The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review
Abstract
:1. Introduction
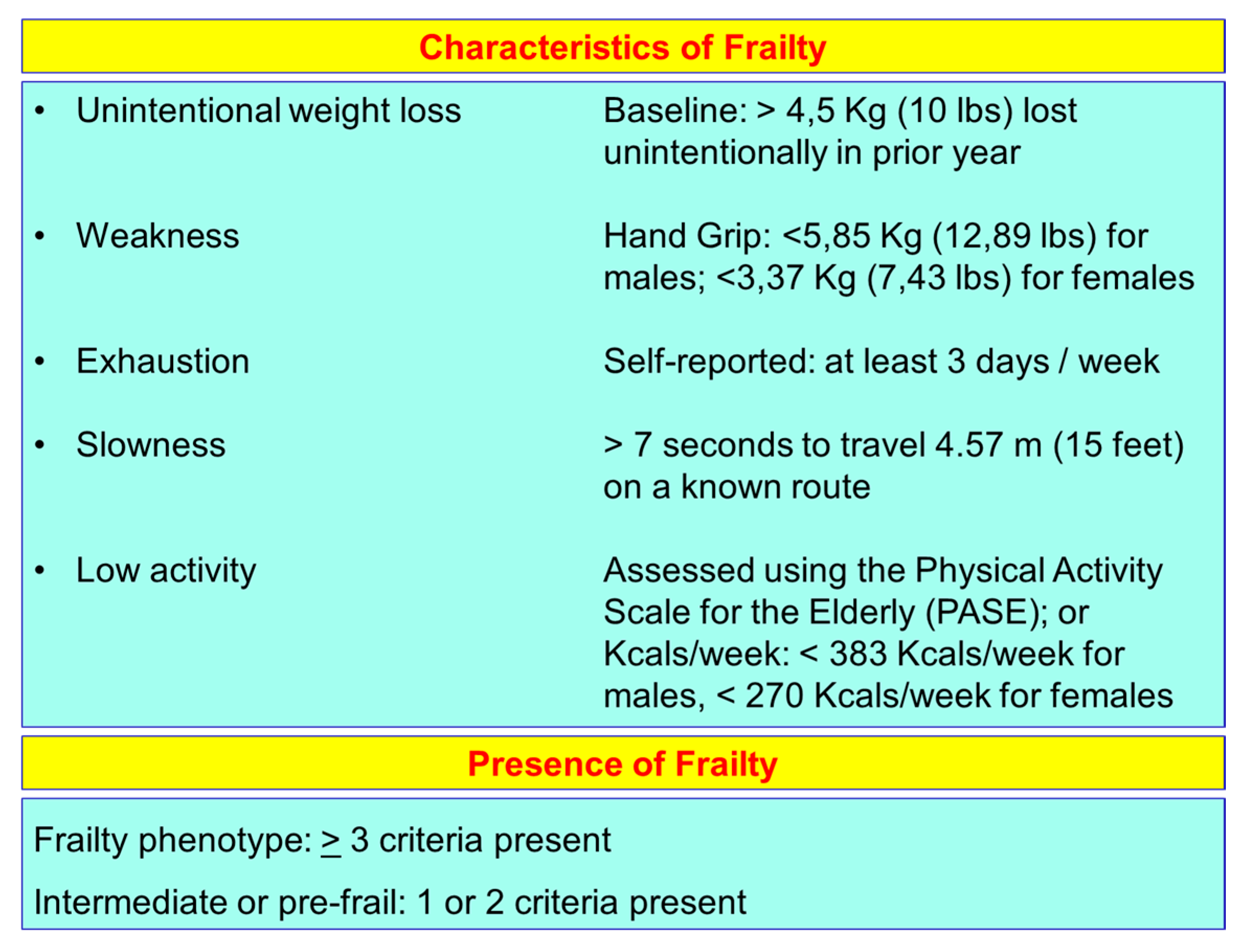
2. Aging and Frailty
3. Aging, or Cellular Senescence, and Health
4. The Role of Senescence in the Progression of Diabetes Mellitus and Atherosclerosis
5. Caloric Restriction, Effects on Metabolism of Adipose Tissue and Increase of Longevity
6. Caloric Restriction and Inflammatory State
7. Caloric Restriction, Mitochondria Activity and Reactive Oxygen Species Production
8. Caloric Restriction, Hormesis and Mitochondria Activity during Aging
9. Caloric Restriction and DNA Methylation
10. Caloric Restriction, Metabolic Adaptation and Oxidative Damage
11. Mediterranean Diet, Cardiovascular Disease and Mortality
12. Omega-3 Poly-Unsaturated Fatty Acids and Aging
13. Mediterranean Diet Increases Lifespan and Improves Aging
14. Diet Patterns and “Inflammaging”
15. Mediterranean Diet Confers Protection Against Sarcopenia
16. Mediterranean Diet Maintains Health Status and Prevents from the Onset of Frailty
17. Conclusion Remarks on Nutrition and Frailty
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Population Structure and Ageing. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Population_structure_and_ageing (accessed on 1 June 2019).
- Kinsella, K.; Phillips, D.R. Global Aging: The Challenge of Success; Population Bulletin; Population Reference Bureau: Washington, DC, USA, 2005; Volume 60. [Google Scholar]
- United Nations. The World at Six Billion. Available online: www.un.org/esa/population/publications/sixbillion/sixbilpart1.pdf (accessed on 1 June 2019).
- Beard, J.R. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.R.; Bloom, D.E. Towards a comprehensive public health response to population ageing. Lancet 2015, 385, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Age Wave, Sun America. Age Wave/Sun America Retirement Reset Study; Age Wave, Sun America: Los Angeles, CA, USA, 2011. [Google Scholar]
- Britton, A.; Shipley, M.; Singh-Manoux, A.; Marmot, M.G. Successful aging: The contribution of early life and midlife risk factors. J. Am. Geriatr. Soc. 2008, 56, 1098–1105. [Google Scholar] [CrossRef] [Green Version]
- Akbaraly, T.; Sabia, S.; Hagger-Johnson, G.; Tabak, A.G.; Shipley, M.J.; Jokela, M.; Brunner, E.J.; Hamer, M.; Batty, G.D.; Singh-Manoux, A.; et al. Does overall diet in midlife predict future aging phenotypes? A cohort study. Am. J. Med. 2013, 126, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Sun, Q.; Townsend, M.K.; Chiuve, S.E.; Okereke, O.I.; Willett, W.C.; Stampfer, M.; Grodstein, F. The association between dietary patterns at midlife and health in aging an observational study. Ann. Intern. Med. 2013, 159, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research agenda for frailty in older adults: Toward a better understanding of physiology and aetiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef]
- Eeles, E.M.; White, S.V.; O’Mahony, S.M.; Bayer, A.J.; Hubbard, R.E. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012, 41, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Strandberg, T.E.; Pitkälä, K.H. Frailty in elderly people. Lancet 2007, 369, 1328–1329. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Clegg, A. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J. Am. Geriatr. Soc. 2010, 58, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. 2004, 59, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Phenotype of frailty: Characterization in the women’s health and aging studies. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 262–266. [Google Scholar]
- Morley, J.E.; Haren, M.T.; Rolland, Y.; Kim, M.J. Frailty. Med. Clin. N. Am. 2006, 90, 837–847. [Google Scholar] [CrossRef]
- Flatt, T. A new definition of aging? Front. Genet. 2012, 3, 148. [Google Scholar] [CrossRef] [Green Version]
- Giaimo, S.; d’Adda di Fagagna, F. Is cellular senescence an example of antagonistic pleiotropy? Aging Cell 2012, 11, 378–383. [Google Scholar] [CrossRef]
- Sharpless, N.E. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 2001, 413, 86–91. [Google Scholar] [CrossRef]
- Sager, R. Senescence as a mode of tumor suppression. Environ. Health Perspect. 1991, 93, 59–62. [Google Scholar] [CrossRef]
- Baker, D.J. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Baker, D.J. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 2008, 10, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004, 36, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Kaszubowska, L. Telomere shortening and ageing of the immune system. J. Physiol. Pharmacol. 2008, 59 (Suppl. S9), 169–186. [Google Scholar] [PubMed]
- Titus, S. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013, 5, 172ra121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.K.; Naidoo, N. The endoplasmic reticulum stress response in aging and age-related diseases. Front. Physiol. 2012, 3, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, I. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 2012, 15, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Minamino, T. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef]
- Ryan, A.S. Insulin resistance with aging: Effects of diet and exercise. Sports Med. 2000, 30, 327–346. [Google Scholar] [CrossRef]
- Walters, M.S. Smoking accelerates aging of the small airway epithelium. Respir. Res. 2014, 15, 94. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Xu, H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Roden, M. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, N. Short telomeres compromise beta-cell signaling and survival. PLoS ONE 2011, 6, e17858. [Google Scholar]
- Yang, T.K. Davallialactone from mushroom reduced premature senescence and inflammation on glucose oxidative stress in human diploid fibroblast cells. J. Agric. Food Chem. 2013, 61, 7089–7095. [Google Scholar] [CrossRef]
- Liu, J. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell. Signal. 2014, 26, 110–121. [Google Scholar] [CrossRef]
- Mortuza, R.; Chen, S.; Feng, B.; Sen, S.; Chakrabarti, S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE 2013, 8, e54514. [Google Scholar] [CrossRef]
- Kim, Y.J. miR-486–5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem. Cells Dev. 2012, 21, 1749–1760. [Google Scholar] [CrossRef]
- Salpea, K.D.; Humphries, S.E. Telomere length in atherosclerosis and diabetes. Atherosclerosis 2010, 209, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, S.; Yokoyama, M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef]
- Barzilai, N.; Banerjee, S.; Hawkins, M.; Chen, W.; Rossetti, L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J. Clin. Investig. 1998, 101, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvestrini, V.; Sell, C.; Lorenzini, A. Obesity May Accelerate the Aging Process. Front. Endocrinol. 2019, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Shah, P.P.; Nativio, R.; Berger, S.L. Epigenetic Mechanisms of Longevity and Aging. Cell 2016, 166, 822–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T. The metabolic regulation of aging. Nat. Med. 2015, 21, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, H.A.; Lynd, F.T.; Masoro, E.J.; Yu, B.P. Changes in adipose mass and cellularity through the adult life of rats fed ad libitum or a life-prolonging restricted diet. J. Gerontol. 1980, 35, 827–835. [Google Scholar] [CrossRef]
- Liao, C.Y.; Rikke, B.A.; Johnson, T.E.; Diaz, V.; Nelson, J.F. Genetic variation in the murine lifespan response to dietary restriction: From life extension to life shortening. Aging Cell 2010, 9, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar] [CrossRef]
- Liao, C.Y. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell 2011, 10, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Vermeij, W.P. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 2016, 537, 427–447. [Google Scholar] [CrossRef] [Green Version]
- Huffman, K.M. Caloric restriction alters the metabolic response to a mixed-meal: Results from a randomized, controlled trial. PLoS ONE 2012, 7, e28190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riera, C.E.; Dillin, A. Tipping the metabolic scales towards increased longevity in mammals. Nat. Cell Biol. 2015, 17, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Willette, A.A. Interleukin-8 and interleukin-10, brain volume and microstructure, and the influence of calorie restriction in old rhesus macaques. Age 2013, 35, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Youm, Y.H.; Dixit, V.D. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J. Immunol. 2009, 183, 3040–3052. [Google Scholar] [CrossRef] [Green Version]
- Masoro, E.J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005, 126, 913–922. [Google Scholar] [CrossRef]
- Dillin, A. Rates of behavior and aging specified by mitochondrial function during development. Science 2002, 298, 2398–2401. [Google Scholar] [CrossRef]
- Lee, S.S. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 2003, 33, 40–48. [Google Scholar] [CrossRef]
- Trifunovic, A. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Kujoth, G.C. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Rea, S.L.; Ventura, N.; Johnson, T.E. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007, 5, e259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barja, G. The mitochondrial free radical theory of aging. Prog. Mol. Biol. Transl. Sci. 2014, 127, 1–27. [Google Scholar] [PubMed]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Ristow, M. Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 2014, 12, 288–341. [Google Scholar] [CrossRef]
- López-Lluch, G. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef] [Green Version]
- Finley, L.W. Skeletal muscle transcriptional coactivator PGC-1α mediates mitochondrial, but not metabolic, changes during calorie restriction. Proc. Natl. Acad. Sci. USA 2012, 109, 2931–2936. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.P. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.M. Autophagy and aging: The importance of maintaining “clean” cells. Autophagy 2005, 1, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, A.; Rera, M.; Walker, D.W. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc. Natl. Acad. Sci. USA 2013, 110, 8638–8643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef] [Green Version]
- Maegawa, S. Caloric restriction delays age-related methylation drift. Nat. Commun. 2017, 8, 539. [Google Scholar] [CrossRef]
- Redman, L.M. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. S6), S1402–S1406. [Google Scholar] [CrossRef]
- Fundación Dieta Mediterránea. 2010. Available online: https://dietamediterranea.com (accessed on 1 June 2019).
- Keys, A.B. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; Harvard University Press: Cambridge, MA, USA, 1980; p. 381. [Google Scholar]
- Trichopoulou, A. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A. Diet and overall survival in the elderly. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Sofi, F. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelker, R. The Mediterranean Diet’s Fight against Frailty. JAMA 2018, 319, 1971–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach-Faig, A. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Kromhout, D. Comparative ecologic relationships of saturated fat, sucrose, food groups, and a Mediterranean food pattern score to 50-year coronary heart disease mortality rates among 16 cohorts of the Seven Countries Study. Eur. J. Clin. Nutr. 2018, 72, 1103–1110. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Yokoyama, M. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Kromhout, D. Alpha Omega Trial Group. N-3 fatty acids and cardiovascular events aftermyocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef]
- Einvik, G. A randomized clinical trial on N-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 588–592. [Google Scholar] [CrossRef]
- Bosch, J. ORIGIN Trial Investigators. N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [PubMed] [Green Version]
- Rauch, B. OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010, 122, 2152–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galan, P. OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonds, D.E. Writing Group for the AREDS2 Research Group. Effect of long-chain ω-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: Results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern. Med. 2014, 174, 763–771. [Google Scholar] [PubMed]
- Deepak, L.; Bhatt, D.L. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar]
- Bucher, H.C. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am. J. Med. 2002, 112, 298–304. [Google Scholar] [CrossRef]
- Rizos, E.C. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Kwak, S.M. Korean Meta-analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: Ameta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 2012, 172, 686–694. [Google Scholar]
- Agency for Heathcare Research and Quality. Omega-3 Fatty Acids and Cardiovascular Disease: An Updated Systemative Review. Evidence Report/Technology Assessment No. 223. Available online: https://effectivehealthcare.ahrq.gov/sites/default/files/related_files/fatty-acids-cardiovascular-disease_executive.pdf (accessed on 3 June 2019).
- Zhang, Y. Association of fish and long-chain omega-3 fatty acids intakes with total and cause-specific mortality: Prospective analysis of 421 309 individuals. J. Intern. Med. 2018, 284, 399–417. [Google Scholar] [CrossRef]
- Siscovick, D.S. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Tavazzi, L. Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Qi, W. The ω-3 fatty acid α-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARα and oxidation to oxylipins. Aging Cell 2017, 16, 1125–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathers, J.C. Nutrition and ageing: Knowledge, gaps and research priorities. Proc. Nutr. Soc. 2013, 72, 246–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffman, D.M. Energetic interventions for healthspan and resiliency with aging. Exp. Gerontol. 2016, 86, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia: A review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Lafortune, L. Behavioural Risk Factors in Mid-Life Associated with Successful Ageing, Disability, Dementia and Frailty in Later Life: A Rapid Systematic Review. PLoS ONE 2016, 11, e0144405. [Google Scholar] [CrossRef]
- Trichopoulou, A. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z. Food Habits, Lifestyle Factors and Mortality among Oldest Old Chinese: The Chinese Longitudinal Healthy Longevity Survey (CLHLS). Nutrients 2015, 7, 7562–7579. [Google Scholar] [CrossRef] [Green Version]
- Carruba, G. Nutrition, aging and cancer: Lessons from dietary intervention studies. Immun. Ageing 2016, 13, 13. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Roman, B.; Estruch, R. Scientific evidence of interventions using the Mediterranean diet: A systematic review. Nutr. Rev. 2006, 64, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Rees, K. “Mediterranean” dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 8, CD009825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sleiman, D.; Al-Badri, M.R.; Azar, S.T. Effect of Mediterranean diet in diabetes control and cardiovascular risk modification: A systematic review. Front. Public Health 2015, 3, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaccio, M.; Cerletti, C.; Iacoviello, L.; de Gaetano, G. Mediterranean diet and low-grade subclinical inflammation: The moli-sani study. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L. Changes in LDL oxidative status and oxidative and inflammatory gene expression after red wine intake in healthy people: A randomized trial. Mediat. Inflamm. 2015, 2015, 317348. [Google Scholar] [CrossRef] [PubMed]
- Milte, C.M.; McNaughton, S.A. Dietary patterns and successful ageing: A systematic review. Eur. J. Nutr. 2016, 55, 423–450. [Google Scholar] [CrossRef] [Green Version]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef] [Green Version]
- Smaga, I. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 2015, 67, 569–580. [Google Scholar] [CrossRef]
- Ticinesi, A. Nutrition and Inflammation in Older Individuals: Focus on Vitamin, D.; n-3 Polyunsaturated Fatty Acids and Whey Proteins. Nutrients 2016, 8, 186. [Google Scholar] [CrossRef] [Green Version]
- Giunta, B. Inflammaging as a prodrome to Alzheimer’s disease. J. Neuroinflamm. 2008, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Stepanova, M. Age-independent rise of inflammatory scores may contribute to accelerated aging in multimorbidity. Oncotarget 2015, 6, 1414–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostan, R. Inflammaging and cancer: A challenge for the Mediterranean diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.J. Inflammation, depression, and slow gait: A high mortality phenotype in later life. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannini, S. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J. Am. Geriatr. Soc. 2011, 59, 1679–1685. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T. The role of malnutrition in older persons with mobility limitations. Curr. Pharm. Des. 2014, 20, 3173–3177. [Google Scholar] [CrossRef]
- Jensen, G.L. Malnutrition and inflammation—“Burning down the house”: Inflammation as an adaptive physiologic response versus self-destruction? J. Parenter Enter. Nutr. 2015, 39, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Batt, J.; Dos Santos, C.C.; Herridge, M.S. Muscle injury during critical illness. J. Am. Med. Assoc. 2013, 310, 1569–1570. [Google Scholar] [CrossRef]
- Nouvenne, A. The prognostic value of high-sensitivity C-reactive protein and prealbumin for short-term mortality in acutely hospitalized multimorbid elderly patients: A prospective cohort study. J. Nutr. Health Aging 2016, 20, 462–468. [Google Scholar] [CrossRef]
- Haran, P.H.; Rivas, D.A.; Fielding, R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Kelaiditi, E. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos. Int. 2016, 27, 3251–3260. [Google Scholar] [CrossRef]
- Huang, R.Y. The Association between Total Protein and Vegetable Protein Intake and Low Muscle Mass among the Community-Dwelling Elderly Population in Northern Taiwan. Nutrients 2016, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S. Differences in Nutrient Intake and Biochemical Nutrient Status Between Sarcopenic and Nonsarcopenic Older Adults-Results From the Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlaan, S. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L. Association between Mediterranean diet and hand grip strength in older adult women. Clin. Nutr. 2019, 38, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiatarone, M.A. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef] [Green Version]
- Bonnefoy, M. Frailty and nutrition: Searching for evidence. J. Nutr. Health Aging 2015, 19, 250–257. [Google Scholar] [CrossRef]
- Kiefte-de Jong, J.C.; Mathers, J.C.; Franco, O.H. Nutrition and healthy ageing: The key ingredients. Proc. Nutr. Soc. 2014, 73, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Milaneschi, Y.; Bandinelli, S.; Corsi, A.M.; Lauretani, F.; Paolisso, G.; Dominguez, L.J.; Semba, R.D.; Tanaka, T.; Abbatecola, A.M.; Talegawkar, S.A.; et al. Mediterranean diet and mobility decline in older persons. Exp. Gerontol. 2011, 46, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Dietary quality is related to frailty in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Rodacki, C.L.N. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J. Nutr. Health Aging 2013, 17, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Takayama, M. Association of marine-origin n-3 polyunsaturated fatty acids consumption and functional mobility in the community-dwelling oldest old. J. Nutr. Health Aging 2013, 17, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G. Short-term bed rest impairs amino acid-induced protein anabolism in humans. J. Physiol. 2004, 558, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [Green Version]
- Magne, H.; Savary-Auzeloux, I.; Rémond, D.; Dardevet, D. Nutritional strategies to counteract muscle atrophy caused by disuse and to improve recovery. Nutr. Res. Rev. 2013, 26, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; Borgonjen-Van den Berg, K.J.; van Loon, L.J.C.; de Groot, L.C. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur. J. Nutr. 2012, 51, 173–179. [Google Scholar] [CrossRef]
- Talegawkar, S.A.; Bandinelli, S.; Bandeen-Roche, K.; Chen, P.; Milaneschi, Y.; Tanaka, T.; Semba, R.D.; Guralnik, J.M.; Ferrucci, L. A Higher Adherence to a Mediterranean-Style Diet Is Inversely Associated with the Development of Frailty in Community-Dwelling Elderly Men and Women. J. Nutr. 2012, 142, 2161–2166. [Google Scholar] [CrossRef] [Green Version]
- León-Muñoz, L.M.; García-Esquinas, E.; López-García, E.; Banegas, J.R.; Rodríguez-Artalejo, F. Major dietary patterns and risk of frailty in older adults: A prospective cohort study. BMC Med. 2015, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Chan, R.; Leung, J.; Woo, J. Dietary Patterns and Risk of Frailty in Chinese Community-Dwelling Older People in Hong Kong: A Prospective Cohort Study. Nutrients 2015, 7, 7070–7084. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.; Leung, S.S.F.; Ho, S.C.; Lam, T.H.; Janus, E.D. A food frequency questionnaire for use in the Chinese population in Hong Kong: Description and examination of validity. Nutr. Res. 1997, 17, 1633–1641. [Google Scholar] [CrossRef]
- Chan, R.; Chan, D.; Woo, J. Associations between dietary patterns and demographics, lifestyle, anthropometry and blood pressure in Chinese community-dwelling older men and women. J. Nutr. Sci. 2012, 1, e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Cheung, B.; Ho, S.; Sham, A.; Lam, T.H. Influence of dietary pattern on the development of overweight in a Chinese population. Eur. J. Clin. Nutr. 2008, 62, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, R.; Chan, D.; Woo, J. The association of a priori and a posterior dietary pattern with the risk of incident stroke in Chinese older people in Hong Kong. J. Nutr. Health Aging 2013, 17, 866–874. [Google Scholar] [CrossRef]
- Rahi, B.; Ajana, S.; Tabue-Teguo, M.; Dartigues, J.F.; Peres, K.; Feart, C. High adherence to a Mediterranean diet and lower risk of frailty among French older adults community-dwellers: Results from the Three-City-Bordeaux Study. Clin. Nutr. 2017, 37, 1293–1298. [Google Scholar] [CrossRef]
- Antoniak, M.; Pugliatti, M.; Hubbard, R.; Britton, J.; Sotgiu, S.; Sadovnick, A.D.; Yee, I.M.; Cumsille, M.A.; Bevilacqua, J.A.; Burdett, S.; et al. The Three City Study Group. Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Rizzoli, R.; Vaona, A.; Demurtas, J.; Crepaldi, G.; Maggi, S. Adherence to a Mediterranean diet is associated with lower incidence of frailty: A longitudinal cohort study. Clin. Nutr. 2017, 37, 1492–1497. [Google Scholar] [CrossRef]
- Felson, D.T.; Nevitt, M.C. Epidemiologic studies for osteoarthritis: New versus conventional study design approaches. Rheum. Dis. Clin. N. Am. 2004, 30, 783–797. [Google Scholar] [CrossRef]
- The Osteoarthritis Initiative—A Multi-Center Observational Study of Men and Women. Available online: http://www.oai.ucsf.edu (accessed on 4 June 2019).
- Block, G.; Hartman, A.M.; Naughton, D. A reduced dietary questionnaire: Development and validation. Epidemiology 1990, 1, 58–64. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Ntanasi, E.; Anastasiou, C.A.; Scarmeas, N. Frailty and nutrition: From epidemiological and clinical evidence to potential mechanisms. Metabolism 2017, 68, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Andriollo-Sanchez, M. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59 (Suppl. S2), S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, V.B. Aging and oxidative stress. Mol. Asp. Med. 2004, 25, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M.; Waskiewicz, A.; Mironczuk-Chodakowska, I. Dietary antioxidant and flavonoid intakes are reduced in the elderly. Oxid. Med. Cell. Longev. 2015, 2015, 843173. [Google Scholar] [CrossRef] [Green Version]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.J.; Oxidative, Y.L.J. Stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [Green Version]




| Author and Year of Publication | Study Design | Sample Size | Risk of Mortality |
|---|---|---|---|
| Trichopoulou, 2003, [84] | Population-based, prospective study | 8895 men and 13,148 women | Death from any cause: HR = 0.75 (95% CI: 0.64–0.87) for a Two-Point Increase in the Mediterranean-Diet Score Death from coronary heart disease: HR = 0.67 (95% CI: 0.47–0.94) for a Two-Point Increase in the Mediterranean-Diet Score Death from cancer: HR = 0.76 (95% CI: 0.59–0.98) for a Two-Point Increase in the Mediterranean-Diet Score |
| Estruch, 2013, [86] | Parallel-group, multicentre, randomized trial | 1050 men and 1493 women with MD with EVOO 1128 men and 1326 women with MD with nuts 987 men and 1463 women with Control Diet | Myocardial infarction, stroke, and death from cardiovascular causes: HR = 0.70 (95% CI: 0.54–0.92, p = 0.01) for MD with EVOO vs. Control Diet HR = 0.72 (95% CI: 0.54–0.96, p = 0.03) for MD with Nuts vs. Control Diet Death from any cause: HR = 0.82 (95% CI: 0.64–1.07, p = 0.15) for MD with EVOO vs. Control Diet HR = 0.97 (95% CI: 0.74–1.26, p = 0.82) for MD with Nuts vs. Control Diet |
| Estruch, 2018, [87] | Parallel-group, multicentre, randomized trial | 1050 men and 1493 women with MD with EVOO 1128 men and 1326 women with MD with nuts 987 men and 1463 women with Control Diet | Myocardial infarction: HR = 0.82 (95% CI: 0.52–1.30) for MD with EVOO vs. Control Diet HR = 0.76 (95% CI: 0.47–1.25) for MD with Nuts vs. Control Diet Stroke: HR = 0.65 (95% CI: 0.44–0.95) for MD with EVOO vs. Control Diet HR = 0.54 (95% CI: 0.35–0.82) for MD with Nuts vs. Control Diet Death from cardiovascular causes: HR = 0.62 (95% CI: 0.36–1.06) for MD with EVOO vs. Control Diet HR = 1.02 (95% CI: 0.63–1.67) for MD with Nuts vs. Control Diet Death from any cause: HR = 0.90 (95% CI: 0.69–1.18) for MD with EVOO vs. Control Diet HR = 1.12 (95% CI: 0.86–1.47) for MD with Nuts vs. Control Diet |
| Sofi, 2008, [88] | Meta-analysis of prospective cohort studies | 1,574,299 subjects from 12 studies | Mortality from cardiovascular diseases: RR = 0.91 (95% CI: 0.87–0.95) Mortality from any cause: RR = 0.91 (95% CI: 0.89–0.94 Mortality from cancer: RR = 0.94 (95% CI: 0.92–0.96) Incidence of Parkinson’s disease and Alzheimer’s disease: RR = 0.87 (95% CI: 0.80–0.96) |
| Sofi, 2010, [89] | Meta-analysis of prospective cohort studies | 508,393 subjects from 7 studies | Mortality from cardiovascular diseases: RR = 0.90 (95% CI: 0.87–0.93) Mortality from any cause: RR = 0.92 (95% CI: 0.90–0.94) Mortality from cancer: RR = 0.94 (95% CI: 0.92–0.96) Incidence of neurodegenerative disease: RR = 0.87 (95% CI: 0.81–0.94) |
| Kromhout, 2018, [92] | Prospective Cohort Study | 12,763 subjects from 16 cohorts of the Seven Countries Study | Mortality from cardiovascular diseases: Inverse association between consumption of cereals, vegetables, legumes, and alcohol and long-term CHD mortality rates (r = −0.52 to −0.62) Positive association between consumption of hard fat plus sweet products, animal foods except fish, and long-term CHD mortality rates (r = 0.68 to 0.84) |
| Author and Year of Publication | Study Design | Sample Size | Risk of Mortality |
|---|---|---|---|
| GISSI Prevention trial, 1999, [93] | Prospective Cohort Study | 8496 cases and 2828 controls from a cohort of 11,324 subjects | Death, non-fatal MI, and non-fatal stroke in two-way analysis: RR = 0.90 (95% CI: 0.82–0.99, p = 0.048) Cardiovascular death, non-fatal MI, and non-fatal stroke in two-way analysis: RR = 0.89 (95% CI: 0.80–1.01, p = 0.053) Death, non-fatal MI, and non-fatal stroke in four-way analysis: RR = 0.85 (95% CI: 0.74–0.98, p = 0.023) Cardiovascular death, non-fatal MI, and non-fatal stroke in four-way analysis: RR = 0.80 (95% CI: 0.68–0.95, p = 0.008) |
| Yokoyama, 2007, [94] | Prospective Randomised Open-Label Cohort Study | 9326 EPA treatments and 9319 controls from a cohort of 18,645 subjects | Incidence of coronary events in the total study population: HR = 0.81 (95% CI: 0.69–0.95, p = 0.011) for EPA treatments vs. controls; Incidence of coronary events in in the primary prevention arm: HR = 0.82 (95% CI: 0.63–1.06, p = 0.132) for EPA treatments vs. controls; Incidence of coronary events in in the secondary prevention arm: HR = 0.81 (95% CI: 0.66–1.00, p = 0.048) for EPA treatments vs. controls |
| Kromhout, 2010, [95] | Prospective Multi-centre, double-blind trial: n−3 fatty acids EPA and DHA and plant-derived ALA vs. placebo | 1212 subjects randomized to receive EPA–DHA and ALA; 1192 subjects randomized to receive EPA–DHA and ALA placebo; 1197 subjects randomized to receive EPA–DHA placebo and ALA; 1236 subjects randomized to receive EPA–DHA placebo and ALA placebo | Major cardiovascular events: HR = 1.01 (95% CI: 0.87–1.17, p = 0.93) with EPA–DHA; HR = 0.91 (95% CI: 0.78–1.05, p = 0.20) with ALA |
| Einvik, 2010, [96] | Interventional Clinical Trial | 563 Norwegian men randomized to a 3-year clinical trial of diet with n-3 PUFA supplementation vs. placebo (corn oil) | Mortality from any cause: HR = 0.57 (95% CI: 0.29–1.10) Mortality from cardiovascular diseases: HR = 0.86 (95% CI: 0.57–1.38) |
| Bosch, 2012, [97] | Prospective multi-centre, double-blind trial: n−3 fatty acids vs. placebo | 6281 subjects randomized to receive n−3 fatty acids; 6255 subjects randomized to receive placebo | Death from cardiovascular causes: HR = 0.98 (95% CI: 0.87–1.10, p = 0.72) Myocardial Infarction, Stroke, or Cardiovascular Death: HR = 1.01 (95% CI: 0.93–1.10, p = 0.81) Death from Any Cause: HR = 0.98 (95% CI: 0.89–1.07, p = 0.63) Death from Arrhythmia: HR = 1.10 (95% CI: 0.93–1.30, p = 0.26) |
| Rauch, 2010, [98] | Prospective randomized, placebo-controlled, double-blind, multicentre trial | 1919 subjects randomized to receive n−3 fatty acids; 1885 subjects randomized to receive placebo | Sudden cardiac death: OR = 0.95 (95% CI: 0.56–1.60, p = 0.84) Total mortality: OR = 1.25 (95% CI: 0.90–1.72, p = 0.18) Major adverse cerebrovascular and cardiovascular Events: OR = 1.21 (95% CI: 0.96–1.52, p = 0.10) Revascularization in survivors: OR = 0.93 (95% CI: 0.80–1.08, p = 0.34) |
| Galan, 2010, [99] | Prospective randomized, placebo-controlled, double-blind trial | 620 subjects randomized to receive B vitamins + omega 3 fatty acids; 633 subjects randomized to receive Omega 3 fatty acids; 622 subjects randomized to receive B vitamins; 626 subjects randomized to receive placebo | Non-fatal myocardial infarction, stroke, or death from cardiovascular disease: HR = 1.08 (95% CI: 0.79–1.47, p = 0.64); Total mortality: HR = 1.03 (95% CI: 0.72–1.48, p = 0.88) |
| Bonds, 2014, [100] | 2 × 2 factorial-designed randomized clinical trial | 1079 subjects randomized to receive lutein + zeaxanthin and DHA + EPA; 1068 subjects randomized to receive DHA + EPA; 1044 subjects randomized to receive lutein + zeaxanthin; 1012 subjects randomized to receive placebo | Time to First Cardiovascular Disease Mortality/Morbidity Event: HR = 0.95 (95% CI: 0.78–1.17) for DHA + EPA vs. No DHA + EPA; HR = 0.94 (95% CI: 0.77–1.15) for Lutein + zeaxanthin vs. No Lutein + zeaxanthin |
| Deepak, 2019, [101] | Multicentre, randomized, double-blind, placebo-controlled trial | 4089 subjects randomized to receive 2 g of Icosapent Ethyl twice daily; 4090 subjects randomized to receive placebo | Cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina: HR = 0.75 (95% CI: 0.68–0.83, p < 0.001) |
| Bucher, 2002, [102] | Meta-analysis from 11 case-control studies | 7951 patients in the treatment groups and 7855 patients in the control groups | Nonfatal myocardial infarction: RR = 0.80 (95% CI: 0.5–1.2, p = 0.16) for n-3 poly-unsaturated fatty acid-enriched diets; Fatal myocardial infarction: RR = 0.70 (95% CI: 0.6–0.8, p < 0.001) for n-3 poly-unsaturated fatty acid-enriched diets; Sudden death: RR = 0.70 (95% CI: 0.6–0.9, p < 0.01) for n-3 poly-unsaturated fatty acid-enriched diets; Overall mortality: RR = 0.80 (95% CI: 0.7–0.9, p < 0.001) for n-3 poly-unsaturated fatty acid-enriched diets |
| Rizos, 2012, [103] | Meta-analysis from 20 case-control studies | 34,388 patients in the treatment groups and 34,292 patients in the control groups | All-cause mortality: RR = 0.96 (95% CI: 0.91–1.02) for n-3 poly-unsaturated fatty acids; Cardiac death: RR = 0.91 (95% CI: 0.85–0.98) for n-3 poly-unsaturated fatty acids; Sudden death: RR = 0.87 (95% CI: 0.75–1.01) for n-3 poly-unsaturated fatty acids; Myocardial infarction: RR = 0.89 (95% CI: 0.76–1.04) for n-3 poly-unsaturated fatty acids; Stroke: RR = 1.05 (95% CI: 0.93–1.18) for n-3 poly-unsaturated fatty acids |
| Kwak, 2012, [104] | Meta-analysis from 14 placebo-control trials | 10,226 patients in the treatment groups and 10,259 patients in the control groups | Overall cardiovascular events: RR = 0.99 (95% CI: 0.89–1.09) for omega-3 fatty acid supplement; All-cause mortality: RR = 0.96 (95% CI: 0.90–1.02) for omega-3 fatty acid supplement; Sudden cardiac death: RR = 0.93 (95% CI: 0.66–1.30) for omega-3 fatty acid supplement; Cardiovascular death: RR = 0.92 (95% CI: 0.35–1.01) for omega-3 fatty acid supplement; Myocardial infarction: RR = 0.81 (95% CI: 0.65–1.01) for omega-3 fatty acid supplement; Angina and unstable angina: RR = 0.77 (95% CI: 0.50–1.18) for omega-3 fatty acid supplement; Congestive heart failure: RR = 0.92 (95% CI: 0.73–1.17) for omega-3 fatty acid supplement; Transient ischemic attack and Stroke: RR = 1.13 (95% CI: 0.77–1.66) for omega-3 fatty acid supplement |
| Agency for Healthcare Research and Quality, 2016, [105] | Meta-analysis from 61 randomized controlled trials and 37 longitudinal observational studies | No available data about sample sizes of cohorts examined | All-cause death: HR = 0.97 (95% CI: 0.92–1.03) for EPA + DHA; Major Adverse Cardiovascular Events: HR = 0.96 (95% CI: 0.91–1.02) for EPA + DHA; Myocardial infarction: HR = 0.88 (95% CI: 0.77–1.02) for EPA + DHA; Cardiovascular Disease Death: HR = 0.92 (95% CI: 0.82–1.02) for EPA + DHA; Sudden Cardiac Death: HR = 1.04 (95% CI: 0.92–1.17) for EPA + DHA; Stroke: HR = 0.98 (95% CI: 0.88–1.09) for EPA + DHA |
| Zhang, 2018, [106] | Prospective cohort study | Total and cause-specific Mortality from a cohort of 240,729 men and 180,580 women | All-cause death: HR = 0.89 (95% CI: 0.86–0.92, p < 0.0001) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in men; HR = 0.90 (95% CI: 0.86–0.94, p < 0.0001) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in women; Cancer death: HR = 0.95 (95% CI: 0.90–1.00, p = 0.040) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in men; HR = 1.01 (95% CI: 0.93–1.09, p = 0.51) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in women; Cardiovascular disease death: HR = 0.85 (95% CI: 0.80–0.90, p < 0.0001) for highest vs. lowest quintiles of of long-chain omega-3 PUFAs intake in men; HR = 0.82 (95% CI: 0.75–0.90, p < 0.0001) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in women; Respiratory disease death: HR = 0.73 (95% CI: 0.65–0.83, p < 0.0001) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in men; HR = 0.74 (95% CI: 0.64–0.87, p < 0.0001) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in women; Alzheimer’s Disease death: HR = 0.70 (95% CI: 0.54–0.89, p = 0.0008) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in men; HR = 0.59 (95% CI: 0.43–0.80, p = 0.0024) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in women; Chronic liver disease death: HR = 0.66 (95% CI: 0.49–0.89, p = 0.0046) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in men; HR = 1.30 (95% CI: 0.78–2.16, p = 0.88) for highest vs. lowest quintiles of long-chain omega-3 PUFAs intake in women |
| Author and Year of Publication | Study Design | Sample Size | Risk of Mortality |
|---|---|---|---|
| Trichopoulou, 1995, [85] | Prospective cohort study | 91 men and 91women | Mortality Rate: RR = 0.83 (95% CI: 0.69–0.99, p = 0.04) for high adherence to MD |
| Britton, 2008, [7] | Longitudinal cohort study | 4140 men and 1823 women | Likelihood of Successful Aging for men: OR = 1.52 (95% CI: 1.34–1.72, p < 0.001) for socioeconomic position OR = 1.19 (95% CI: 1.06–1.33, p = 0.003) for early-life factors OR = 1.29 (95% CI: 1.16–1.44, p < 0.001) for health behaviours OR = 1.12 (95% CI: 1.01–1.24, p = 0.03) for psychosocial factors Likelihood of Successful Aging for women: OR = 1.58 (95% CI: 1.31–1.92, p < 0.001) for socioeconomic position. OR = 1.23 (95% CI: 1.01–1.49, p = 0.04) for early-life factors OR = 1.29 (95% CI: 1.09–1.54, p = 0.003) for health behaviours OR = 1.10 (95% CI: 0.94–1.28, p = 0.25) for psychosocial factors |
| Akbaraly, 2013, [8] | Longitudinal cohort study | 3775 men and 1575 women | Ideal Aging with Healthy-foods diet: OR = 1.19 (95% CI: 0.82–1.73, p = 0.35) for higher vs. lower tertile; Non-fatal cardiovascular disease with Healthy-foods diet: OR = 1.10 (95% CI: 0.89–1.35, p = 0.39) for higher vs. lower tertile; Cardiovascular disease death with Healthy-foods diet: OR = 0.66 (95% CI: 0.43–1.01, p = 0.05) for higher vs. lower tertile; Non-cardiovascular disease death with Healthy-foods diet: OR = 0.61 (95% CI: 0.47–0.80, p < 0.0001) for higher vs. lower tertile; Ideal Aging with Western-type diet: OR = 0.52 (95% CI: 0.33–0.82, p = 0.005) for higher vs. lower tertile; Non-fatal cardiovascular disease with Western-type diet: OR = 1.08 (95% CI: 0.83–1.41, p = 0.56) for higher vs. lower tertile; Cardiovascular disease death with Western-type diet: OR = 1.66 (95% CI: 0.95–2.89, p = 0.07) for higher vs. lower tertile; Non-cardiovascular disease death with Western-type diet: OR = 1.23 (95% CI: 0.87–1.72, p = 0.24) for higher vs. lower tertile |
| Samieri, 2013, [9] | Cross-sectional observational study | 1171 “Healthy agers” vs. 9499 “Usual agers” | Healthy aging and component of healthy aging, according to Alternative Healthy Eating Index-2010: Healthy aging: OR = 1.34 (95% CI: 1.09–1.66, p < 0.001) for higher vs. lower quintile; No chronic disease: OR = 1.01 (95% CI: 0.97–1.05, p = 0.26) for higher vs. lower quintile; No cognitive impairment: OR = 0.99 (95% CI: 0.97–1.01, p = 0.09) for higher vs. lower quintile; No impairment of physical function: OR = 1.23 (95% CI: 1.11–1.36, p < 0.001) for higher vs. lower quintile; No limitation of mental health: OR = 1.13 (95% CI: 1.05–1.22, p < 0.001) for higher vs. lower quintile; Healthy aging and component of healthy aging, according to MD: Healthy aging: OR = 1.46 (95% CI: 1.17–1.83, p = 0.0022) for higher vs. lower quintile; No chronic disease: OR = 1.04 (95% CI: 1.00–1.09, p = 0.13) for higher vs. lower quintile; No cognitive impairment: OR = 0.97 (95% CI: 0.95–1.00, p = 0.02) for higher vs. lower quintile; No impairment of physical function: OR = 1.14 (95% CI: 1.03–1.26, p = 0.005) for higher vs. lower quintile; No limitation of mental health: OR = 1.12 (95% CI: 1.04–1.20, p < 0.001) for higher vs. lower quintile |
| Trichopoulou, 2005, [115] | Multicentre, prospective cohort study | 24,545 men and 50,062 women from the EPIC-elderly cohort | Mortality ratios (MR) for all countries: MR = 0.92 (95% CI: 0.88–0.97, p value for heterogeneity = 0.328) for 2 unit increase of modified MD score; Mortality ratios (MR) calibrated across countries: MR = 0.93 (95% CI: 0.88–0.99, p value for heterogeneity = 0.091) for 2 unit increase of modified MD score |
| Shi, 2015, [116] | Longitudinal cohort study | 3567 men and 5392 women from the Chinese Longitudinal Healthy Longevity Survey (CLHLS) | Hazard ratios for all-cause mortality: HR = 0.73 (95% CI: 0.68–0.77, p < 0.01) for physical activity vs. no physical activity; HR = 0.85 (95% CI: 0.77–0.92, p < 0.01) for daily fruit intake; HR = 0.74 (95% CI: 0.66–0.83, p < 0.01) for daily vegetable intake; HR = 1.05 (95% CI: 0.97–1.14, p > 0.05) for daily meat intake; HR = 1.06 (95% CI: 1.00–1.13, p < 0.05) for occasionally fish intake; HR = 1.04 (95% CI: 0.97–1.12, p > 0.05) for daily sugar intake; HR = 1.10 (95% CI: 1.03–1.18, p < 0.01) for daily salt-preserved vegetable intake |
| Author and Year of Publication | Study Design | Sample Size | Muscle Mass and Muscle Strength |
|---|---|---|---|
| Kelaiditi, 2016, [137] | Cross-sectional study | 2570 women from the Twins UK study | Fat-free mass (%): 0.9 ± 0.4 P-trend = 0.012 for highest vs. lowest adherence to MD in women ≤ 50 years; 1.0 ± 0.4 P-trend = 0.008 for highest vs. lowest adherence to MD in women ≥ 50 years; Grip strength (kg): 0.3 ± 1.0 P-trend = 0.912 for highest vs. lowest adherence to MD in women ≤ 50 years; −0.1 ± 0.5 P-trend = 0.975 for highest vs. lowest adherence to MD in women ≥ 50 years; Leg explosive power (watts/kg): 7.4 ± 3.2 P-trend = 0.010 for highest vs. lowest adherence to MD in women ≤ 50 years; 9.5 ± 3.0 P-trend = 0.005 for highest vs. lowest adherence to MD in women ≥ 50 years |
| Huang, 2016, [138] | Cross-sectional study | 327 community-dwelling elderly people | Odds ratios for total protein and vegetable protein density for Low Muscle Mass (LMM): OR = 3.11 (95% CI: 1.42–6.84, p = 0.005) for lowest vs. highest total protein density intake; OR = 2.50 (95% CI: 1.22–5.10, p = 0.012) for lowest vs. highest vegetable protein density intake; Adjusted least square (LS) means for LMM vs. normal groups: 14.5 vs. 15.5, p = 0.008 for total protein density intake; 7.0 vs. 8.2, p = 0.002 for vegetable protein density intake |
| Ter Borg, 2016, [139] | Cross-sectional study | 227 community-dwelling adults aged over 65 years from the Maastricht Sarcopenia Study | Mean(SD) of daily dietary and supplement intake of nutrients for sarcopenic vs. nonsarcopenic subjects: Protein (g): 68 (22) vs. 74 (20), p = 0.048; N-3 fatty acids (g): 1.7 (0.7) vs. 2.1 (0.8), p = 0.005; ALA, 18:3n-3 (g): 1.47 (0.59) vs. 1.73 (0.72), p = 0.018; Folic acid equivalents (g): 312 (160) vs. 375 (167), p = 0.016 Magnesium (mg): 305 (132) vs. 350 (125), p = 0.024; Mean(SD) of biochemical nutrient levels for sarcopenic vs. nonsarcopenic subjects: 25-hydroxyvitamin D (nmol/l): 56.2 (31.3) vs. 70.1 (30.3), p = 0.004; EPA, 20:5n-3(%): 0.79 (0.27) vs. 0.94 (0.38), p = 0.007; LA, 18:2n-6, %: 10.6 (1.6) vs. 9.9 (1.6), p = 0.016; Homocysteine, mmol/l: 12.1 (4.2) vs. 15.2 (7.9), p < 0.001 |
| Verlaan, 2017, [140] | Matched case-control observational study | 66 sarcopenic older adults vs. 66 non-sarcopenic older adults from the PROVIDE Study | Mean (SD) of daily dietary nutrient intakes for sarcopenic vs. nonsarcopenic subjects: Protein (g): 72.5 (19.6) vs. 75.3 (20.7), p = 0.359; Protein (g/kg): 0.99 (0.24) vs. 1.0 9 (0.29), p = 0.044 Carbohydrate (g): 212 (61) vs. 208 (76), p = 0.906; Total Fat (g): 63.3 (19.0) vs. 65.8 (22.1), p = 0.403; Vitamin B-12 (g): 3.9 (2.6) vs. 5.3 (3.6), p = 0.011 Vitamin D (mg): 2.6 (2.1) vs. 4.0 (3.4), p = 0.007 Magnesium (mg): 260 (96) vs. 295 (86), p = 0.015; Phosphorus (mg): 1196 (330) vs. 1325 (338), p = 0.014 Selenium (mg): 39.1 (17.1) vs. 46.5 (21.2), p = 0.039 |
| Barrea, 2019, [141] | Cross-sectional observational study | 84 not hospitalized elderly women from the PERSSILAA project | Daily nutrients (SD, range) intake of participants according the HGS cut-point: Protein (%): 12.24 (2.04) for HGS < 20 Kg vs. 14.75 (1.45) for HGS > 20 Kg, p < 0.001; Carbohydrate (%): 55.1 (range 50.91–60.00) for HGS < 20 Kg vs. 56.00 (range 51.00–61.90) for HGS > 20 Kg, p < 0.001; Total Fat (%): 32.34 (3.38) for HGS < 20 Kg vs. 29.50 (3.27) for HGS > 20 Kg, p < 0.001; Unsaturated Fat (%): 20.98 (3.96) for HGS < 20 Kg vs. 22.83 (3.05) for HGS > 20 Kg, p = 0.018; N-3 PUFA (g/day): 4.28 (2.85) for HGS < 20 Kg vs. 5.54 (2.42) for HGS > 20 Kg, p = 0.031; Cholesterol (mg/day): 332.42 (34.91) for HGS < 20 Kg vs. 309.78 (38.24) for HGS > 20 Kg, p = 0.006; Association of adherence to MD with the HGS, after adjusting for BMI: Low adherence to MD: OR = 0.73 (95% CI: 0.61–0.86), p < 0.001; Average adherence to MD: OR = 1.02 (95% CI: 0.95–1.09), p = 0.611 High adherence to MD: OR = 1.14 (95% CI: 1.04–1.25), p = 0.003 |
| Author and Year of Publication | Study Design | Sample Size | Risk of Frailty |
|---|---|---|---|
| Milaneschi, 2011, [146] | Prospective population-based study | 935 community-living subjects aged over 65 years from the InCHIANTI Study cohort | Adjusted odds of developing mobility disability: OR = 0.73 (95% CI: 0.41–1.28, p = 0.27) for highest vs. lowest adherence to MD; Decrease in SPPB scores at 9 years of follow up: Average Score = 0.9 (SE = 0.41, p = 0.03) for highest vs. lowest adherence to MD; Adjusted incidence of mobility disability: HR = 0.71 (95% CI: 0.51–0.98, p = 0.04) for highest vs. lowest adherence to MD |
| Bollwein, 2013, [147] | Cross-sectional study | 192 community-dwelling volunteers aged over 75 years | Odds Ratio for Frailty: OR = 0.19 (95% CI: 0.05–0.82, p = 0.011) for highest vs. lowest adherence to MD |
| Talegawkar, 2012, [155] | Prospective population-based study | 690 community-living subjects aged over 65 years from the InCHIANTI Study cohort | Odds Ratio for Frailty: OR = 0.30 (95% CI: 0.14–0.66) for highest vs. lowest adherence to MD |
| Luz, 2015, [156] | Prospective cohort study | 1872 non-institutionalized subjects aged over 60 years from the Seniors-ENRICA cohort Study | Odds Ratio for Frailty: OR = 0.40 (95% CI: 0.20–0.81, p = 0.009) for highest adherence to a “prudent pattern” diet; 0.40 (0.20–0.81) 0.009 OR = 1.61 (95% CI: 0.85–3.03, p = 0.14) for highest adherence to a “westernized pattern” diet |
| Rahi, 2017, [163] | Population-based prospective cohort study | 560 non-institutionalized subjects aged over 65 years from the cohort of Three-City-Bordeaux Study | Odds Ratio for Frailty: OR = 0.32 (95% CI: 0.14–0.72, p = 0.006) for highest vs. lowest adherence to MD |
| Veronese, 2017, [165] | Population-based prospective cohort study | 1857 men and 2564 women from the The Osteoarthritis Initiative cohort Study | Odds Ratio for Frailty: OR = 0.71 (95% CI: 0.50–0.99, p = 0.047) for highest vs. lowest adherence to MD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capurso, C.; Bellanti, F.; Lo Buglio, A.; Vendemiale, G. The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review. Nutrients 2020, 12, 35. https://doi.org/10.3390/nu12010035
Capurso C, Bellanti F, Lo Buglio A, Vendemiale G. The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review. Nutrients. 2020; 12(1):35. https://doi.org/10.3390/nu12010035
Chicago/Turabian StyleCapurso, Cristiano, Francesco Bellanti, Aurelio Lo Buglio, and Gianluigi Vendemiale. 2020. "The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review" Nutrients 12, no. 1: 35. https://doi.org/10.3390/nu12010035






