessive gastrointestinal losses, whereas calcium, phosphate and potassium can be elevated as a consequence of dehydration [20]. Screening of electrolytes, vitamins, and trace elements should be performed at baseline and monitored thereafter. Electrolytes should be monitored every 1–3 months or more frequently when indicated (e.g., in the case of recent PN composition change or increased gastro-intestinal losses); vitamins and trace elements should be monitored every 6–12 months [21,22]. |
| Medication | Some medication may increase intestinal losses (e.g., non-steroidal anti-inflammatory drugs, proton pump inhibitors, antibiotics) [23]. Proton pump inhibitors are frequently used to reduce gastric PH and gastric fluid production which is most markedly increased in the hypersecretory acute phase of IF [24]. Also, because of the decreased enteral absorption of nutrients and fluids by the small intestine, medication dosages may have to be adjusted or converted to intravenous supplementation. If it is uncertain whether the medication will be enterally absorbed, the intravenous route is the preferred one [25]. |
| Core Members | Roles |
| Supervising physician * | Supervision and overall responsibility of care provided by the team Understands underlying diseases and prognosis Prescribes PN solutions and medication |
| Gastroenterologist * | Understands and treats underlying diseases (Aids in) operative insertion of gastrostomies/jejunostomies |
| Surgeon * | Operative insertion of CVCs and gastrostomies/jejunostomies Surgical management of IF (e.g., restoration of bowel continuity, surgical lengthening procedures, and management of anastomotic strictures) Is responsible for postsurgical care |
| Interventional radiologist */Anesthesiologist * | Assists in challenging pediatric cases of central venous access. In adults, interventional radiologists are the primary consultant regarding CVC placement. |
| Nurse specialist * | Teaches and trains patients and/or caregivers in care of tubes, stomas, and CVCs and in home PN administration when applicable Recognizes and manages complications of CVCs etc. Is case manager for patients and their caregivers |
| Dietitian * | Conducts nutritional screening and assessment Designs and implements feeding regimens based on measurement or calculation of individual requirements Monitors patient’s response with nutritional, laboratory, and fluid status Is case manager for patients and their caregivers |
| Pharmacist * | Is responsible for providing enteral formulations and PN solutions and for composition optimization Advises on compatibility and stability issues and drug/nutrient interactions |
| Additional Members | Roles |
| Endocrinologist * | Advises on preventing and treating complications of PN (and malnutrition) such as growth problems in children, metabolic bone disease, osteoporosis, and diabetes mellitus |
| Hematologist * | Advises on prevention and treatment of catheter-related thrombosis |
| Psychologist * | Provides psychological support and therapy for patients (and caregivers) |
| Speech therapist * | Advises on oral feeding in case of oral aversion or swallowing difficulties |
| Social worker * | Provides emotional support for patients (and caregivers) |
| Physiotherapist * | Assesses motor development in pediatric patients Provides training programs focused on weight-bearing exercise |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlug, L.E.; Nagelkerke, S.C.J.; Jonkers-Schuitema, C.F.; Rings, E.H.H.M.; Tabbers, M.M. The Role of a Nutrition Support Team in the Management of Intestinal Failure Patients. Nutrients 2020, 12, 172. https://doi.org/10.3390/nu12010172
Vlug LE, Nagelkerke SCJ, Jonkers-Schuitema CF, Rings EHHM, Tabbers MM. The Role of a Nutrition Support Team in the Management of Intestinal Failure Patients. Nutrients. 2020; 12(1):172. https://doi.org/10.3390/nu12010172
Chicago/Turabian StyleVlug, Lotte E., Sjoerd C. J. Nagelkerke, Cora F. Jonkers-Schuitema, Edmond H. H. M. Rings, and Merit M. Tabbers. 2020. "The Role of a Nutrition Support Team in the Management of Intestinal Failure Patients" Nutrients 12, no. 1: 172. https://doi.org/10.3390/nu12010172
APA StyleVlug, L. E., Nagelkerke, S. C. J., Jonkers-Schuitema, C. F., Rings, E. H. H. M., & Tabbers, M. M. (2020). The Role of a Nutrition Support Team in the Management of Intestinal Failure Patients. Nutrients, 12(1), 172. https://doi.org/10.3390/nu12010172





