Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Living Kidney Donors
2.2. Tissue Samples
2.3. Fatty Acid Composition
2.4. Biochemistry
2.5. Subjects Diet Stratification
2.6. Statistical Methods
3. Results
3.1. Study Subjects
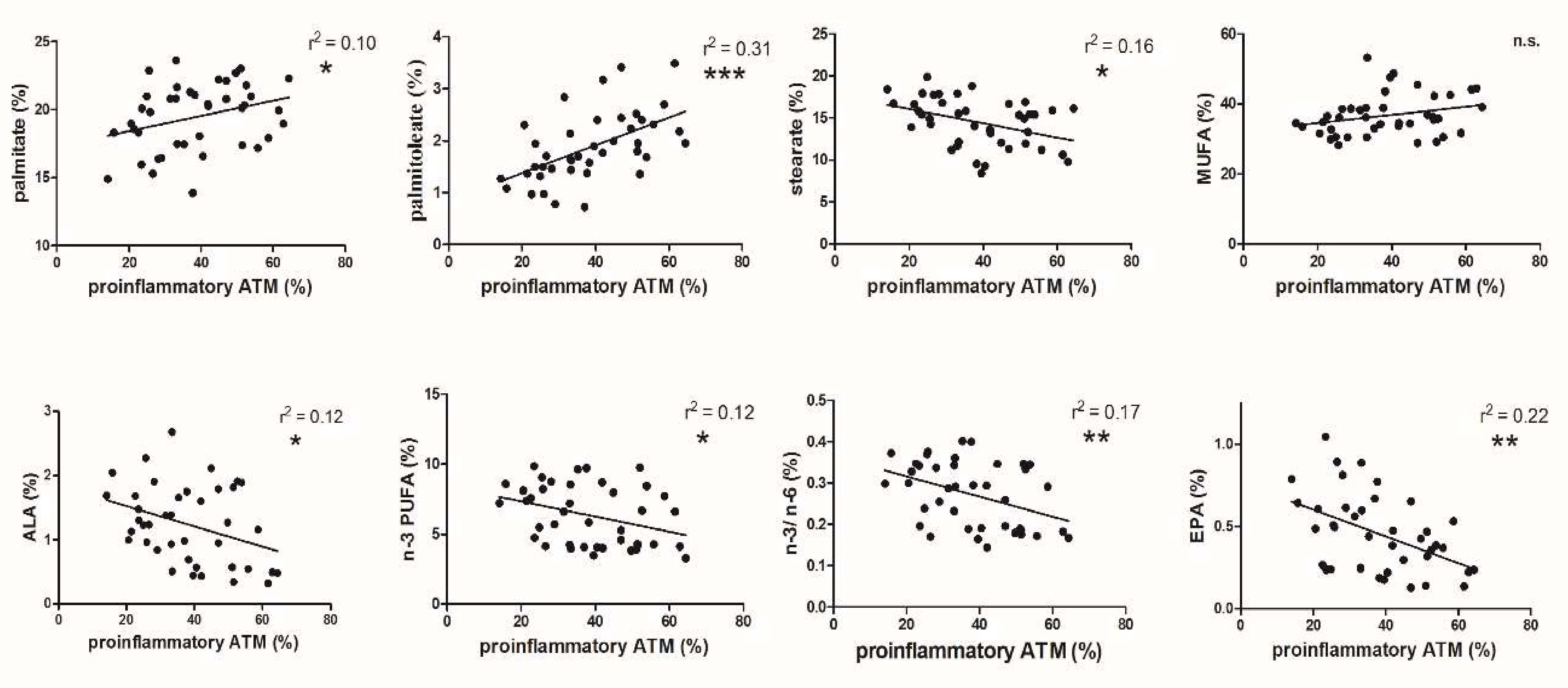
3.2. FA and ATM Relations
3.3. Dietary Score and Proinflammatory ATM Proportion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cifkova, R.; Skodova, Z.; Bruthans, J.; Adamkova, V.; Jozifova, M.; Galovcova, M.; Wohlfahrt, P.; Krajcoviechova, A.; Poledne, R.; Stavek, P.; et al. Longitudinal trends in major cardiovascular risk factors in the Czech population between 1985 and 2007/8. Czech MONICA and Czech post-MONICA. Atherosclerosis 2010, 211, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Rossmeisl, M.; Kopecky, J. Omega-3 fatty acids and adipose tissue biology. Mol. Asp. Med. 2018, 64, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.; Banete, A.; Basta, S. Spleen-derived macrophages are readily polarized into classically activated (M1) or alternatively activated (M2) states. Immunobiology 2014, 219, 737–745. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Ramkhelawon, B.; Hennessy, E.J.; Menager, M.; Ray, T.D.; Sheedy, F.J.; Hutchison, S.; Wanschel, A.; Oldebeken, S.; Geoffrion, M.; Spiro, W.; et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med. 2014, 20, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Cochain, C.; Zernecke, A. Macrophages in vascular inflammation and atherosclerosis. Pflügers Arch. 2017, 469, 485–499. [Google Scholar] [CrossRef]
- Lesna, I.K.; Cejkova, S.; Kralova, A.; Fronek, J.; Petras, M.; Sekerkova, A.; Thieme, F.; Janousek, L.; Poledne, R. Human adipose tissue accumulation is associated with pro-inflammatory changes in subcutaneous rather than visceral adipose tissue. Nutr. Diabetes 2017, 7, e264. [Google Scholar] [CrossRef] [Green Version]
- Kralova Lesna, I.; Poledne, R.; Fronek, J.; Kralova, A.; Sekerkova, A.; Thieme, F.; Pitha, J. Macrophage subsets in the adipose tissue could be modified by sex and the reproductive age of women. Atherosclerosis 2015, 241, 255–258. [Google Scholar] [CrossRef]
- Poledne, R.; Kralova Lesna, I.; Kralova, A.; Fronek, J.; Cejkova, S. The relationship between non-HDL cholesterol and macrophage phenotypes in human adipose tissue. J. Lipid Res. 2016, 57, 1899–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Y.; Lowy, C.; Islam, S.; Khan, F.S.; Swaminathan, R. Relationship between red cell membrane fatty acids and adipokines in individuals with varying insulin sensitivity. Eur. J. Clin. Nutr. 2011, 65, 690–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan, J.; Papandreou, C.; Sala-Vila, A.; Rosique-Esteban, N.; Fito, M.; Estruch, R.; Angel Martinez-Gonzalez, M.; Corella, D.; Ros, E.; Razquin, C.; et al. Fatty Acids Composition of Blood Cell Membranes and Peripheral Inflammation in the PREDIMED Study: A Cross-Sectional Analysis. Nutrients 2019, 11, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron-Wisnewsky, J.; Tordjman, J.; Poitou, C.; Darakhshan, F.; Hugol, D.; Basdevant, A.; Aissat, A.; Guerre-Millo, M.; Clement, K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J. Clin. Endocrinol. Metab. 2009, 94, 4619–4623. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.H.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [Green Version]
- Kovacikova, M.; Sengenes, C.; Kovacova, Z.; Siklova-Vitkova, M.; Klimcakova, E.; Polak, J.; Rossmeislova, L.; Bajzova, M.; Hejnova, J.; Hnevkovska, Z.; et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int. J. Obes. 2011, 35, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Kralova Lesna, I.; Kralova, A.; Cejkova, S.; Fronek, J.; Petras, M.; Sekerkova, A.; Thieme, F.; Janousek, L.; Poledne, R. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J. Tansl. Med. 2016, 14, 208. [Google Scholar] [CrossRef] [Green Version]
- Malinska, H.; Huttl, M.; Oliyarnyk, O.; Bratova, M.; Kazdova, L. Conjugated linoleic acid reduces visceral and ectopic lipid accumulation and insulin resistance in chronic severe hypertriacylglycerolemia. Nutrition 2015, 31, 1045–1051. [Google Scholar] [CrossRef]
- Eder, K. Gas chromatographic analysis of fatty acid methyl esters. J. Chromatogr. B Biomed. Appl. 1995, 671, 113–131. [Google Scholar] [CrossRef]
- Menegaut, L.; Thomas, C.; Lagrost, L.; Masson, D. Fatty acid metabolism in macrophages: A target in cardio-metabolic diseases. Curr. Opin. Lipidol. 2017, 28, 19–26. [Google Scholar]
- Thomas, D.; Apovian, C. Macrophage functions in lean and obese adipose tissue. Metabolism 2017, 72, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Poledne, R. Inflammation and atherogenic effects due to saturated fatty acids. In Handbook of Lipids in Human Function: Fatty Acids; Watson, R.R., De Meester, F., Eds.; AOCS Press, Elsevier: Amsterdam, The Netherlands, 2016; pp. 163–179. [Google Scholar]
- Ecker, J.; Liebisch, G.; Englmaier, M.; Grandl, M.; Robenek, H.; Schmitz, G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 7817–7822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, L.; Karpe, F. Is there something special about palmitoleate? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.S.; Lavrador, M.S.; Koike, M.K.; Cintra, D.E.; Ferreira, F.D.; Nunes, V.S.; Castilho, G.; Gioielli, L.A.; Paula Bombo, R.; Catanozi, S.; et al. Dietary interesterified fat enriched with palmitic acid induces atherosclerosis by impairing macrophage cholesterol efflux and eliciting inflammation. J. Nutr. Biochem. 2016, 32, 91–100. [Google Scholar] [CrossRef]
- Knutsen, S.F.; Fraser, G.E.; Beeson, W.L.; Lindsted, K.D.; Shavlik, D.J. Comparison of adipose tissue fatty acids with dietary fatty acids as measured by 24-hour recall and food frequency questionnaire in Black and White Adventists: The Adventist Health Study. Ann. Epidemiol. 2003, 13, 119–127. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef]
- Chan, K.L.; Pillon, N.J.; Sivaloganathan, D.M.; Costford, S.R.; Liu, Z.; Theret, M.; Chazaud, B.; Klip, A. Palmitoleate Reverses High Fat-induced Proinflammatory Macrophage Polarization via AMP-activated Protein Kinase (AMPK). J. Biol. Chem. 2015, 290, 16979–16988. [Google Scholar] [CrossRef] [Green Version]
- Foryst-Ludwig, A.; Kreissl, M.C.; Benz, V.; Brix, S.; Smeir, E.; Ban, Z.; Januszewicz, E.; Salatzki, J.; Grune, J.; Schwanstecher, A.K.; et al. Adipose Tissue Lipolysis Promotes Exercise-induced Cardiac Hypertrophy Involving the Lipokine C16:1n7-Palmitoleate. J. Biol. Chem. 2015, 290, 23603–23615. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.H.; Pryor, M.; Noguchi, A.; Sampson, M.; Johnson, B.; Pryor, M.; Donkor, K.; Amar, M.; Remaley, A.T. Dietary Palmitoleic Acid Attenuates Atherosclerosis Progression and Hyperlipidemia in Low-Density Lipoprotein Receptor-Deficient Mice. Mol. Nutr. Food Res. 2019, 63, e1900120. [Google Scholar] [CrossRef]
- Gong, J.; Campos, H.; McGarvey, S.; Wu, Z.; Goldberg, R.; Baylin, A. Adipose tissue palmitoleic acid and obesity in humans: Does it behave as a lipokine? Am. J. Clin. Nutr. 2011, 93, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Cimen, I.; Yildirim, Z.; Dogan, A.E.; Yildirim, A.D.; Tufanli, O.; Onat, U.I.; Nguyen, U.; Watkins, S.M.; Weber, C.; Erbay, E. Double bond configuration of palmitoleate is critical for atheroprotection. Mol. Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.A.; Monk, J.M.; Liddle, D.M.; Power, K.A.; Ma, D.W.; Robinson, L.E. Fish Oil-Derived Long-Chain n-3 Polyunsaturated Fatty Acids Reduce Expression of M1-Associated Macrophage Markers in an ex vivo Adipose Tissue Culture Model, in Part through Adiponectin. Front. Nutr. 2015, 2, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, J.T.; Toohey, T.; Clandinin, M.T. Does a threshold for the effect of dietary omega-3 fatty acids on the fatty acid composition of nuclear envelope phospholipids exist? Lipids 1992, 27, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colson, C.; Ghandour, R.A.; Dufies, O.; Rekima, S.; Loubat, A.; Munro, P.; Boyer, L.; Pisani, D.F. Diet Supplementation in omega3 Polyunsaturated Fatty Acid Favors an Anti-Inflammatory Basal Environment in Mouse Adipose Tissue. Nutrients 2019, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Hagi, A.; Nakayama, M.; Shinzaki, W.; Haji, S.; Ohyanagi, H. Effects of the omega-6: Omega-3 fatty acid ratio of fat emulsions on the fatty acid composition in cell membranes and the anti-inflammatory action. JPEN J. Parenter. Enter. Nutr. 2010, 34, 263–270. [Google Scholar] [CrossRef]
- Yaqoob, P.; Pala, H.S.; Cortina-Borja, M.; Newsholme, E.A.; Calder, P.C. Encapsulated fish oil enriched in alpha-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur. J. Clin. Investig. 2000, 30, 260–274. [Google Scholar] [CrossRef]
- Manzanares, W.; Langlois, P.L.; Dhaliwal, R.; Lemieux, M.; Heyland, D.K. Intravenous fish oil lipid emulsions in critically ill patients: An updated systematic review and meta-analysis. Crit. Care 2015, 19, 167. [Google Scholar] [CrossRef] [Green Version]
- Rogacev, K.S.; Zawada, A.M.; Emrich, I.; Seiler, S.; Bohm, M.; Fliser, D.; Woollard, K.J.; Heine, G.H. Lower Apo A-I and lower HDL-C levels are associated with higher intermediate CD14++CD16+ monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2120–2127. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hou, R.; Xi, Y.; Kowalski, A.; Wang, T.; Yu, Z.; Hu, Y.; Chandrasekar, E.K.; Sun, H.; Ali, M.K. The association and dose-response relationship between dietary intake of alpha-linolenic acid and risk of CHD: A systematic review and meta-analysis of cohort studies. Br. J. Nutr. 2018, 119, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camell, C.; Smith, C.W. Dietary oleic acid increases m2 macrophages in the mesenteric adipose tissue. PLoS ONE 2013, 8, e75147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robblee, M.M.; Kim, C.C.; Porter Abate, J.; Valdearcos, M.; Sandlund, K.L.; Shenoy, M.K.; Volmer, R.; Iwawaki, T.; Koliwad, S.K. Saturated Fatty Acids Engage an IRE1alpha-Dependent Pathway to Activate the NLRP3 Inflammasome in Myeloid Cells. Cell Rep. 2016, 14, 2611–2623. [Google Scholar] [CrossRef] [Green Version]


| LKDs | Controls | |
|---|---|---|
| Age (years) | 46.30 ± 9.87 | 45.77 ± 9.73 |
| BMI (kg/m2) | 25.95 ± 3.77 | 27.45 ± 6.03 |
| Cholesterol (mmol/L) | 4.40 ± 0.94 | 5.43 ± 1.09 *** |
| HDL-cholesterol (mmol/L) | 1.16 ± 0.38 | 1.47 ± 0.34 *** |
| non-HDL-cholesterol (mmol/L) | 3.24 ± 0.91 | 3.96 ± 1.08 |
| Triglycerides (mmol/L) | 1.51 ± 0.82 | 1.77 ± 1.21 |
| hsCRP (mg/L) | 1.24 ± 1.79 | 1.86 ± 2.36 |
| Hypertension (Yes/No) | 8/35 | 16/27 |
| Diabetes mellitus (Yes/No) | 0/43 | 3/40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poledne, R.; Malinska, H.; Kubatova, H.; Fronek, J.; Thieme, F.; Kauerova, S.; Kralova Lesna, I. Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients 2020, 12, 8. https://doi.org/10.3390/nu12010008
Poledne R, Malinska H, Kubatova H, Fronek J, Thieme F, Kauerova S, Kralova Lesna I. Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients. 2020; 12(1):8. https://doi.org/10.3390/nu12010008
Chicago/Turabian StylePoledne, Rudolf, Hana Malinska, Hana Kubatova, Jiri Fronek, Filip Thieme, Sona Kauerova, and Ivana Kralova Lesna. 2020. "Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids" Nutrients 12, no. 1: 8. https://doi.org/10.3390/nu12010008
APA StylePoledne, R., Malinska, H., Kubatova, H., Fronek, J., Thieme, F., Kauerova, S., & Kralova Lesna, I. (2020). Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients, 12(1), 8. https://doi.org/10.3390/nu12010008





