Estrogenic, Antiestrogenic and Antiproliferative Activities of Euphorbia bicolor (Euphorbiaceae) Latex Extracts and Its Phytochemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Preparation of Latex Extract
2.2. Identification of Latex Phytochemicals by UPLC-ESI-MS/MS
2.3. Estrogenic and Antiestrogenic Assays
2.4. Cell Lines and Cell Culture Conditions
2.5. Cell Culture Treatments
2.6. Statistical Analyses
3. Results
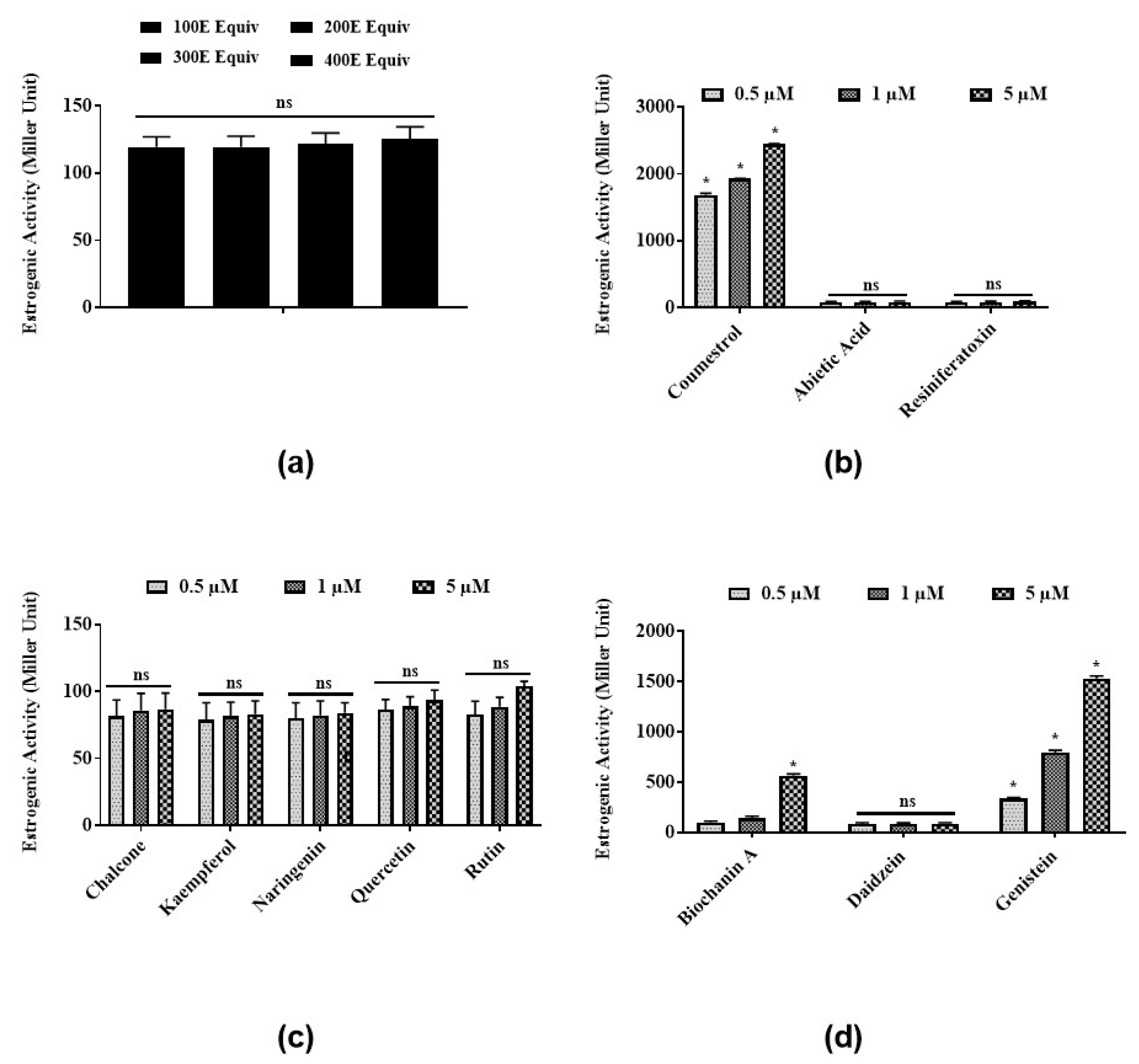
3.1. Estrogenic Activities of Latex Extract and Its Phytochemicals in a Steroid-Regulated Yeast System
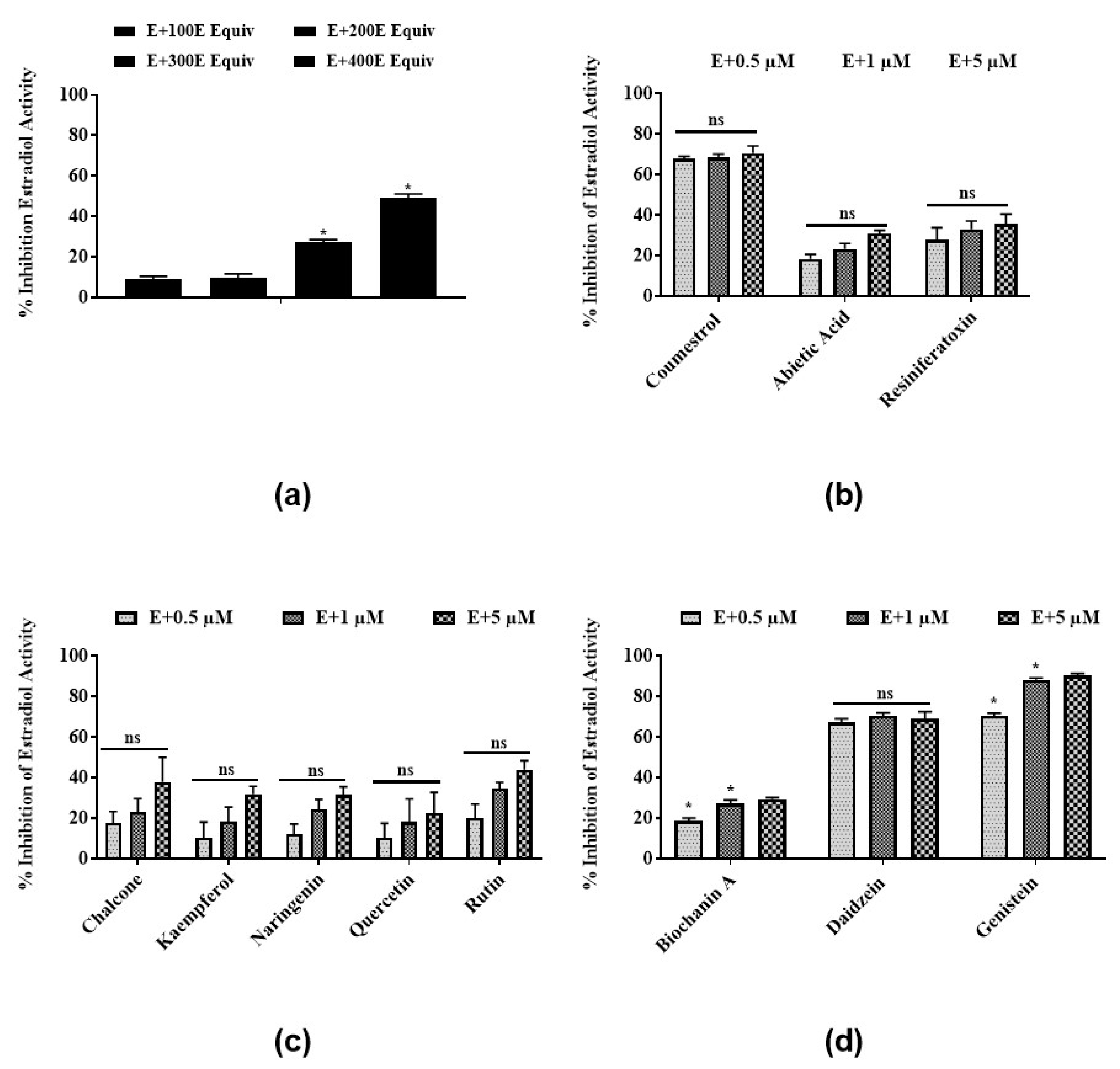
3.2. Antiestrogenic Activities of Latex Extract and Its Phytochemicals in a Steroid-Regulated Yeast System
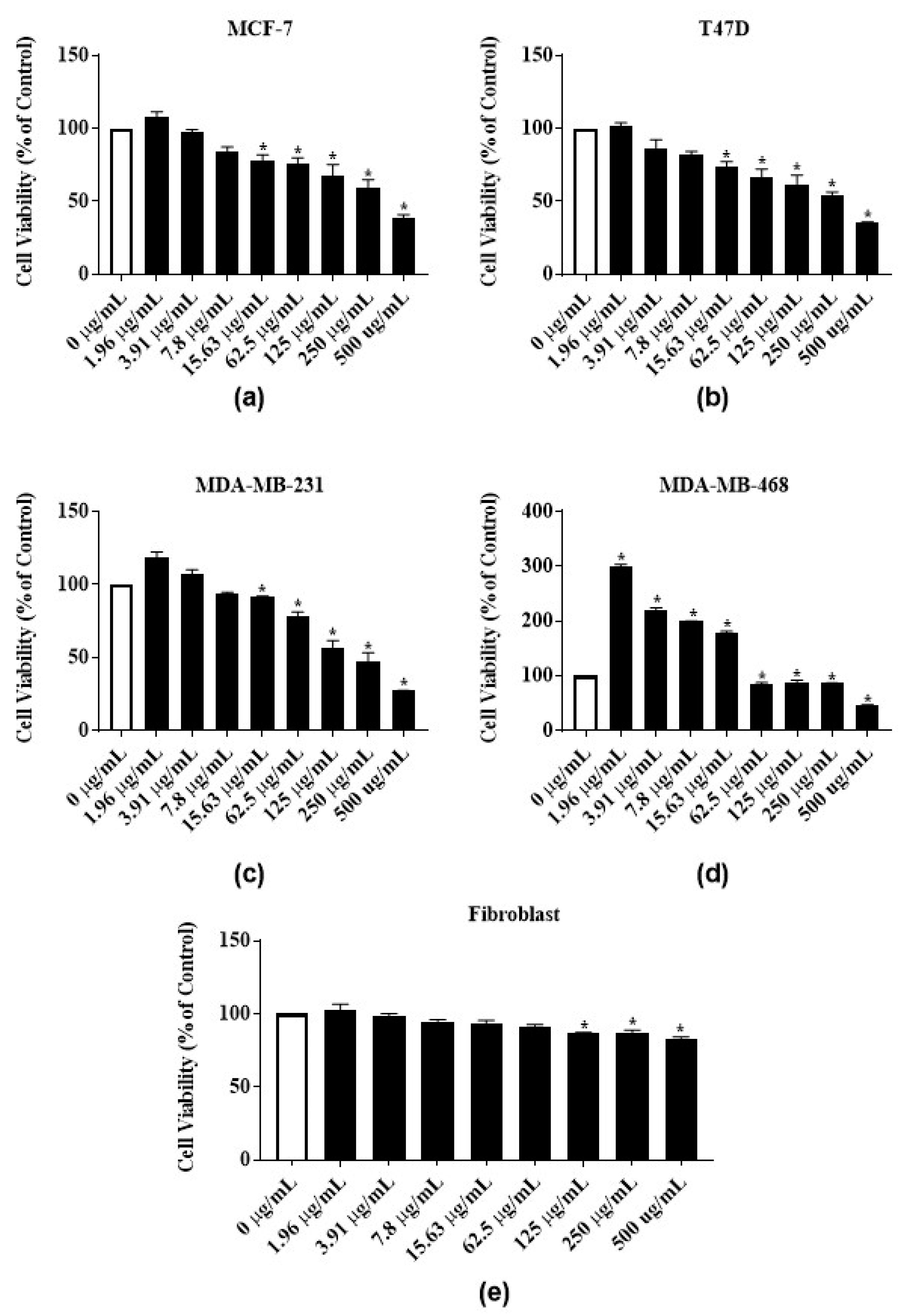
3.3. Antiproliferative Activities of Latex Extract and Its Phytochemicals on ER-Positive and Triple Negative Breast Cancer Cell Lines
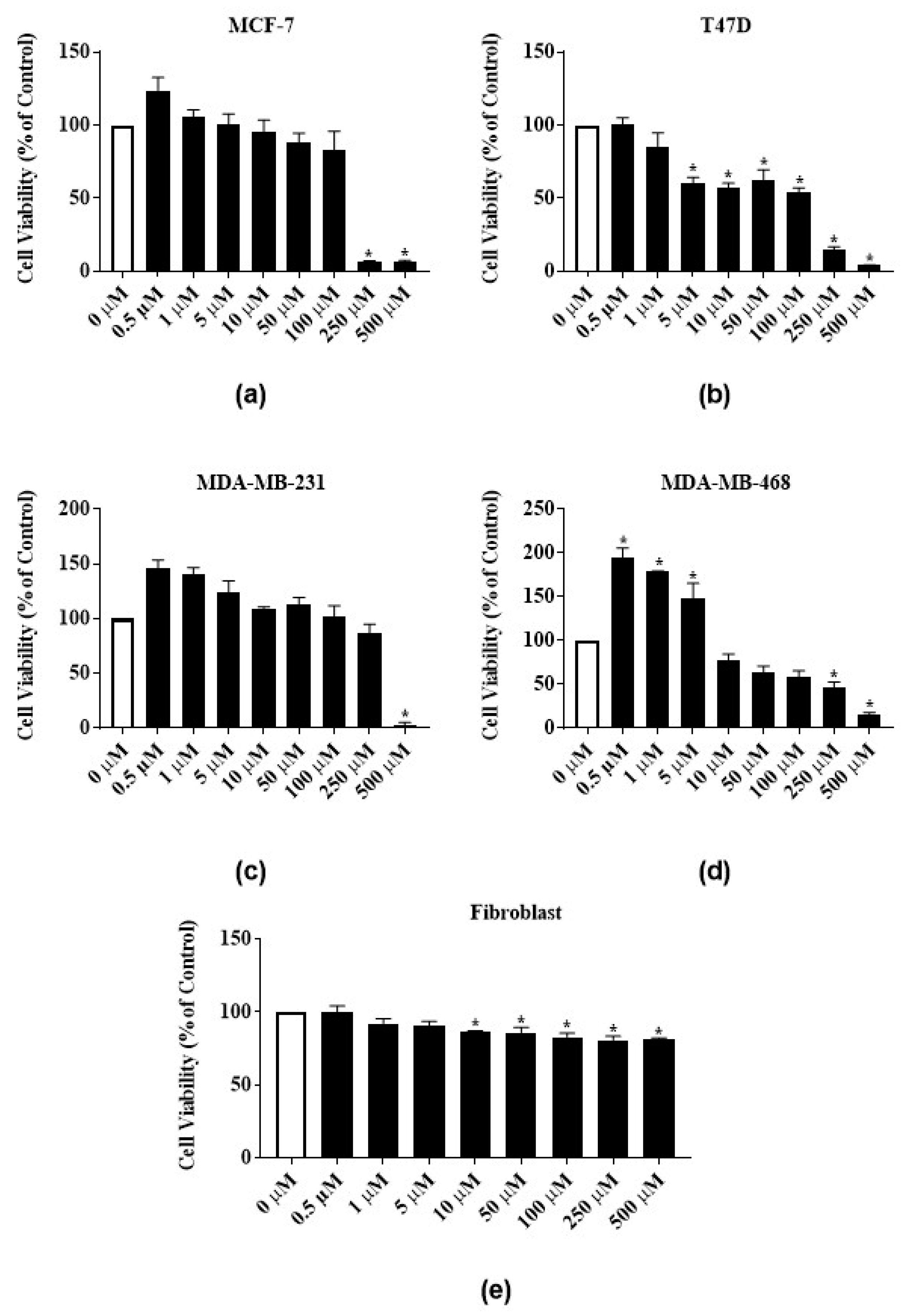
3.4. Antiproliferative Activity of Latex Extract in Combination with RTX or Rutin in MCF-7 Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Institute for Cancer Research. Worldwide Cancer Data—Global Cancer Statistics for the Most Common Cancers. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data (accessed on 10 October 2019).
- National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 10 October 2019).
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Peng, Y.; Kiselar, J.; Zhao, X.; Albaqami, A.; Mendez, D.; Chen, Y.; Chakravarthy, S.; Gupta, S.; Ralston, C.; et al. Multidomain architecture of estrogen receptor reveals interfacial cross-talk between its DNA-binding and ligand-binding domains. Nat. Commun. 2018, 9, 3520. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.; Winer, E.; Viale, G.; Cameron, D.; Gianni, L. Triple-negative breast cancer: Disease entity or title of convenience? Nat. Rev. Clin. Oncol. 2010, 7, 683. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Norris, J.D. Connections and regulation of the human estrogen receptor. Science 2002, 296, 1642–1644. [Google Scholar] [CrossRef]
- Siersbæk, R.; Kumar, S.; Carroll, J.S. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018, 32, 1141–1154. [Google Scholar] [CrossRef]
- Ali, S.; Coombes, R.C. Estrogen receptor alpha in human breast cancer: Occurrence and significance. J. Mammary Gland Biol. Neoplasia 2000, 5, 271–281. [Google Scholar] [CrossRef]
- Jensen, E.V.; Jordan, V.C. The estrogen receptor: A model for molecular medicine. Clin. Cancer Res. 2003, 9, 1980–1989. [Google Scholar]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef]
- Lumachi, F.; Brunello, A.; Maruzzo, M.; Basso, U.; Basso, S.M. Treatment of estrogen receptor-positive breast cancer. Curr. Med. Chem. 2013, 20, 596–604. [Google Scholar] [CrossRef]
- Monsuez, J.-J.; Charniot, J.-C.; Vignat, N.; Artigou, J.-Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Dropcho, E.J. The neurologic side effects of chemotherapeutic agents. CONTINUUM Lifelong Learn. Neurol. 2011, 17, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, M.; Yazdiniapour, Z.; Ghanadian, M.; Zolfaghari, B.; Lanzotti, V.; Mirsafaee, V. Obtusifoliol related steroids from Euphorbia sogdiana with cell growth inhibitory activity and apoptotic effects on breast cancer cells (MCF-7 and MDA-MB231). Steroids 2016, 115, 90–97. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Lorigooini, Z.; Shirzad, H. The effect of Euphorbia szovitsii Fisch. & C.A. Mey extract on the viability and the proliferation of MDA-MB-231 cell line. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Choene, M.; Motadi, L. Validation of the antiproliferative effects of Euphorbia tirucalli extracts in breast cancer cell lines. Mol. Biol. 2016, 50, 98–110. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Liu, X.; Wink, M.; Ma, Y.; Guo, Y. A myrsinol diterpene isolated from Euphorbia prolifera reverses multidrug resistance in breast cancer cells. Pharmazie 2016, 71, 537–539. [Google Scholar]
- Basu, P.; Tongkhuya, S.; Harris, T.L.; Riley, A.R.; Maier, C.; Granger, J.; Wojtaszek, J.; Averitt, D. Euphorbia bicolor (Euphorbiaceae) latex phytochemicals induce long-lasting non-opioid peripheral analgesia in a rat model of inflammatory pain. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Santiso-Mere, D.N.; Nawaz, Z.; McDonnell, D.P.; O’Malley, B.W. The development of S. cerevisiae as a host system for steroid hormone action studies. In Hormone Action and Molecular Endocrinology; Huges, M.R., Schrader, W.T., O’Malley, B.W., Eds.; Houston Biological Associates Inc.: Houston, TX, USA, 1991; pp. 23-1–23-24. [Google Scholar]
- Maier, C.; Chapman, K.; Smith, D.W. Differential estrogenic activities of male and female plant extracts from two dioecious species. Plant Sci. 1995, 109, 31–43. [Google Scholar] [CrossRef]
- Basu, P.; Dixon, D.; Varghese, S.; Maier, C. Detection of estrogenic, antiestrogenic, and drug synergistic activities of seven commercially available fruits by in vitro reporter assays. Pharmacogn. Res. 2018, 10, 137–142. [Google Scholar]
- Boonchird, C.; Mahapanichkul, T.; Cherdshewasart, W. Differential binding with ERα and ERβ of the phytoestrogen-rich plant Pueraria mirifica. Braz. J. Med. Biol. Res. 2010, 43, 195–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Halawany, A.M.; El Dine, R.S.; Chung, M.H.; Nishihara, T.; Hattori, M. Screening for estrogenic and antiestrogenic activities of plants growing in Egypt and Thailand. Pharmacogn. Res. 2011, 3, 107–113. [Google Scholar]
- Shutt, D.A.; Cox, R.I. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J. Endocrinol. 1972, 52, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Resende, F.A.; de Oliveira, A.P.S.; de Camargo, M.S.; Vilegas, W.; Varanda, E.A. Evaluation of estrogenic potential of flavonoids using a recombinant yeast strain and MCF7/BUS cell proliferation assay. PLoS ONE 2013, 8, e74881. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.M.; McLachlan, J.A.; Arnold, S.F. The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids 1997, 62, 365–372. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef]
- Prietsch, R.F.; Monte, L.G.; da Silva, F.A.; Beira, F.T.; Del Pino, F.A.B.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.; Ein Oon, C.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016, 54, 1223–1236. [Google Scholar]
- Taş, A.; Şahin-Bölükbaşı, S.; Çevik, E.; Özmen, E.; Gümüş, E.; Siliğ, Y. An in vitro study of cytotoxic activity of Euphorbia macroclada boiss on MCF–7 cells. Indian J. Pharm. Educ. Res. 2018, 52, S119–S123. [Google Scholar] [CrossRef]
- Sadeghi-Aliabadi, H.; Sajjadi, S.E.; Khodamoradi, M. Cytotoxicity of Euphorbia macroclada on MDA-MB-468 breast cancer cell line. Iran. J. Pharm. Sci. 2009, 5, 103–108. [Google Scholar]
- Shin, S.Y.; Kim, C.G.; Jung, Y.J.; Jung, Y.; Jung, H.; Im, J.; Lim, Y.; Lee, Y.H. Euphorbia humifusa Willd exerts inhibition of breast cancer cell invasion and metastasis through inhibition of TNFα-induced MMP-9 expression. BMC Complement. Altern. Med. 2016, 16, 413. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Li, W.; Kanno, Y.; Yamashita, N.; Kikkawa, S.; Azumaya, I.; Nemoto, K.; Asada, Y.; Koike, K. Euphorins A–H: Bioactive diterpenoids from Euphorbia fischeriana. J. Nat. Med. 2016, 70, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Raisinghani, M.; Pabbidi, R.M.; Premkumar, L.S. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J. Physiol. 2005, 567, 771–786. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Racca, S.; Abbadessa, G.; Piccione, F.; Re, G. Transient receptor potential vanilloid 1 expression and functionality in MCF-7 cells: A preliminary investigation. J. Breast Cancer 2014, 17, 332–338. [Google Scholar] [CrossRef]
- Vercelli, C.; Barbero, R.; Cuniberti, B.; Odore, R.; Re, G. Expression and functionality of TRPV1 receptor in human MCF-7 and canine CF.41 cells. Vet. Comp. Oncol. 2015, 13, 133–142. [Google Scholar] [CrossRef]
- Farfariello, V.; Liberati, S.; Morelli, M.B.; Tomassoni, D.; Santoni, M.; Nabissi, M.; Giannantoni, A.; Santoni, G.; Amantini, C. Resiniferatoxin induces death of bladder cancer cells associated with mitochondrial dysfunction and reduces tumor growth in a xenograft mouse model. Chem. Biol. Interact. 2014, 224, 128–135. [Google Scholar] [CrossRef]
- Pecze, L.; Jósvay, K.; Blum, W.; Petrovics, G.; Vizler, C.; Oláh, Z.; Schwaller, B. Activation of endogenous TRPV1 fails to induce overstimulation-based cytotoxicity in breast and prostate cancer cells but not in pain-sensing neurons. Biochim. Biophys. Acta 2016, 1863, 2054–2064. [Google Scholar] [CrossRef]
- Sahpazidou, D.; Geromichalos, G.D.; Stagos, D.; Apostolou, A.; Haroutounian, S.A.; Tsatsakis, A.M.; Tzanakakis, G.N.; Hayes, A.W.; Kouretas, D. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol. Lett. 2014, 230, 218–224. [Google Scholar] [CrossRef]
- Elsayed, H.E.; Ebrahim, H.Y.; Mohyeldin, M.M.; Siddique, A.B.; Kamal, A.M.; Haggag, E.G.; El Sayed, K.A. Rutin as a novel c-Met inhibitory lead for the control of triple negative breast malignancies. Nutr. Cancer 2017, 69, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Pashikanti, S.; de Alba, D.R.; Boissonneault, G.A.; Cervantes-Laurean, D. Rutin metabolites: Novel inhibitors of nonoxidative advanced glycation end products. Free Radic. Biol. Med. 2010, 48, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Valiveti, C.K.; Kumar, D.R.; Kesharwani, S.S.; Seefeldt, T.; Scaria, J.; Tumala, H.; Bhat, G.J. The flavonoid metabolite 2, 4, 6-trihydroxybenzoic acid is a CDK inhibitor and an anti-proliferative agent: A potential role in cancer prevention. Cancers 2019, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Bernacik, K.; Typek, R. Rutin transformation during its analysis involving extraction process for sample preparation. Food Anal. Methods 2016, 9, 213–224. [Google Scholar] [CrossRef]
- Mecenas, A.S.; Malafaia, C.R.A.; Sangenito, L.S.; Simas, D.L.R.; de Barros Machado, T.; Amaral, A.C.F.; dos Santos, A.J.S.; Freire, D.M.G.; Leal, I.C.R. Rutin derivatives obtained by transesterification reactions catalyzed by Novozym 435: Antioxidant properties and absence of toxicity in mammalian cells. PLoS ONE 2018, 13, e0203159. [Google Scholar] [CrossRef]
- Maggiolini, M.; Bonofiglio, D.; Marsico, S.; Panno, M.L.; Cenni, B.; Picard, D.; Andò, S. Estrogen receptor α mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol. Pharm. 2001, 60, 595–602. [Google Scholar]
- Seo, H.S.; DeNardo, D.G.; Jacquot, Y.; Laïos, I.; Vidal, D.S.; Zambrana, C.R.; Leclercq, G.; Brown, P.H. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res. Treat. 2006, 99, 121–134. [Google Scholar] [CrossRef]
- Ju, Y.H.; Fultz, J.; Allred, K.F.; Doerge, D.R.; Helferich, W.G. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 2006, 27, 856–863. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, X.; Chen, X.; Zhong, H.; Liang, C.; Xu, X.; Xu, W.; Cheng, W.; Wang, W.; Yu, L.; et al. Individual factors define the overall effects of dietary genistein exposure on breast cancer patients. Nutr. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Deveci, H.A.; Nazıroğlu, M.; Nur, G. 5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in MCF-7 human breast cancer cells by TRPV1 channel activation but not Hypericum perforatum treatment. Mol. Cell. Biochem. 2018, 439, 189–198. [Google Scholar] [CrossRef]
- Koşar, P.A.; Nazıroğlu, M.; Övey, İ.S.; Çiğ, B. Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: Involvement of TRPV1 channels. J. Membr. Biol. 2016, 249, 129–140. [Google Scholar] [CrossRef]
- Rodrigues, T.; Sieglitz, F.; Bernardes, G.J. Natural product modulators of transient receptor potential (TRP) channels as potential anti-cancer agents. Chem. Soc. Rev. 2016, 45, 6130–6137. [Google Scholar] [CrossRef]
- Peterson, J.; Doughty, H.; Eells, A.; Johnson, T.; Hastings, J.P.; Crowther, C.M.; Andrus, M.B.; Kenealey, J.D. The effects of 4′-esterified resveratrol derivatives on calcium dynamics in breast cancer cells. Molecules 2017, 22, 1968. [Google Scholar] [CrossRef]


| E. bicolor Latex Extract | |
| Breast Carcinoma | GI50 (µg/mL) |
| MCF-7 | 498.7 ± 1.3 |
| T47D | 315.7 ± 36.6 |
| MDA-MB-231 | 258.3 ± 18 |
| MDA-MB-468 | 499 ± 0.8 |
| RTX | |
| Breast Carcinoma | GI50 (µM) |
| MCF-7 | 139 ± 7.8 |
| T47D | 100 ± 23.6 |
| MDA-MB-231 | 246.7 ± 3.4 |
| MDA-MB-468 | 248.5 ± 1.5 |
| Rutin | |
| Breast Carcinoma | GI50 (µM) |
| MCF-7 | 77.5 ± 18.8 |
| T47D | 65.7 ± 14 |
| MDA-MB-231 | 160 ± 8.2 |
| MDA-MB-468 | 383.3 ± 54.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, P.; Meza, E.; Bergel, M.; Maier, C. Estrogenic, Antiestrogenic and Antiproliferative Activities of Euphorbia bicolor (Euphorbiaceae) Latex Extracts and Its Phytochemicals. Nutrients 2020, 12, 59. https://doi.org/10.3390/nu12010059
Basu P, Meza E, Bergel M, Maier C. Estrogenic, Antiestrogenic and Antiproliferative Activities of Euphorbia bicolor (Euphorbiaceae) Latex Extracts and Its Phytochemicals. Nutrients. 2020; 12(1):59. https://doi.org/10.3390/nu12010059
Chicago/Turabian StyleBasu, Paramita, Elizabeth Meza, Michael Bergel, and Camelia Maier. 2020. "Estrogenic, Antiestrogenic and Antiproliferative Activities of Euphorbia bicolor (Euphorbiaceae) Latex Extracts and Its Phytochemicals" Nutrients 12, no. 1: 59. https://doi.org/10.3390/nu12010059
APA StyleBasu, P., Meza, E., Bergel, M., & Maier, C. (2020). Estrogenic, Antiestrogenic and Antiproliferative Activities of Euphorbia bicolor (Euphorbiaceae) Latex Extracts and Its Phytochemicals. Nutrients, 12(1), 59. https://doi.org/10.3390/nu12010059






