Observed Dietary Intake in Adults with Intellectual Disability Living in Group Homes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Setting and Design
2.2. Anthropometric Measurements
2.3. Measurement and Assessment of Dietary Intake
2.4. Assessment of the Adequacy of Dietary Intake
2.5. Statistical Analysis
3. Results
3.1. Participant Recruitment, Demographic and Anthropometric Characteristics
3.2. Adequacy of Dietary Intake against Nutrient Reference Value
3.2.1. Energy and Macronutrient Intake
3.2.2. Micronutrient Intake
3.3. Adequacy of Dietary Intake against the Australian Guide to Healthy Eating
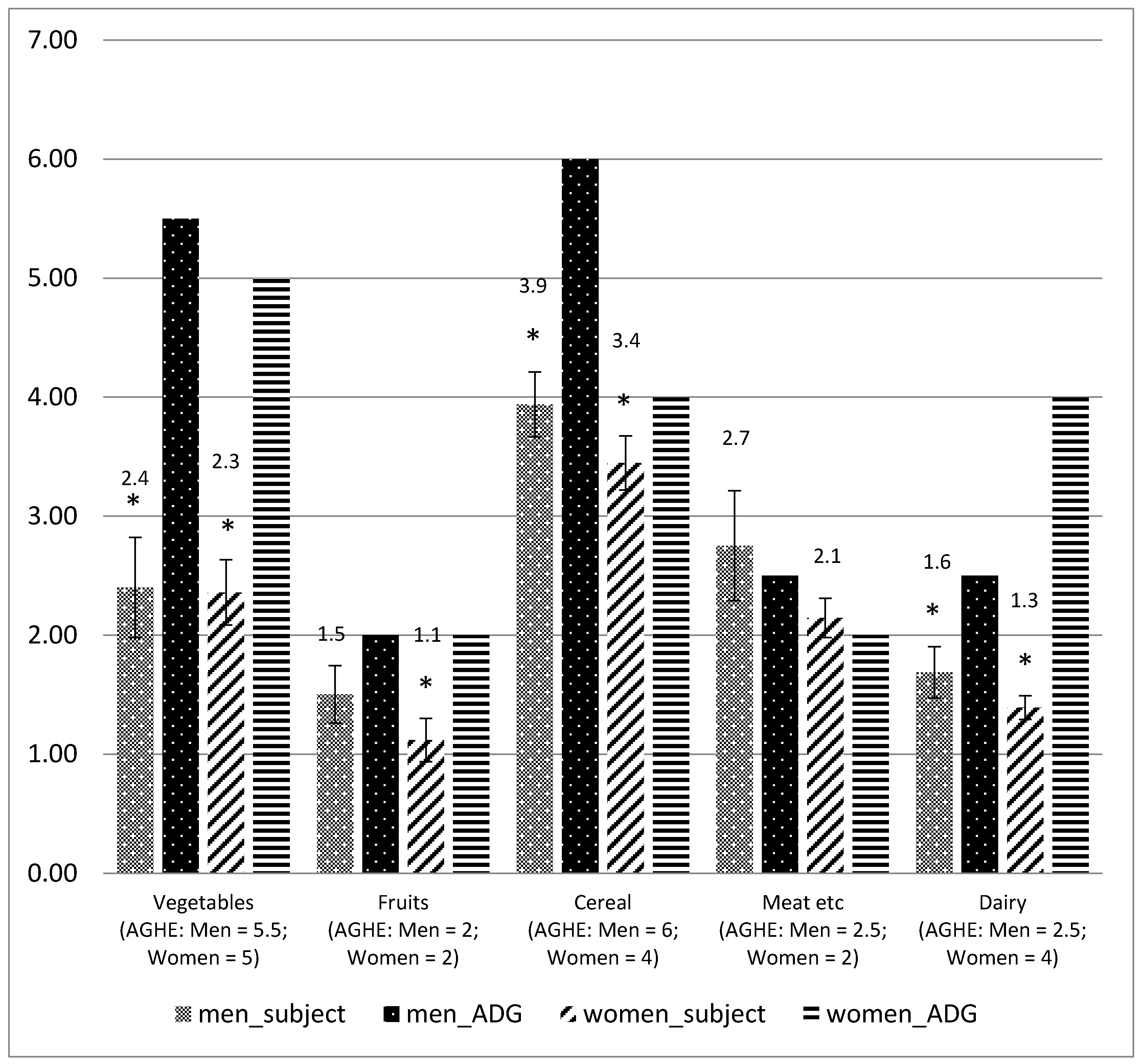
3.3.1. Intake of Core Food against Australian Guide to Healthy Eating
3.3.2. Intake of Discretionary Food against Australian Guide to Healthy Eating
4. Discussion
4.1. Energy Intake
4.2. Macronutrient Intake
4.3. Micronutrient Intake
4.4. Intake of Servings According to the Australian Guide to Healthy Eating
4.5. Strengths and Limitations
4.6. Nutrition Recommendations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, L. Community and cluster centre residential services for adults with intellectual disability: Long-term results from an Australian-matched sample. J. Intellect. Disabil. Res. 2006, 50, 419–431. [Google Scholar] [CrossRef]
- Mansell, J.; Beadle-Brown, J. Deinstitutionalisation and community living: Position statement of the comparative policy and practice special interest research group of the international association for the scientific study of intellectual disabilities. J. Intellect. Disabil. Res. 2010, 54, 104–112. [Google Scholar] [CrossRef]
- McCarron, M.; Lombard-Vance, R.; Murphy, E.; May, P.; Webb, N.; Sheaf, G.; McCallion, P.; Stancliffe, R.; Normand, C.; Smith, V.; et al. Effect of deinstitutionalisation on quality of life for adults with intellectual disabilities: A systematic review. BMJ Open 2019, 9, e025735. [Google Scholar] [CrossRef]
- Adolfsson, P.; Sydner, Y.M.; Fjellström, C.; Lewin, B.; Andersson, A. Observed dietary intake in adults with intellectual disability living in the community. Food Nutr. Res. 2008, 52, 1–7. [Google Scholar] [CrossRef]
- Emerson, E.; Hatton, C. Deinstitutionalization in the UK and Ireland: Outcomes for service users. J. Intellect. Dev. Disabil. 1996, 21, 17–37. [Google Scholar] [CrossRef]
- Kim, S.; Larson, S.A.; Lakin, K.C. Behavioural outcomes of deinstitutionalisation for people with intellectual disability: A review of US studies conducted between 1980 and 1999. J. Intellect. Dev. Disabil. 2001, 26, 35–50. [Google Scholar] [CrossRef]
- Kozma, A.; Mansell, J.; Beadle-Brown, J. Outcomes in different residential settings for people with intellectual disability: A systematic review. Am. J. Intellect. Dev. Disabil. 2009, 114, 193–222. [Google Scholar] [CrossRef] [PubMed]
- McConkey, R.; Kelly, F.; Craig, S.; Mannan, H. A longitudinal study of the intra-country variations in the provision of residential care for adult persons with an intellectual disability. J. Intellect. Disabil. Res. 2013, 57, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Molony, H.; Taplin, J. Deinstitutionalization of people with developmental disability. J. Intellect. Dev. Disabil. 1988, 14, 109–122. [Google Scholar] [CrossRef]
- Young, L. Residential and Lifestyle Changes for Adults with an Intellectual Disability in Queensland 1960-2001. Int. J. Disabil. Dev. Educ. 2003, 50, 93–106. [Google Scholar] [CrossRef]
- Young, L.; Sigafoos, J.; Grevell, P. Deinstitutionalisation of persons with intellectual disabilities: A review of Australian studies. J. Intellect. Dev. Disabil. 1998, 23, 155–170. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Disability Support Services: Appendix 2012–13. Disability Series; Cat. no.: AUS 182; AIHW: Canberra, Australia, 2014. Available online: http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129547853 (accessed on 16 September 2015).
- Australian Institute of Health and Welfare. Disability in Australia: Intellectual Disability; AIHW Bulletin no. 67; Cat. no.: AUS 110; AIHW: Canberra, Australia, 2008. Available online: http://www.aihw.gov.au/publication-detail/?id=6442468183 (accessed on 16 September 2015).
- Trollor, J.; Srasuebkul, P.; Xu, H.; Howlett, S. Cause of death and potentially avoidable deaths in Australian adults with intellectual disability using retrospective linked data. BMJ Open 2017, 7, e013489. [Google Scholar] [CrossRef] [PubMed]
- Bryan, F.; Allan, T.; Russell, L. The move from a long-stay learning disabilities hospital to community homes: A comparison of clients’ nutritional status. J. Hum. Nutr. Diet. 2000, 13, 265–270. [Google Scholar] [CrossRef]
- Frey, B.; Rimmer, J.H. Comparison of body composition between German and American adults with mental retardation. Med. Sci. Sports Exer. 1995, 27, 1439–1443. [Google Scholar] [CrossRef]
- Hsieh, K.; Rimmer, J.H.; Heller, T. Obesity and associated factors in adults with intellectual disability. J. Intellect. Disabil. Res. 2014, 58, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Battezzati, A.; Merati, G.; Margonato, V.; Maggioni, M.; Testolin, G.; Veicsteinas, A. Nutritional status and dietary patterns in disabled people. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 100–112. [Google Scholar] [CrossRef]
- Draheim, C.C.; McCubbin, J.A.; Williams, D.P. Differences in cardiovascular disease risk between nondiabetic adults with mental retardation with and without Down syndrome. Am. J. Ment. Retard. 2002, 107, 201–211. [Google Scholar] [CrossRef]
- Teasdale, S.B.; Burrows, T.L.; Hayes, T.; Hsia, C.Y.; Watkins, A.; Curtis, J.; Ward, P.B. Dietary intake, food addiction and nutrition knowledge in young people with mental illness. Nutr. Diet. 2019. [Google Scholar] [CrossRef]
- Robertson, J.; Emerson, E.; Gregory, N.; Hatton, C.; Kessissoglou, S.; Hallam, A. Receipt of psychotropic medication by people with intellectual disability in residential settings. J. Intellect. Disabil. Res. 2000, 44, 666–676. [Google Scholar] [CrossRef]
- Holland, A.J.; Wong, J. Genetically determined obesity in Prader-Willi syndrome: The ethics and legality of treatment. J. Med. Ethics 1999, 25, 230–236. [Google Scholar] [CrossRef]
- Pereira, R.; Schalk, A.; Geraghty, M.E. Prader-Willi Syndrome a Review for Pediatric Nutrition Professionals. Infant Child Adolesc. Nutr. 2009, 1, 282–287. [Google Scholar] [CrossRef]
- Sinnema, M.; Einfeld, S.L.; Schrander-Stumpel, C.T.; Maaskant, M.A.; Boer, H.; Curfs, L.M. Behavioral phenotype in adults with Prader–Willi syndrome. Res. Dev. Disabil. 2011, 32, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Beange, H.; Mackerras, D. A survey of dietary problems of adults with learning disabilities in the community. J. Appl. Res. Intellect. Disabil. 2006, 7, 41–50. [Google Scholar] [CrossRef]
- Humphries, K.; Traci, M.A.; Seekins, T. A preliminary assessment of the nutrition and food-system environment of adults with intellectual disabilities living in supported arrangements in the community. Ecol. Food Nutr. 2004, 43, 517–532. [Google Scholar] [CrossRef]
- Melville, C.A.; Hamilton, S.; Hankey, C.R.; Miller, S.; Boyle, S. The prevalence and determinants of obesity in adults with intellectual disabilities. Obes. Rev. 2007, 8, 223–230. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Yamaki, K. Obesity and intellectual disability. Dev. Disabil. Res. Rev. 2006, 12, 22–27. [Google Scholar] [CrossRef]
- De Winter, C.F.; Bastiaanse, L.P.; Hilgenkamp, T.I.M.; Evenhuis, H.M.; Echteld, M.A. Overweight and obesity in older people with intellectual disability. Res. Dev. Disabil. 2012, 33, 398–405. [Google Scholar] [CrossRef]
- Stancliffe, R.J.; Lakin, K.C.; Larson, S.; Engler, J.; Bershadsky, J.; Taub, S.; Fortune, J.; Ticha, R. Overweight and obesity among adults with intellectual disabilities who use intellectual disability/developmental disability services in 20 U.S. States. Am. J. Intellect. Dev. Disabil. 2011, 116, 401–418. [Google Scholar] [CrossRef]
- Ptomey, L.; Goetz, J.; Lee, J.; Donnelly, J.; Sullivan, D. Diet Quality of Overweight and Obese Adults with Intellectual and Developmental Disabilities as Measured by the Healthy Eating Index-2005. J. Dev. Phys. Disabil. 2013, 25, 625–636. [Google Scholar] [CrossRef]
- Hamzaid, N.H.; Flood, V.M.; Prvan, T.; O’Connor, H.T. General nutrition knowledge among carers at group homes for people with intellectual disability. J. Intellect. Disabil. Res. 2018, 62, 422–430. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. J. Am. Med. Assoc. 2003, 289, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, Lifestyle, and Risk of Type 2 Diabetes Mellitus in Women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H. Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Agency of Clinical Innovation. Nutrition Standards for Consumers of Inpatient Mental Health Services in NSW; ACI: Chatswood, Australia, 2013. Available online: https://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0013/201091/ACI-Nutrition-Mental-Health-Inpatients-web-final.pdf (accessed on 16 September 2015).
- Van Riper, C. Position of the American Dietetic Association: Providing Nutrition Services for People with Developmental Disabilities and Special Health Care Needs. J. Am. Diet. Assoc. 2010, 110, 296–307. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and World Bank. World Report on Disability; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 1994. [Google Scholar]
- National Health and Medical Research Council. Australian Dietary Guidelines Educator Guide; NHMRC: Canberra, Australia, 2013. Available online: https://www.eatforhealth.gov.au/sites/default/files/content/The%20Guidelines/n55b_educator_guide_140321_1.pdf (accessed on 30 September 2019).
- Draheim, C.C.; Stanish, H.I.; Williams, D.P.; McCubbin, J.A. Dietary intake of adults with mental retardation who reside in community settings. Am. J. Ment. Retard. 2007, 112, 392–400. [Google Scholar] [CrossRef]
- Humphries, K.; Traci, M.A.; Seekins, T. Nutrition and adults with intellectual or developmental disabilities: Systematic literature review results. Intellect. Dev. Disabil. 2009, 47, 163–185. [Google Scholar] [CrossRef]
- Bergström, H.; Hagströmer, M.; Hagberg, J.; Elinder, L.S. A multi-component universal intervention to improve diet and physical activity among adults with intellectual disabilities in community residences: A cluster randomised controlled trial. Res. Dev. Disabil. 2013, 34, 3847–3857. [Google Scholar] [CrossRef]
- Elinder, L.S.; Brunosson, A.; Bergström, H.; Hagströmer, M.; Patterson, E. Validation of personal digital photography to assess dietary quality among people with intellectual disabilities. J. Intellect. Disabil. Res. 2012, 56, 221–226. [Google Scholar] [CrossRef]
- Ptomey, L.T.; Willis, E.A.; Goetz, J.R.; Lee, J.; Sullivan, D.K.; Donnelly, J.E. Digital photography improves estimates of dietary intake in adolescents with intellectual and developmental disabilities. Disabil. Health J. 2015, 8, 146–150. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. AUSNUT 2007. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/Pages/ausnut2007.aspx (accessed on 30 September 2019).
- Food Standards Australia & New Zealand. Food Nutrient Database. 2019. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/foodnutrient/Pages/defaul.aspx (accessed on 30 September 2019).
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; NHMRC: Canberra, Australia, 2006. Available online: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/n35.pdf (accessed on 30 September 2019).
- Atherton, M.; Bellis-Smith, N.; Cichero, J.; Suter, M. Texture-modified foods and thickened fluids as used for individuals with dysphagia: Australian standardised labels and definitions. Nutr. Diet. 2007, 64, 53–76. [Google Scholar]
- National Health and Medical Research Council. Recommended Number of Serves for Adults; NHMRC: Canberra, Australia, 2015. Available online: https://www.eatforhealth.gov.au/food-essentials/how-much-do-we-need-each-day/recommended-number-serves-adults (accessed on 30 November 2019).
- Lindeman, A.K. Resident manager’s nutrition concerns of staff and residents of group homes for mentally retarded adults. J. Am. Diet. Assoc. 1991, 91, 602–604. [Google Scholar] [PubMed]
- Mikulovic, J.; Vanhelst, J.; Salleron, J.; Marcellini, A.; Compte, R.; Fardy, P.S.; Bui-Xuan, G. Overweight in intellectually-disabled population: Physical, behavioral and psychological characteristics. Res. Dev. Disabil. 2013, 35, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lante, K.; Reece, J.; Walkley, J. Energy expended by adults with and without intellectual disabilities during activities of daily living. Res. Dev. Disabil. 2010, 31, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Theodoro, M.F.; Bittel, D.C.; Donnelly, J.E. Energy Expenditure and Physical Activity in Prader-Willi Syndrome: Comparison with Obese Subjects. Am. J. Med. Genet. A 2007, 459, 449–459. [Google Scholar] [CrossRef]
- Hinckson, E.A.; Dickinson, A.; Water, T.; Sands, M.; Penman, L. Physical activity, dietary habits and overall health in overweight and obese children and youth with intellectual disability or autism. Res. Dev. Disabil. 2013, 34, 1170–1178. [Google Scholar] [CrossRef]
- Emerson, E. Underweight, obesity and exercise among adults with intellectual disabilities in supported accomodation in Northern England. J. Intellect. Disabil. Res. 2005, 49, 134–143. [Google Scholar] [CrossRef]
- Braunschweig, C.L.; Gomez, S.; Sheean, P.; Tomey, K.M.; Rimmer, J.; Heller, T. Nutritional status and risk factors for chronic disease in urban-dwelling adults with Down syndrome. Am. J. Ment. Retard. 2004, 109, 186–193. [Google Scholar] [CrossRef]
- Böhmer, C.J.; Taminiau, J.A.; Klinkenberg-Knol, E.C.; Meuwissen, S.G. The prevalence of constipation in institutionalized people with intellectual disability. J. Intellect. Disabil. Res. 2001, 45, 212–218. [Google Scholar] [CrossRef]
- Baptista, F.; Varela, A.; Sardinha, L.B. Bone mineral mass in males and females with and without Down syndrome. Osteoporos. Int. 2005, 16, 380–388. [Google Scholar] [CrossRef]
- Bastiaanse, L.P.; Mergler, S.; Evenhuis, H.M.; Echteld, M.A. Bone quality in older adults with intellectual disabilities. Res. Dev. Disabil. 2014, 35, 1927–1933. [Google Scholar] [CrossRef]
- Lin, L.P.; Hsu, S.W.; Yao, C.H.; Lai, W.J.; Hsu, P.J.; Wu, J.L.; Lin, J.D. Risk for osteopenia and osteoporosis in institution-dwelling individuals with intellectual and/or developmental disabilities. Res. Dev. Disabil. 2015, 36, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, R.; Cassidy, G.; Joiner, C.; Teeluckdharry, S. Osteoporosis in people with intellectual disabilities: A review and a brief study of risk factors for osteoporosis in a community sample of people with intellectual disabilities. J. Intellect. Disabil. Res. 2011, 55, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Australian Health Survey (AHS). Australian Bureau of Statistics: Updated Results, 2011–2012; ABS: Canberra, Australia, 2012. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.003main+features12011-2012 (accessed on 10 October 2019).
- He, J.; Ogden, L.G.; Vupputuri, S.; Bazzano, L.A.; Loria, C.; Whelton, P.K. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. J. Am. Med. Assoc. 1999, 282, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Tinker, L.; Shaw, P.A.; Schoeller, D.; Bingham, S.A.; Horn, L.V.; Beresford, S.A.; Caan, B.; Thomson, C.; Satterfield, S.; et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am. J. Epidemiol. 2008, 167, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, D.; Jaceldo-Siegl, K.; Beeson, W.; Fraser, G.; Oda, K.; Haddad, E. Foods and supplements associated with vitamin B12 biomarkers among vegetarian and non-vegetarian participants of the Adventist Health Study-2 (AHS-2) Calibration Study. Nutrients 2018, 10, 722. [Google Scholar] [CrossRef]
- Zhu, K.; Oddy, W.H.; Holt, P.; Ping-Delfos, W.C.S.; McVeigh, J.; Straker, L.; Mori, T.A.; Lye, S.; Pennell, C.; Walsh, J.P. Relationship between vitamin D status from childhood to early adulthood with body composition in young Australian adults. J. Endocr. Soc. 2019, 3, 563–576. [Google Scholar] [CrossRef]

| Total Sample (n = 33) | ||
|---|---|---|
| Classification | Men (n = 14) n (%) | Women (n = 19) n (%) |
| aAge (years), mean ± SD (median) | 48 ± 9 (50) | 53 ± 17 (58) |
| 19–30 | 1 (7) | 2 (11) |
| 31–50 | 7 (50) | 6 (32) |
| 51–70 | 6 (42) | 10 (53) |
| >70 | 0 | 1 (5) |
| bBody Mass Index (kgm−2), mean ±SD (median) | 26 ± 4 (27) | 29 ± 7 (28) |
| 18.50–24.99 (normal weight) | 5 (36) | 7 (37) |
| 25.00–29.99 (overweight) | 8 (57) | 5 (26) |
| 30.00–40.00 (obese) | 1 (7) | 7 (37) |
| cDisability Level | ||
| Mild | 1 (7) | 2 (10) |
| Moderate | 6 (42) | 8 (42) |
| Severe | 6 (42) | 6 (32) |
| * Profound | 1 (7) | 3 (16) |
| dTexture Modification | ||
| Normal | 11 (79) | 13 (68) |
| Soft | 1 (7) | 4 (21) |
| Minced and Moist | 2 (14) | 2 (10) |
| Smooth Pureed | 0 | 0 |
| Mobility | ||
| Independent | 11 (79) | 16 (84) |
| Dependent/Assisted | 2 (14) | 3 (16) |
| Wheelchair dependent | 1 (7) | 0 |
| Liquid Meal Supplement Used | ||
| Yes | 2 (14) | 0 |
| No | 12 (86) | 19 (100) |
| Macronutrients | Mean ± SD [95% CI] | Median (Inter-Quartile Range) | ||
|---|---|---|---|---|
| Men (n = 14) | Women (n = 19) | Men | Women | |
| Energy (kJ per day) | 7439 (6676–8202) | 7034 (6461–7607) | 7365 (5520–9134) | 6846 (5444–9797) |
| kJ/kg | 101.8 ± 30.2 (95.2–137.7) | 111.2 ± 27.8 (92.2–136.1) | 102.5 (82.4–197.9) | 113.9 (55.0–165.1) |
| % ≥PAL 1.2 a | 29 | 89 | ||
| % ≥PAL 1.4 a | 17 | 47 | ||
| Carbohydrate total (g/d) | 206.2 ± 55.2 (174.1–237.9) | 187.2 ± 55.2 (162.6–211.8) | 203 (130–332) | 180 (108–302) |
| Recommendation | 45%–65% of energy | 45%–65% of energy | ||
| % of energy | 47.6 ±11.1 | 46.5 ± 9.7 | ||
| g/kg/d | 3.2 ±1.3 | 3.2 ± 1.2 | ||
| Total sugars (g/d) | 81.5 ± 32.1 (62.2–106.0) | 77.8 ± 30.9 (59.0–103.1) | 79 (38–146) | 70 (28–139) |
| Recommendation | <10% of energy | <10% of energy | ||
| % of energy | 21.1 ± 10.8 | 16.8 ± 6.6 | ||
| Protein (g/d) | 89.5 ± 15.5 (80.6–98.4) | 75.6 ± 17.2 (63.5–97.1) | 93 (61–118) | 75 (47–105) |
| Recommendation | 15%–25% of energy | 15%–25% of energy | ||
| % of energy | 17.3 ± 2.4 | 17.2 ± 2.2 | ||
| g/kg/day | 1.3 ± 0.3 | 1.1 ± 0.3 | ||
| Fat total (g/d) | 63.9 ± 23.2 (50.6–77.3) | 66.1 ± 11.6 (60.1–71.1) | 67 (27–100) | 66 (42–87) |
| Recommendation | 20%–35% of energy | 20%–35% of energy | ||
| % of energy | 32.6 ± 9.6 | 35.8 ±7.6 | ||
| Saturated fat (g/d) | 24.2 ± 10.7 [14.1–27.2] | 25.6 ± 8.0 [15.2–28.9] | 26 (7–40) | 30 (13–41) |
| Recommendation | <10% of energy | <10% of energy | ||
| % of energy | 11.9 ± 5.3 | 14.9 ± 5.2 | ||
| g/kg/day | [8.8–14.9] | [12.4–17.5] | ||
| Dietary fibre (g/d) | 23.0 ± 4.8 (19.3–24.6) | 20.9 ± 5.9 (18.0–23.8) | 24 (18–31) | 21 (12–33) |
| Recommendation | 30 g per day | 25 g per day | ||
| Micronutrient | Mean ± SD [95% CI] | p-Value | a EAR | a RDI/a AI | % < EAR | % < RDI/AI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 14) | Women (n = 19) | M | W | M | W | M | W | M | W | ||
| Thiamin (mg/d) | 1.7 ± 0.6 (1.4–2.1) | 1.7 ± 1.1 (1.2–2.2) | 0.929 | 1.0 | 0.9 | 1.2 | 1.1 | 7 | 21 | 13 | 26 |
| Riboflavin (mg/d) | 2.2 ± 0.6 (1.9–2.5) | 2.2 ± 1.0 (1.8–2.7) | 0.945 | 1.1 | 0.9 | 1.3 | 1.1 | 6 | 0 | 6 | 0 |
| Niacin Eq (mg/d) | 44.6 ± 7.7 (40.2–49.1) | 39.9 ± 8.7 (35.7–44.0) | 0.109 | 12 | 11 | 16 | 14 | 0 | 0 | 0 | 0 |
| Vitamin C (mg/d) | 111.7 ± 47.2 (84.5–138.9) | 94.4 ± 58.4 (66.2–122.5) | 0.369 | 30 | 30 | 45 | 45 | 0 | 7 | 0 | 13 |
| Vitamin E (mg/d) | 6.9 ± 2.0 (5.8–8.1) | 6.0±2.5 (4.7–7.2) | 0.219 | - | - | c 10 | c 7 | - | - | 93 | 89 |
| b Folate (µg/day) | 531.1 ± 137.7 (451.5–610.5) | 572.1 ± 302.4 (426.3–717.8) | 0.640 | 320 | 320 | 400 | 400 | 0 | 16 | 13 | 26 |
| Magnesium (mg/d) | 295.9 ± 51.6 (266.1–325.6) | 260.0 ± 76.5 (223.1–296.8) | 0.139 | 350 | 265 | 420 | 320 | 86 | 63 | 100 | 78 |
| Calcium (mg/d) | 763.2 ± 321.8 (577.4–949.0) | 709.6 ± 211.2 (607.9–811.4) | 0.593 | 840 | 1100 | 1000 | 1300 | 43 | 78 | 86 | 94 |
| Phosphorus (mg/d) | 1384.5 ± 242.1 (1244.7–1524.2) | 1272.3 ± 313.7 (1121.1–1423.5) | 0.256 | 580 | 580 | 1000 | 1000 | 0 | 0 | 26 | 26 |
| Iron (mg/d) | 12.6 ± 4.0 (10.3–15.0) | 10.1 ± 3.5 (8.4–11.7) | 0.065 | 6 | 5 | 8 | 8 | 0 | 5 | 12 | 42 |
| Zinc (mg/d) | 13.7 ± 3.9 (11.5–16.0) | 10.1 ± 2.5 (9.0–11.4) | 0.007 | 12 | 6.5 | 14 | 8 | 43 | 0 | 66 | 21 |
| Iodine (µg/d) | 136.9 ± 48.6 (108.8–164.9) | 128.6 ± 39.0 (109.8–147.4) | 0.593 | 100 | 100 | 150 | 150 | 43 | 47 | 67 | 74 |
| Sodium (mg/d) | 2565.7 ± 981.1 (1999.2–132.1) | 2404.5 ± 1241.6 (1806.1–3002.2) | 0.691 | - | - | c 460–920 | c 460–920 | - | - | 0 | 0 |
| Potassium (mg/d) | 2774.4 ± 458 (2510.1–3038.7) | 2417.3 ± 908.7 (1979.3–2855.3) | 0.188 | - | - | c 3800 | c 2800 | - | - | 100 | 79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzaid, N.H.; O’Connor, H.T.; Flood, V.M. Observed Dietary Intake in Adults with Intellectual Disability Living in Group Homes. Nutrients 2020, 12, 37. https://doi.org/10.3390/nu12010037
Hamzaid NH, O’Connor HT, Flood VM. Observed Dietary Intake in Adults with Intellectual Disability Living in Group Homes. Nutrients. 2020; 12(1):37. https://doi.org/10.3390/nu12010037
Chicago/Turabian StyleHamzaid, Nur Hana, Helen T. O’Connor, and Victoria M. Flood. 2020. "Observed Dietary Intake in Adults with Intellectual Disability Living in Group Homes" Nutrients 12, no. 1: 37. https://doi.org/10.3390/nu12010037
APA StyleHamzaid, N. H., O’Connor, H. T., & Flood, V. M. (2020). Observed Dietary Intake in Adults with Intellectual Disability Living in Group Homes. Nutrients, 12(1), 37. https://doi.org/10.3390/nu12010037






