The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine
Abstract
:1. Introduction
2. Polyamine Synthesis and Catabolism
2.1. Bacterial Polyamine Production in the Colon Is Dictated by Microbiome, Diet and Host Factors
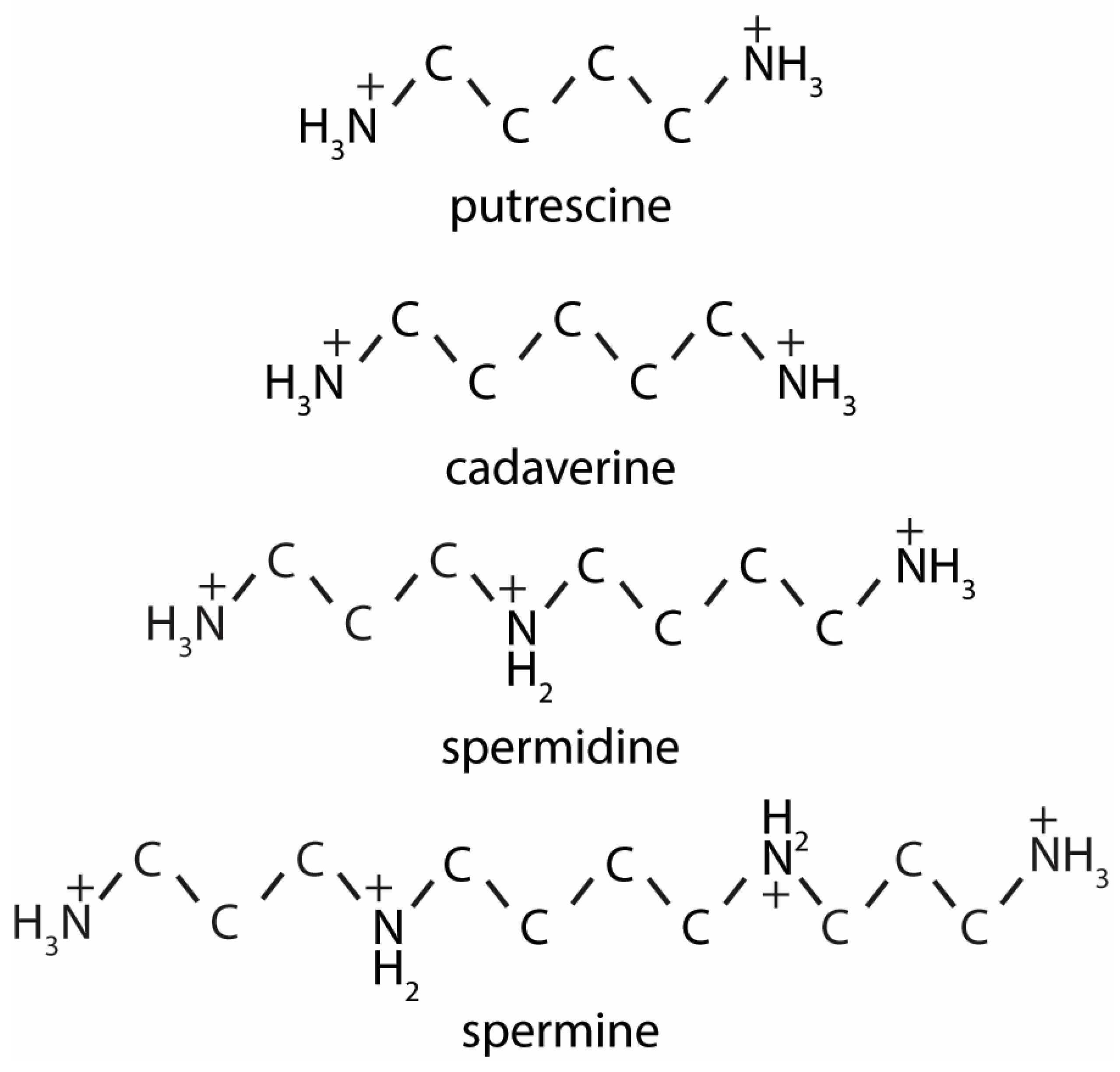
2.2. Mammalian Polyamines Synthesis Pathways
3. Regulation of Polyamine Levels
3.1. Regulation through Intracellular Polyamine Metabolism
3.2. Regulation through Uptake and Transport
4. Polyamines, Gene-Transcription and Proliferation
4.1. Polyamines Are the Substrates for the Post-Translational Modification Hypusine
4.2. Regulation of Eukaryotic Translation Initiation Factor 5A (EIF5A) Hypusination through Acetylation
4.3. EIF5A Hypusination Regulates Polyamine Synthesis
5. Polyamines and Metabolic Functions
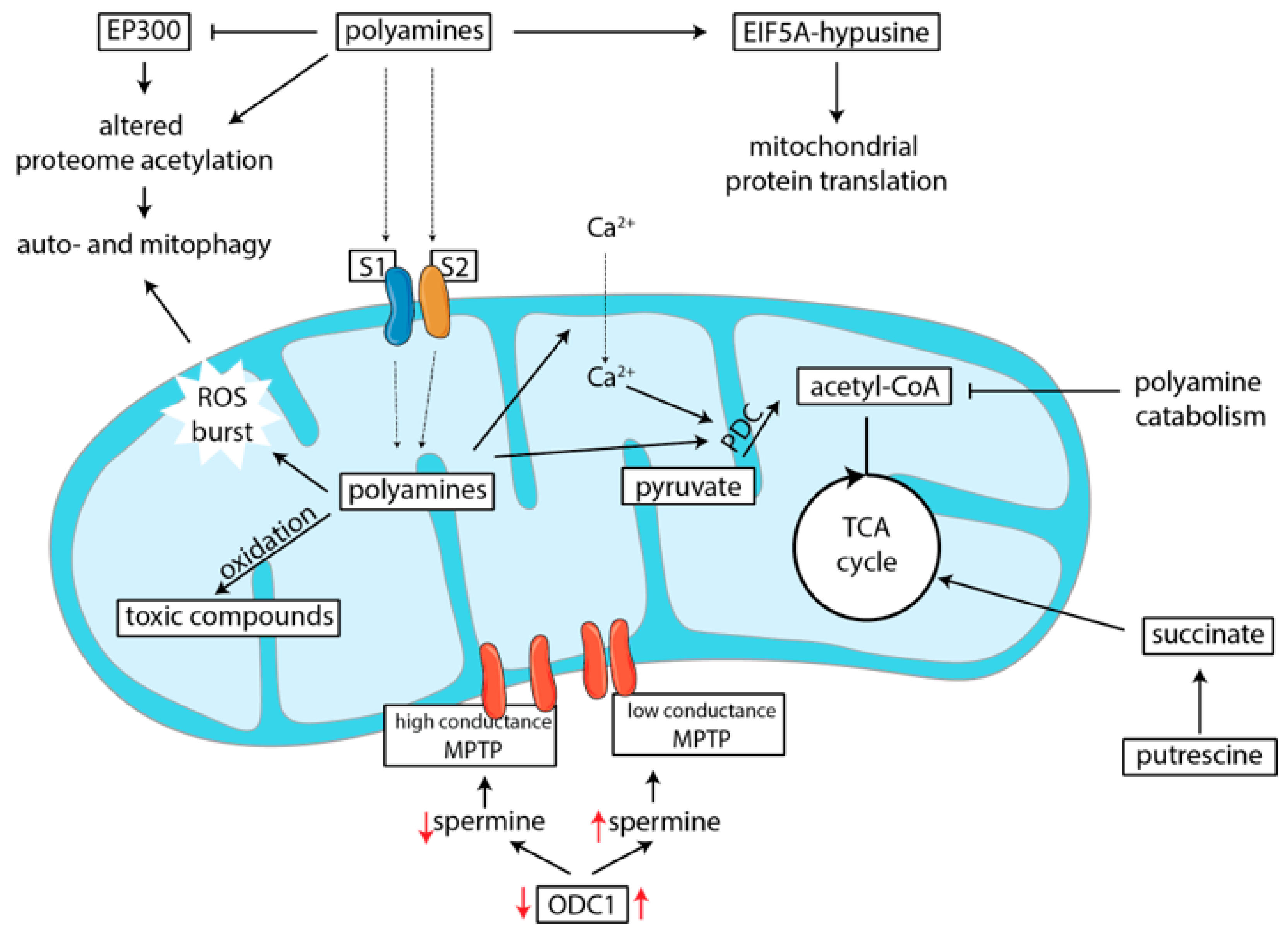
5.1. Polyamines Induce Mitochondrial Protein and Gene Transcription
5.2. Polyamines Can Induce Mitophagy
5.3. Polyamines Serve as Energy Scource for Enterocytes
5.4. Polyamines Influence Metabolism by Depleting Acetyl-CoA Levels
5.5. Polyamines Are Transported into Mitochondria and Influence Oxidation
5.6. Polyamine Catabolism Leads to Toxic By-Product Formation
5.7. Polyamines Regulate Formation of the Mitochondrial Permeability Transition Pore
6. Polyamines as Regulators of Intestinal Physiology
6.1. Polyamines Regulate Intestinal Barrier Integrity
6.2. Polyamines Stimulate Gut Development and Longevity
6.3. Do luminal Polyamine Levels Influence Intracellular Concentrations in Enterocytes?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berryman, C.E.; Lieberman, H.R.; Fulgoni, V.L., III; Pasiakos, S.M. Protein intake trends and conformity with the dietary reference intakes in the united states: Analysis of the national health and nutrition examination survey, 2001–2014. Am. J. Clin. Nutr. 2018, 108, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D. Digestibility issues of vegetable versus animal proteins: Protein and amino acid requirements—functional aspects. Food Nutr. Bull. 2013, 34, 272–274. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, N.; Moughan, P.J.; Mensink, M. Amino acid absorption in the large intestine of humans and porcine models. J. Nutr. 2017, 147, 1493–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; van Mil, S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G159–G170. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef] [Green Version]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Wang, L.; Zheng, C.; Yang, X.; Ma, X.; Wu, Y.; Chen, Z.; Jiang, Z. Fecal scores and microbial metabolites in weaned piglets fed different protein sources and levels. Anim. Nutr. 2017, 4, 31–36. [Google Scholar] [CrossRef]
- Pieper, R.; Boudry, C.; Bindelle, J.; Vahjen, W.; Zentek, J. Interaction between dietary protein content and the source of carbohydrates along the gastrointestinal tract of weaned piglets. Arch. Anim. Nutr. 2014, 68, 263–280. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [Green Version]
- Seiler, N.; Raul, F. Polyamines and the intestinal tract. Crit. Rev. Clin. Lab. Sci. 2007, 44, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Benno, Y. The relationship between microbiota and polyamine concentration in the human intestine: A pilot study. Microbiol. Immunol. 2007, 51, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forget, P.; Sinaasappel, M.; Bouquet, J.; Deutz, N.E.; Smeets, C. Fecal polyamine concentration in children with and without nutrient malabsorption. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 285–288. [Google Scholar] [CrossRef]
- Di Martino, M.L.; Campilongo, R.; Casalino, M.; Micheli, G.; Colonna, B.; Prosseda, G. Polyamines: Emerging players in bacteria–host interactions. Int. J. Med. Microbiol. 2013, 303, 484–491. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H.; Allison, C. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 1986, 132, 1647–1656. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Ooga, T.; Matsumoto, M. Intestinal luminal putrescine is produced by collective biosynthetic pathways of the commensal microbiome. Gut Microbes 2019, 10, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Estellé, J.; Kiilerich, P.; Ramayo-Caldas, Y.; Xia, Z.; Feng, Q.; Liang, S.; Pedersen, A.Ø.; Kjeldsen, N.J.; Liu, C.; et al. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 2016, 1, 16161. [Google Scholar] [CrossRef]
- Wang, W.; Higuchi, C.M. Dietary soy protein is associated with reduced intestinal mucosal polyamine concentration in male wistar rats. J. Nutr. 2000, 130, 1815–1820. [Google Scholar] [CrossRef] [Green Version]
- Benamouzig, R.; Mahe, S.; Meziani, K.; Martin, A.; Juste, C.; Catala, I.; Tome, D. Effects of soy protein diet on digestive lumenal polyamines and colonic cell proliferation in pigs. Reprod. Nutr. Dev. 1999, 39, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.J.; Chew, Y.V.; Colakoglu, F.; Cliff, J.B.; Klaassens, E.; Read, M.N.; Solon-Biet, S.M.; McMahon, A.C.; Cogger, V.C.; Ruohonen, K.; et al. Diet-microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 2017, 25, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. Ph and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Murray-Stewart, T.; Devereux, W.; Hacker, A.; Frydman, B.; Woster, P.M.; Casero, R.A. Properties of purified recombinant human polyamine oxidase, paoh1/smo. Biochem. Biophys. Res. Commun. 2003, 304, 605–611. [Google Scholar] [CrossRef]
- Pegg, A.E. Spermidine/spermine-n1-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casero, R.A., Jr.; Pegg, A.E. Spermidine/spermine n1-acetyltransferase--the turning point in polyamine metabolism. FASEB J. 1993, 7, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Tomar, P.C.; Lakra, N.; Mishra, S.N. Cadaverine: A lysine catabolite involved in plant growth and development. Plant Signal. Behav. 2013, 8, e25850. [Google Scholar] [CrossRef] [Green Version]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining mysteries of molecular biology: The role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Elitsur, Y.; Gesell, M.; Luk, G.D. Odc activity and polyamine levels in isolated human colonocytes. Life Sci. 1993, 53, 945–952. [Google Scholar] [CrossRef]
- Hölttä, E.; Pohjanpelto, P. Polyamine starvation causes accumulation of cadaverine and its derivatives in a polyamine-dependent strain of chinese-hamster ovary cells. Biochem. J. 1983, 210, 945–948. [Google Scholar] [CrossRef] [Green Version]
- Pegg, A.E.; McGill, S. Decarboxylation of ornithine and lysine in rat tissues. Biochim. Biophys. Acta Enzym. 1979, 568, 416–427. [Google Scholar] [CrossRef]
- Alhonen-Hongisto, L.; Jänne, J. Polyamine depletion induces enhanced synthesis and accumulation of cadaverine in cultured ehrlich ascites carcinoma cells. Biochem. Biophys. Res. Commun. 1980, 93, 1005–1013. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsufuji, S.; Kameji, T.; Hayashi, S.-I.; Igarashi, K.; Tamura, T.; Tanaka, K.; Ichihara, A. Ornithine decarboxylase is degraded by the 26s proteasome without ubiquitination. Nature 1992, 360, 597. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Ichiba, T.; Matsufuji, S.; Hayashi, S.-I. Cloning of antizyme inhibitor, a highly homologous protein to ornithine decarboxylase. J. Biol. Chem. 1996, 271, 3340–3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, H.; Pegg, A.E. Mechanistic studies of the processing of human s-adenosylmethionine decarboxylase proenzyme: Isolation of an ester intermediate. J. Biol. Chem. 1999, 274, 35059–35066. [Google Scholar] [CrossRef] [Green Version]
- Bale, S.; Lopez, M.M.; Makhatadze, G.I.; Fang, Q.; Pegg, A.E.; Ealick, S.E. Structural basis for putrescine activation of human s-adenosylmethionine decarboxylase. Biochemistry. 2008, 47, 13404–13417. [Google Scholar] [CrossRef]
- Shirahata, A.; Pegg, A.E. Increased content of mrna for a precursor of s-adenosylmethionine decarboxylase in rat prostate after treatment with 2-difluoromethylornithine. J. Biol. Chem. 1986, 261, 13833–13837. [Google Scholar]
- Shantz, L.M.; Holm, I.; Jänne, O.A.; Pegg, A.E. Regulation of s-adenosylmethionine decarboxylase activity by alterations in the intracellular polyamine content. Biochem. J. 1992, 288, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Condon, K.J.; Sabatini, D.M. Nutrient regulation of mtorc1 at a glance. J. Cell Sci. 2019, 132, jcs222570. [Google Scholar] [CrossRef]
- Zabala-Letona, A.; Arruabarrena-Aristorena, A.; Martín-Martín, N.; Fernandez-Ruiz, S.; Sutherland, J.D.; Clasquin, M.; Tomas-Cortazar, J.; Jimenez, J.; Torres, I.; Quang, P.; et al. Mtorc1-dependent amd1 regulation sustains polyamine metabolism in prostate cancer. Nature 2017, 547, 109–113. [Google Scholar] [CrossRef]
- Basu Roy, U.K.; Rial, N.S.; Kachel, K.L.; Gerner, E.W. Activated k-ras increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol. Carcinog. 2008, 47, 538–553. [Google Scholar]
- Cheng, F.; Mani, K.; van den Born, J.; Ding, K.; Belting, M.; Fransson, L.A. Nitric oxide-dependent processing of heparan sulfate in recycling s-nitrosylated glypican-1 takes place in caveolin-1-containing endosomes. J. Biol. Chem. 2002, 277, 44431–44439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belting, M.; Mani, K.; Jonsson, M.; Cheng, F.; Sandgren, S.; Jonsson, S.; Ding, K.; Delcros, J.G.; Fransson, L.A. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: A pivital role for nitrosothiol-derived nitric oxide. J. Biol. Chem. 2003, 278, 47181–47189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uemura, T.; Yerushalmi, H.F.; Tsaprailis, G.; Stringer, D.E.; Pastorian, K.E.; Hawel, L., 3rd; Byus, C.V.; Gerner, E.W. Identification and characterization of a diamine exporter in colon epithelial cells. J. Biol. Chem. 2008, 283, 26428–26435. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, M.K.; Tabor, C.W.; Tabor, H. Spermidine but not spermine is essential for hypusine biosynthesis and growth in saccharomyces cerevisiae: Spermine is converted to spermidine in vivo by the fms1-amine oxidase. Proc. Natl. Acad. Sci. USA 2003, 100, 13869–13874. [Google Scholar] [CrossRef] [Green Version]
- Zanelli, C.F.; Valentini, S.R. Is there a role for eif5a in translation? Amino Acids 2007, 33, 351–358. [Google Scholar] [CrossRef]
- Schuller, A.P.; Wu, C.C.-C.; Dever, T.E.; Buskirk, A.R.; Green, R. Eif5a functions globally in translation elongation and termination. Mol. Cell 2017, 66, 194–205.e5. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Nishimura, K.; Zanelli, C.F.; Valentini, S.R. Functional significance of eif5a and its hypusine modification in eukaryotes. Amino Acids 2010, 38, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Zanelli, C.F.; Maragno, A.L.C.; Gregio, A.P.B.; Komili, S.; Pandolfi, J.R.; Mestriner, C.A.; Lustri, W.R.; Valentini, S.R. Eif5a binds to translational machinery components and affects translation in yeast. Biochem. Biophys. Res. Commun. 2006, 348, 1358–1366. [Google Scholar] [CrossRef]
- Timmons, J.; Chang, E.T.; Wang, J.-Y.; Rao, J.N. Polyamines and gut mucosal homeostasis. J. Gastrointestin. Dig. Syst. 2012, 2, 001. [Google Scholar] [CrossRef]
- Sievert, H.; Venz, S.; Platas-Barradas, O.; Dhople, V.M.; Schaletzky, M.; Nagel, C.-H.; Braig, M.; Preukschas, M.; Pällmann, N.; Bokemeyer, C.; et al. Protein-protein-interaction network organization of the hypusine modification system. Mol. Cell. Proteom. 2012, 11, 1289–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.R.; Kim, Y.S.; Wolff, E.C.; Park, M.H. Specificity of the deoxyhypusine hydroxylase-eukaryotic translation initiation factor (eif5a) interaction: Identification of amino acid residues of the enzyme required for binding of its substrate, deoxyhypusine-containing eif5a. J. Biol. Chem. 2007, 282, 8300–8308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joe, Y.A.; Park, M.H. Structural features of the eif-5a precursor required for posttranslational synthesis of deoxyhypusine. J. Biol. Chem. 1994, 269, 25916–25921. [Google Scholar] [PubMed]
- Nishimura, K.; Murozumi, K.; Shirahata, A.; Park, M.H.; Kashiwagi, K.; Igarashi, K. Independent roles of eif5a and polyamines in cell proliferation. Biochem. J. 2005, 385, 779–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.B.; Park, J.H.; Folk, J.E.; Deck, J.A.; Pegg, A.E.; Sokabe, M.; Fraser, C.S.; Park, M.H. Inactivation of eukaryotic initiation factor 5a (eif5a) by specific acetylation of its hypusine residue by spermidine/spermine acetyltransferase 1 (ssat1). Biochem. J. 2010, 433, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.P.; Shin, B.-S.; Loughran, G.; Tzani, I.; Young-Baird, S.K.; Cao, C.; Atkins, J.F.; Dever, T.E. Polyamine control of translation elongation regulates start site selection on antizyme inhibitor mrna via ribosome queuing. Mol. Cell 2018, 70, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.P.; Loughran, G.; Atkins, J.F. Uorfs with unusual translational start codons autoregulate expression of eukaryotic ornithine decarboxylase homologs. Proc. Natl. Acad. Sci. USA 2008, 105, 10079–10084. [Google Scholar] [CrossRef] [Green Version]
- Venkataramanan, S.; Floor, S.N. The traffic jam: Polyamine prevalence pauses protein production. Mol. Cell 2018, 70, 191–192. [Google Scholar] [CrossRef]
- Shin, B.-S.; Katoh, T.; Gutierrez, E.; Kim, J.-R.; Suga, H.; Dever, T.E. Amino acid substrates impose polyamine, eif5a, or hypusine requirement for peptide synthesis. Nucleic Acids Res. 2017, 45, 8392–8402. [Google Scholar] [CrossRef] [Green Version]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic instruction of immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef]
- Puleston, D.J.; Buck, M.D.; Klein Geltink, R.I.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eif5a hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab. 2019, 30, 352–363. [Google Scholar] [CrossRef] [Green Version]
- Hardbower, D.M.; Asim, M.; Luis, P.B.; Singh, K.; Barry, D.P.; Yang, C.; Steeves, M.A.; Cleveland, J.L.; Schneider, C.; Piazuelo, M.B.; et al. Ornithine decarboxylase regulates m1 macrophage activation and mucosal inflammation via histone modifications. Proc. Natl. Acad. Sci. USA 2017, 114, E751–E760. [Google Scholar] [CrossRef] [Green Version]
- Melis, N.; Rubera, I.; Cougnon, M.; Giraud, S.; Mograbi, B.; Belaid, A.; Pisani, D.F.; Huber, S.M.; Lacas-Gervais, S.; Fragaki, K.; et al. Targeting eif5a hypusination prevents anoxic cell death through mitochondrial silencing and improves kidney transplant outcome. J. Am. Soc. Nephrol. 2017, 28, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Qiu, Q.; Gu, X.; Tian, Y.; Zhang, Y. Atm mediates spermidine-induced mitophagy via pink1 and parkin regulation in human fibroblasts. Sci. Rep. 2016, 6, 24700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Jing, Y.-H.; Yan, J.-L.; Wang, Q.-J.; Chen, H.-C.; Ma, X.-Z.; Yin, J.; Gao, L.-P. Spermidine ameliorates the neuronal aging by improving the mitochondrial function in vitro. Exp. Gerontol. 2018, 108, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Mariño, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Bénit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [Green Version]
- Pietrocola, F.; Lachkar, S.; Enot, D.P.; Niso-Santano, M.; Bravo-San Pedro, J.M.; Sica, V.; Izzo, V.; Maiuri, M.C.; Madeo, F.; Mariño, G.; et al. Spermidine induces autophagy by inhibiting the acetyltransferase ep300. Cell Death Differ. 2015, 22, 509–516. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Buttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Bardócz, S.; Grant, G.; Brown, D.S.; Pusztai, A. Putrescine as a source of instant energy in the small intestine of the rat. Gut 1998, 42, 24–28. [Google Scholar] [CrossRef]
- Kramer, D.L.; Diegelman, P.; Jell, J.; Vujcic, S.; Merali, S.; Porter, C.W. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J. Biol. Chem. 2008, 283, 4241–4251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, K.; Foster, B.A.; Merali, S.; Kramer, D.L.; Hensen, M.L.; Diegelman, P.; Kisiel, N.; Vujcic, S.; Mazurchuk, R.V.; Porter, C.W. Activated polyamine catabolism depletes acetyl-coa pools and suppresses prostate tumor growth in tramp mice. J. Biol. Chem. 2004, 279, 40076–40083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jell, J.; Merali, S.; Hensen, M.L.; Mazurchuk, R.; Spernyak, J.A.; Diegelman, P.; Kisiel, N.D.; Barrero, C.; Deeb, K.K.; Alhonen, L.; et al. Genetically altered expression of spermidine/spermine n1-acetyltransferase affects fat metabolism in mice via acetyl-coa. J. Biol. Chem. 2007, 282, 8404–8413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byczkowski, J.Z.; Zychlinski, L.; Porter, C.W. Inhibition of the bioenergetic functions of isolated rat liver mitochondria by polyamines. Biochem. Pharmacol. 1982, 31, 4045–4053. [Google Scholar] [CrossRef]
- Dalla Via, L.; Di Noto, V.; Toninello, A. Binding of spermidine and putrescine to energized liver mitochondria. Arch. Biochem. Biophys. 1999, 365, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Grancara, S.; Dalla Via, L.; García-Argáez, A.N.; Ohkubo, S.; Pacella, E.; Manente, S.; Bragadin, M.; Toninello, A.; Agostinelli, E. Spermine cycling in mitochondria is mediated by adenine nucleotide translocase activity: Mechanism and pathophysiological implications. Amino Acids 2016, 48, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Toninello, A.; Dalla Via, L.; Testa, S.; Siliprandi, D. Electrophoretic polyamine transport in rat liver mitochondria. Amino Acids 1992, 2, 69–76. [Google Scholar] [CrossRef]
- Pezzato, E.; Battaglia, V.; Brunati, A.M.; Agostinelli, E.; Toninello, A. Ca2+-independent effects of spermine on pyruvate dehydrogenase complex activity in energized rat liver mitochondria incubated in the absence of exogenous Ca2+ and Mg2+. Amino Acids 2009, 36, 449–456. [Google Scholar] [CrossRef]
- Phillips, J.E.; Chaffee, R.R.J. Restorative effects of spermine on oxidative phosphorylation and respiration in heat-aged mitochondria. Biochem. Biophys. Res. Commun. 1982, 108, 174–181. [Google Scholar] [CrossRef]
- Solaini, G.; Tadolini, B. Spermine binding to submitochondrial particles and activation of adenosine triphosphatase. Biochem. J. 1984, 218, 495–499. [Google Scholar] [CrossRef] [Green Version]
- Nicchitta, C.V.; Williamson, J.R. Spermine. A regulator of mitochondrial calcium cycling. J. Biol. Chem. 1984, 259, 12978–12983. [Google Scholar] [PubMed]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, R.; Cardillo, S.; Braga, M.; De Iuliis, A.; Battaglia, V.; Toninello, A.; Agostinelli, E.; Vianello, F. Preliminary kinetic characterization of a copper amine oxidase from rat liver mitochondria matrix. Amino Acids 2011, 40, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bonaiuto, E.; Grancara, S.; Martinis, P.; Stringaro, A.; Colone, M.; Agostinelli, E.; Macone, A.; Stevanato, R.; Vianello, F.; Toninello, A.; et al. A novel enzyme with spermine oxidase properties in bovine liver mitochondria: Identification and kinetic characterization. Free Radic. Biol. Med. 2015, 81, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Elustondo, P.A.; Negoda, A.; Kane, C.L.; Kane, D.A.; Pavlov, E.V. Spermine selectively inhibits high-conductance, but not low-conductance calcium-induced permeability transition pore. Biochim. Biophys. Acta 2015, 1847, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sava, I.G.; Battaglia, V.; Rossi, C.A.; Salvi, M.; Toninello, A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic. Biol. Med. 2006, 41, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Grancara, S.; Battaglia, V.; Martinis, P.; Viceconte, N.; Agostinelli, E.; Toninello, A.; Deana, R. Mitochondrial oxidative stress induced by Ca2+ and monoamines: Different behaviour of liver and brain mitochondria in undergoing permeability transition. Amino Acids 2012, 42, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xue, G.; Zhang, W.; Wang, L.; Li, H.; Zhang, L.; Lu, F.; Bai, S.; Lin, Y.; Lou, Y.; et al. Akt and erk1/2 activate the ornithine decarboxylase/polyamine system in cardioprotective ischemic preconditioning in rats: The role of mitochondrial permeability transition pores. Mol. Cell. Biochem. 2014, 390, 133–142. [Google Scholar] [CrossRef]
- Wei, C.; Li, H.; Wang, Y.; Peng, X.; Shao, H.; Li, H.; Bai, S.; Xu, C. Exogenous spermine inhibits hypoxia/ischemia-induced myocardial apoptosis via regulation of mitochondrial permeability transition pore and associated pathways. Exp. Biol. Med. 2016, 241, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Madsen, K.L.; Brockway, P.D.; Johnson, L.R.; Hardin, J.A.; Gall, D.G. Role of ornithine decarboxylase in enterocyte mitochondrial function and integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 270, G789–G797. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Xiong, X.; Yin, Y. Energy metabolism in intestinal epithelial cells during maturation along the crypt-villus axis. Sci. Rep. 2016, 6, 31917. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Masuyama, Y.; Kitagawa, T. Immunocytochemical localization of polyamines in the gastrointestinal tracts of rats and mice. Histochem. Cell Biol. 1996, 106, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, X.; Rao, J.N.; Zou, T.; Xiao, L.; Yu, T.; Timmons, J.A.; Turner, D.J.; Wang, J.-Y. Polyamines regulate e-cadherin transcription through c-myc modulating intestinal epithelial barrier function. Am. J. Physiol. Cell Physiol. 2009, 296, C801–C810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Rao, J.N.; Liu, L.; Zou, T.; Keledjian, K.M.; Boneva, D.; Marasa, B.S.; Wang, J.-Y. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1159–G1169. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Rao, J.N.; Liu, L.; Zou, T.-T.; Turner, D.J.; Bass, B.L.; Wang, J.-Y. Regulation of adherens junctions and epithelial paracellular permeability: A novel function for polyamines. J. Physiol. Cell Physiol. 2003, 285, C1174–C1187. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Zamora, J.; Sabater-Molina, M.; Rodríguez-Palmero, M.; Rivero, M.; Bosch, V.; Nadal, J.M.; Zamora, S.; Larqué, E. Polyamines in human breast milk for preterm and term infants. Br. J. Nutr. 2013, 110, 524–528. [Google Scholar] [CrossRef] [Green Version]
- Sabater-Molina, M.; Larque, E.; Torrella, F.; Plaza, J.; Lozano, T.; Munoz, A.; Zamora, S. Effects of dietary polyamines at physiologic doses in early-weaned piglets. Nutrition 2009, 25, 940–946. [Google Scholar] [CrossRef]
- Fang, T.; Liu, G.; Cao, W.; Xianjian, W.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Wang, J. Spermine: New insights into the intestinal development and serum antioxidant status of suckling piglets. RSC Adv. 2016, 6, 31323–31335. [Google Scholar] [CrossRef]
- van Wettere, W.H.; Willson, N.L.; Pain, S.J.; Forder, R.E. Effect of oral polyamine supplementation pre-weaning on piglet growth and intestinal characteristics. Animal 2016, 10, 1655–1659. [Google Scholar] [CrossRef] [Green Version]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Yu, K.; Yu, M.; Zhang, C.; Su, Y.; Zhu, W. Alteration of metabolomic markers of amino-acid metabolism in piglets with in-feed antibiotics. Amino Acids 2017, 49, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Pi, Y.; Peng, Y.; Mu, C.L.; Zhu, W.Y. Time-course responses of ileal and fecal microbiota and metabolite profiles to antibiotics in cannulated pigs. Appl. Microbiol. Biotechnol. 2018, 102, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Gioscia-Ryan, R.A.; Hearon, C.M., Jr.; Seals, D.R. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Dev. 2013, 134, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viltard, M.; Durand, S.; Pérez-Lanzón, M.; Aprahamian, F.; Lefevre, D.; Leroy, C.; Madeo, F.; Kroemer, G.; Friedlander, G. The metabolomic signature of extreme longevity: Naked mole rats versus mice. Aging 2019, 11, 4783–4800. [Google Scholar] [CrossRef] [PubMed]
- Uda, K.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Rapid absorption of luminal polyamines in a rat small intestine ex vivo model. J. Gastroenterol. Hepatol. 2003, 18, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Kurihara, S.; Sawaki, E.; Koga, Y.; Benno, Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2012, 2, 233. [Google Scholar] [CrossRef] [Green Version]
- Uemura, T.; Stringer, D.E.; Blohm-Mangone, K.A.; Gerner, E.W. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G517–G522. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.H.; Dejea, C.M.; Edler, D.; Hoang, L.T.; Santidrian, A.F.; Felding, B.H.; Ivanisevic, J.; Cho, K.; Wick, E.C.; Hechenbleikner, E.M.; et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015, 21, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Löser, C.; Eisel, A.; Harms, D.; Fölsch, U.R. Dietary polyamines are essential luminal growth factors for small intestinal and colonic mucosal growth and development. Gut 1999, 44, 12–16. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ooga, T.; Kibe, R.; Aiba, Y.; Koga, Y.; Benno, Y. Colonic absorption of low-molecular-weight metabolites influenced by the intestinal microbiome: A pilot study. PLoS ONE 2017, 12, e0169207. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; Boer, V.C.J.d. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12, 197. https://doi.org/10.3390/nu12010197
Bekebrede AF, Keijer J, Gerrits WJJ, Boer VCJd. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients. 2020; 12(1):197. https://doi.org/10.3390/nu12010197
Chicago/Turabian StyleBekebrede, Anna F., Jaap Keijer, Walter J. J. Gerrits, and Vincent C. J. de Boer. 2020. "The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine" Nutrients 12, no. 1: 197. https://doi.org/10.3390/nu12010197
APA StyleBekebrede, A. F., Keijer, J., Gerrits, W. J. J., & Boer, V. C. J. d. (2020). The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients, 12(1), 197. https://doi.org/10.3390/nu12010197




