Sea Buckthorn and Rosehip Oils with Chokeberry Extract to Prevent Hypercholesterolemia in Mice Caused by a High-Fat Diet In Vivo
Abstract
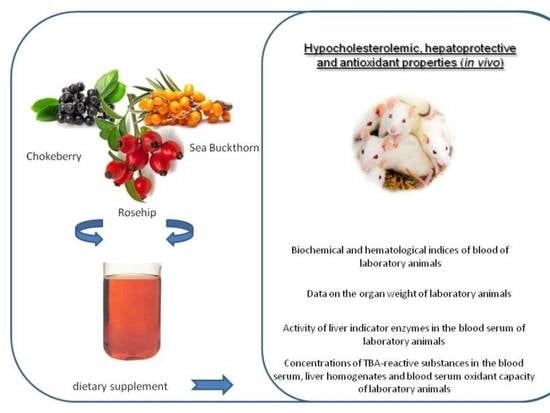
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feed Mixtures
2.3. Experimental Animals
2.4. Experimental Design
2.5. Evaluation of Biochemical Parameters of Blood Serum
2.6. Testing of Hepatoprotective Properties
2.7. Testing of Antioxidant Activity
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Somma, C.D.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bolori, P.; Setaysh, L.; Rasaei, N.; Jarrahi, F.; Yekaninejad, M.; Mirzaei, K. Adherence to a healthy plant diet may reduce inflammatory factors in obese and overweight women-a cross-sectional study. Diabetes Metab. Syndr. 2019, 13, 2795–2802. [Google Scholar] [CrossRef] [PubMed]
- Kovell, L.C.; Yeung, E.H.; Miller, E.R., III; Appel, L.J.; Christenson, R.H.; Rebuck, H.; Schulman, S.P.; Juraschek, S.P. Healthy diet reduces markers of cardiac injury and inflammation regardless of macronutrients: Results from the OmniHeart trial. Int. J. Cardiol. 2020, 299, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Baoru, Y.; Tahvonen, R.; Kallio, H.; Rimbach, G.H.; Minihane, A.M. Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J. Nutr. Biochem. 2002, 13, 346–354. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Mark, R.; Lyua, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Food 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Baboota, R.K.; Bishnoi, M.; Ambalam, P.; Kondepudi, K.K.; Sarma, S.M.; Boparai, R.K.; Podili, K. Functional food ingredients for the management of obesity and associated co-morbidities—A review. J. Funct. Foods 2013, 5, 997–1012. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Basli, A.; Belkacem, N.; Amrani, I. Health benefits of phenolic compounds against cancers. In Phenolic Compounds—Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, R., Eds.; InTech: Rijeka, Croatia, 2017; Chapter 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D. Dietary n-6 polyunsaturated fatty acids and cardiovascular disease: Epidemiologic evidence. Prostaglandins Leukot. Essent. Fatty Acids 2015, 135, 5–9. [Google Scholar] [CrossRef]
- Mollenhauer, M.; Mehrkens, D.; Rudolph, V. Nitrated fatty acids in cardiovascular diseases. Nitric Oxide 2018, 78, 146–153. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Albuquerque, T.G.; Finglas, P.; Ribeiro, T.; Valente, A.; Vasilopoulou, E.; Trichopoulou, A.; Alexieva, I.; Boyko, N.; Costea, C.-E.; et al. Carotenoids, vitamins (A, B2, C and E) and total folate of traditional foods from Black Sea Area countries. J. Sci. Food Agric. 2013, 93, 3545–3557. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Lim, V.; Silva, A.S.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Babich, O.; Dyshlyuk, L.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Chaplygina, T. The effect of postharvest ultraviolet irradiation on the content of antioxidant compounds and the activity of antioxidant enzymes in tomato. Heliyon 2020, 6, e03288. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.A. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Taka, A.; Togashi, H. Effects of a herbal medicine, Hippophae rhamnoides, on cardiovascular functions and coronary microvessels in the spontaneously hypertensive stroke-prone rat. Clin. Hemorheol. Microcirc. 2009, 41, 17–26. [Google Scholar] [CrossRef]
- Larmo, P.S.; Yang, B.; Hurme, S.A.; Alin, J.A.; Kallio, H.P.; Salminen, E.K.; Tahvonen, R.L. Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonoids in healthy adults. Eur. J. Nutr. 2009, 48, 277–282. [Google Scholar] [CrossRef]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef]
- Saggu, S.; Divekar, H.M.; Gupta, V.; Sawhney, R.C.; Banerjee, P.K.; Kumar, R. Adaptogenic and safety evaluation of sea buckthorn (Hippophae rhamnoides) leaf extract: A dose dependent study. Food Chem. Toxicol. 2007, 45, 609–617. [Google Scholar] [CrossRef]
- Upadhyay, N.K.; Kumar, R.; Mandotra, S.K.; Meena, R.N.; Siddiqui, M.S.; Sawhney, R.C.; Gupta, A. Safety and healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem. Toxicol. 2009, 47, 1146–1153. [Google Scholar] [CrossRef]
- Tulsawani, R. Ninety day repeated gavage administration of Hippophae rhamnoides extract in rats. Food Chem. Toxicol. 2010, 48, 2483–2489. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J. Funct. Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Ding, J.; Ruan, C.J.; Guan, Y.; Sham, J.Y.; Li, H.; Bao, Y.H. Characterization and identification of ISSR markers associated with oil content in sea buckthorn berries. Genet. Mol. Res. 2016, 15, gmr.15038278. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E. Hippophae rhamnoides L. (Sea buckthorn): A potential source of nutraceuticals. Food Public Health 2012, 2, 69–72. [Google Scholar] [CrossRef]
- Zeb, A. Anticarcinogenic potential of lipids from hippophae—Evidence from the recent literature. Asian Pac. J. Cancer Prev. 2006, 7, 32–35. [Google Scholar]
- Kumar, R.; Kumar, G.P.; Chaurasia, O.P.; Singh, S. Phytochemical and pharmacological profile of seabuckthorn oil: A review. Res. J. Med. Plant 2011, 5, 491–499. [Google Scholar] [CrossRef]
- Yang, B.; Kalimo, K.O.; Tahvonen, R.L.; Mattil, L.M.; Katajisto, J.K.; Kallio, H.P. Effect of dietary supplementation with sea buckthorn (Hippophaë rhamnoides) seed and pulp oils on the fatty acid composition of skin glycerophospholipids of patients with atopic dermatitis. J. Nutr. Biochem. 2000, 11, 338–340. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdylo, A.; Rudzinska, M.; Oszmiański, J.; Golis, T. Analysis of lipophilic and hydrophilic bioactive compounds content in sea buckthorn (Hippophae rhamnoids L.) berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Huo, Y.; Zhou, F.; Wu, W.; Lu, F.; Yang, X.; Guo, X.; Chen, P.; Deng, Q.; et al. Protective effect of proanthocyanidins from sea buckthorn (Hippophae rhamnoides L.) seed against visible light-induced retinal degeneration in vivo. Nutrients 2016, 8, 245. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Demir, N.; Yıldız, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rosehip (Rosa L.) fruits in Turkey. LWT-Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Salgın, U.; Salgın, S.; Ekici, D.D.; Uludal, G. Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. J. Supercrit. Fluids 2016, 118, 194–202. [Google Scholar] [CrossRef]
- Grajer, M.; Prescha, A.; Korzonek, K.; Wojakowska, A.; Dziadas, M.; Kulma, A.; Grajeta, H. Characteristics of rose hip (Rosa canina L.) cold pressed oil and its oxidative stability studied by the differential scanning calorimetry method. Food Chem. 2015, 188, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Nadpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Analkov, G.T.; Ŀetojevil-Simin, D.D.; Mimica-Dukil, N.M.; Beara, I.N. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Kharazmi, A. Laboratory and preclinical studies on the anti-inflammatory and anti-oxidant properties of rosehip powder-identification and characterization of the active component GOPO®. Osteoarthr. Cartil. 2008, 16, S5–S7. [Google Scholar] [CrossRef] [Green Version]
- Bhave, A.; Schulzova, V.; Chmelarova, H.; Mrnka, L.; Hajslova, J. Assessment of rosehips based on the content of their biologically active compounds. J. Food Drug Anal. 2017, 25, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Kohlmeier, L.; Kark, J.D.; Gomez-Gracia, E.; Martin, B.C.; Steck, S.E.; Kardinaal, A.F.; Ringstad, J.; Thamm, M.; Masaev, V.; Riemersma, R.; et al. Lycopene and myocardial infraction risk in the EURAMIC study. Am. J. Epidemiol. 1997, 146, 618–626. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef]
- Böhm, V.; Fröhlich, K.; Bitsch, R. Rosehip—A “new” source of lycopene? Mol. Aspects Med. 2003, 24, 385–389. [Google Scholar] [CrossRef]
- Alam, P.; Raka, M.A.; Khan, S.; Sarker, J.; Ahmed, N.; DevNath, P.; Hasan, N.; Mohib, M.d.M.; Tisha, A.; Sagor, M.d.A.T. A clinical review of the effectiveness of tomato (Solanum lycopersicum) against cardiovascular dysfunction and related metabolic syndrome. J. Herb. Med. 2019, 16, 100235. [Google Scholar] [CrossRef]
- Babich, O.; Dyshlyuk, L.; Sukhikh, S.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Chaplygina, T.; Kriger, O. Effects of Biopreservatives Combined with Modified Atmosphere Packaging on the Quality of Apples and Tomatoes. Pol. J. Food Nutr. Sci. 2019, 69, 110564. [Google Scholar] [CrossRef]
- Chrubasik, C.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Wenzig, E.M.; Widowitz, U.; Kunert, O.; Churbasik, S.; Bucar, F.; Knauder, E.; Bauer, R. Phytochemical composition and in vivo pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef]
- Ege, I.; Sánchez-Bel, P.; Romojaro, F.; Pretel, M.T. Six edible wild fruits as potential antioxidant additives or nutritional supplements. Plant. Foods Hum. Nutr. 2010, 65, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Exotic fruits as a source of important phytochemicals: Improving the traditional use of Rosa canina fruits in Portugal. Food Res. Int. 2011, 44, 2233–2236. [Google Scholar] [CrossRef]
- Tumbas, V.T.; Ŀanadovil-Brunet, J.M.; Ŀetojevil-Simin, D.D.; Ŀetkovil, G.S.; Ŀilas, S.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Food Compost. Anal. 2012, 92, 1273–1281. [Google Scholar] [CrossRef]
- Guimaraes, R.; Barros, L.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of different enriched phenolic extracts of wild fruits from northeastern Portugal: A comparative study. Plant Foods Hum. Nutr. 2014, 69, 37–42. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Tolić, M.T.; Krbavčić, I.P.; Vujević, P.; Milinović, B.; Jurčević, I.L.; Vahčić, N. Effects of weather conditions on phenolic content and antioxidant capacity in juice of chokeberries (Aronia melanocarpa L.). Polish J. Food Nutr. Sci. 2017, 67, 67–74. [Google Scholar] [CrossRef]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Babich, O.; Prosekov, A.; Zaushincena, A.; Sukhikh, A.; Dyshlyuk, L.; Ivanova, S. Identification and quantification of phenolic compounds of Western Siberia Astragalus danicus in different regions. Heliyon 2019, 5, e02245. [Google Scholar] [CrossRef] [Green Version]
- Madić, V.; Stojanović-Radić, Z.; Jušković, M.; Jugović, D.; Žabar Popović, A.; Vasiljević, P. Genotoxic and antigenotoxic potential of herbal mixture and five medicinal plants used in ethnopharmacology. S. Afr. J. Bot. 2019, 125, 290–297. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Yang, Y. In vivo study of medical and biological properties of functional bakery products with the addition of pumpkin flour. Bioact. Carbohydr. Diet. Fibre 2017, 12, 20–24. [Google Scholar] [CrossRef]
- Babich, O.; Dyshlyuk, L.; Noskova, S.; Sukhikh, S.; Prosekov, A.; Ivanova, S.; Pavsky, V. In vivo study of the potential of the carbohydrate-mineral complex from pine nut shells as an ingredient of functional food products. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100185. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Yagi, K. Lipid Peroxides and Human Diseases. Chem. Phys. Lipids 1987, 45, 337–351. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relation ships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Tereshchuk, L.V.; Starovoitova, K.V. Aspects of production of functional emulsion foods. Foods Raw Mater. 2013, 1, 67–75. [Google Scholar] [CrossRef]
- Ivanova, S.A.; Pavskii, V.A. Stochastic modeling of protein solution foaming process. Theor. Found. Chem. Eng. 2014, 48, 848–854. [Google Scholar] [CrossRef]
- Dyshlyuk, L.S.; Sukhikh, S.A.; Ivanova, S.A.; Smirnova, I.A.; Subbotina, M.A.; Pozdnyakova, A.V.; Neverov, E.N.; Garmashov, S.Y. Prospects for using pine nut products in the dairy industry. Food Raw Mater. 2018, 6, 264–280. [Google Scholar] [CrossRef]
- Prosekov, A.; Babich, O.; Kriger, O.; Ivanova, S.; Pavsky, V.; Sukhikh, S.; Yang, Y.; Kashirskih, E. Functional properties of the enzyme-modified protein from oat bran. Food Biosci. 2018, 24, 46–49. [Google Scholar] [CrossRef]
- Terechuk, L.; Starovoytova, K.; Ivanova, S.; Sergeeva, I. Obtaining Functional Products from Sea Buckthorn Berries. In Proceedings of the 2nd International Conference on Education Science and Social Development, 2019 (ESSD 2019), Changsha, China, 20–21 July 2019. [Google Scholar] [CrossRef] [Green Version]
- Babich, O.O.; Milent’eva, I.S.; Ivanova, S.A.; Pavsky, V.A.; Kashirskikh, E.V.; Yang, Y. The potential of pine nut as a component of sport nutrition. Foods Raw Mater. 2017, 5, 170–177. [Google Scholar] [CrossRef]
- Prosekov, A.Y.; Dyshlyuk, L.S.; Milent`eva, I.S.; Pavsky, V.A.; Ivanova, S.A.; Garmashov, S.Y. Study of the biofunctional properties of cedar pine oil with the use of testing cultures. Food Raw Mater. 2018, 6, 136–143. [Google Scholar] [CrossRef]
- OECD Guidelines for Testing of Chemicals; Organisation for Economic Co-operation and Development: Paris, France, 1981; Available online: http://www.oecd.org/department/0,3355,en_2649_34377_1_1_1_1_1,00.html (accessed on 24 December 2018).
- Istrati, D.; Lacatusu, I.; Bordei, N.; Badea, G.; Oprea, O.; Stefan, L.M.; Stan, R.; Badea, N.; Meghea, A. Phyto-mediated nanostructured carriers based on dual vegetable actives involved in the prevention of cellular damage. Mater. Sci. Eng. C 2016, 64, 249–259. [Google Scholar] [CrossRef]
- Lavinia, S.; Gabi, D.; Drinceanu, D.; Daniela, D.; Stef, D.; Daniela, M.; Julean, C.; Romana, T.; Corionovoschi, N. The effect of medicinal plants and plant extracted oils on broiler duodenum morphology and immunological profile. Rom. Biotechnol. Let. 2009, 14, 4606–4614. [Google Scholar]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Basu, M.; Prasad, R.; Jayamurthy, P.; Pal, K.; Arumughan, C.; Sawhney, R.C. Anti-atherogenic effects of sea buckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 2007, 14, 770–777. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Lu, F.J. Protective effects of seabuckthorn (Hippophae rhamnoides L.) seed oil against carbon tertrachloride-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 2281–2288. [Google Scholar] [CrossRef]
- Liu, Y.T.; Lu, B.N.; Xu, L.N.; Yin, L.H.; Wang, X.N.; Peng, J.Y.; Liu, K.X. The antioxidant activity and hypolipidemic activity of the total flavonoids from the fruit of Rosa laevigata Michx. Nat. Sci. 2010, 2, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Ji, L.; Zhang, S.; Xu, L.; Yin, L.H.; Li, L.; Zhao, Y.; Peng, J. Total flavonoids from Rosa Laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem. Toxicol. 2012, 50, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, B.; Han, X.; Xu, L.; Qi, Y.; Yin, L.; Xu, Y.; Zhao, Y.; Liu, K.; Penga, J. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013, 55, 60–69. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black chokeberry (Aronia melanocarpa) polyphenols reveal different antioxidant, antimicrobial and neutrophil-modulating activities. Food Chem. 2019, 284, 101–117. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Borisova, P.; Galunska, B.; Krasnaliev, I.; Belcheva, A. Hepatoprotective effect of the natural fruit juice from Aronia melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp. Toxicol. Pathol. 2004, 56, 195–201. [Google Scholar] [CrossRef]
- Mardones, M.; Valenzuela, R.; Romanque, P.; Covarrubias, N.; Anghileri, F.; Fernández, V.; Videla, L.A.; Tapia, G. Prevention of liver ischemia reperfusion injury by a combined thyroid hormone and fish oil protocol. J. Nutr. Biochem. 2012, 23, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Illesca, P.; Echeverría, F.; Espinosa, A.; Rincón-Cervera, M.Á.; Ortiz, M.; Hernandez-Rodas, M.C.; Valenzuela, A.; Videla, L.A. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef]
- Hernández-Rodas, M.C.; Valenzuela, R.; Echeverría, F.; Rincón-Cervera, M.Á.; Espinosa, A.; Illesca, P.; Muñoz, P.; Corbari, A.; Romero, N.; Gonzalez-Mañan, D.; et al. Supplementation with Docosahexaenoic Acid and Extra Virgin Olive Oil Prevents Liver Steatosis Induced by a High-Fat Diet in Mice through PPAR-α and Nrf2 Upregulation with Concomitant SREBP-1c and NF-kB Downregulation. Mol. Nutr. Food Res. 2017, 61, 1700479. [Google Scholar] [CrossRef]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-γ and NF-κB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef]
- Soto-Alarcón, S.A.; Ortiz, M.; Orellana, P.; Echeverría, F.; Bustamante, A.; Espinosa, A.; Illesca, P.; Gonzalez-Mañán, D.; Valenzuela, R.; Videla, L.A. Docosahexaenoic acid and hydroxytyrosol co-administration fully prevents liver steatosis and related parameters in mice subjected to high-fat diet: A molecular approach. BioFactors 2019, 45, 930–943. [Google Scholar] [CrossRef]



| Active Substance | Content |
|---|---|
| PUFA ω-3, not less | 380 mg |
| including α-linolenic acid, not less than | 175 mg |
| PUFA ω-6, not less than | 380 mg |
| including linoleic acid, not less than | 265 m |
| γ-linolenic acid, not less than | 110 mg |
| ω-9 (oleic acid), not less than | 180 mg |
| essential phospholipids | 150 mg |
| α-tocopherol | 400 IU |
| β-carotene | 2 mg |
| rutin | 50 mg |
| Ingredients | Feed Mixture | ||||||
|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | |
| Corn starch | 510.7 | 509.7 | 508.6 | 506.5 | 456.5 | 458.7 | 454.5 |
| Dextrinate corn starch | 155.0 | 155.0 | 155.0 | 155.0 | 155.0 | 155.0 | 155.0 |
| Casein | 145.0 | 145.0 | 145.0 | 145.0 | 145.0 | 145.0 | 145.0 |
| Saccharose | 90.0 | 90.0 | 90.0 | 90.0 | 90.0 | 90.0 | 90.0 |
| Soybean oil | 50.0 | 50.0 | 50.0 | 50.0 | 10.0 | 10.0 | 10.0 |
| Ghee | - | - | - | - | 90.0 | 90.0 | 90.0 |
| AIN-93M (mixture of mineral salts) | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| AIN-93-VX (vitamin mix) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| L-cysteine | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Cholesterol | - | - | - | - | - | 2.0 | 2.0 |
| “ESB-1” dietary supplement | - | 1.0 | 2.1 | 4.2 | 4.2 | - | 4.2 |
| 2-tert-butylhydroquinone in soy oil, mg | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Group | Number of Animals | Subcutaneous Injection | Diet |
|---|---|---|---|
| I | 15 | - | No. 1 |
| II | 15 | - | No. 2 |
| III | 15 | - | No. 3 |
| IV | 15 | - | No. 4 |
| V | 15 | - | No. 5 |
| VI | 15 | - | No. 6 |
| VII | 15 | - | No. 7 |
| VIII | 15 | Sterile refined and deodorized sunflower oil, 1 mL/kg of weight | No. 1 |
| IX | 15 | Oil solution of carbon tetrachloride, 1 mL/kg of weight | No. 1 |
| X | 15 | Oil solution of carbon tetrachloride, 1 mL/kg of weight | No. 4 |
| Duration of Incubation, after Subcutaneous Administration of Oil/Oil Solution of Carbon Tetrachloride, h | Groups | |||
|---|---|---|---|---|
| I | VIII | IX | X | |
| Lactate dehydrogenase (U/L) | ||||
| Control | 208.92 ± 1.92 a | - | - | - |
| 24 | - | 234.67 ± 1.94 a | 463.98 ± 3.02 a | 265.47 ± 1.92 a |
| 48 | - | 243.02 ± 1.93 a | 508.57 ± 2.97 a | 304.31 ± 1.92 a |
| 72 | - | 266.04 ± 2.00 a | 586.12 ± 3.17 a | 316.22 ± 1.92 a |
| Aspartate aminotransferase (U/L) | ||||
| Control | 9.24 ± 0.17 a | - | - | - |
| 24 | - | 11.03 ± 0.32 a | 32.76 ± 0.63 a | 15.06 ± 0.18 a |
| 48 | - | 41.97 ± 0.34 b | 54.22 ± 0.38 b | 20.04 ± 0.23 b |
| 72 | - | 21.34 ± 0.24 b | 72.18 ± 0.51 b | 23.11 ± 0.22 b |
| Alanine aminotransferase (U/L) | ||||
| Control | 8.36 ± 0.21 a | - | - | - |
| 24 | - | 10.51 ± 0.32 a | 23.18 ± 0.42 b | 12.23 ± 0.72 a |
| 48 | - | 47.46 ± 0.54 b | 42.44 ± 0.38 b | 22.46 ± 0.78 b |
| 72 | - | 25.84 ± 0.57 b | 47.36 ± 0.45 b | 25.47 ± 0.45 b |
| TBA-reactive products (umol/dm3 MDA) | ||||
| Control | 3.31 ± 0.16 a | - | - | - |
| 24 | - | 3.95 ± 0.21 b | 4.23 ± 0.21 b | 3.27 ± 0.17 a |
| 48 | - | 6.86 ± 0.33 b | 8.46 ± 0.43 b | 3.43 ± 0.17 a |
| 72 | - | 6.92 ± 0.34 b | 11.18 ± 0.57 b | 3.66 ± 0.17 a |
| Homogenates of liver (nmol MDA/g) | ||||
| Control | 41.7 ± 4.2 a | - | - | - |
| 24 | - | 50.0 ± 5.1 b | 53.4 ± 5.4 b | 40.8 ± 4.1 a |
| 48 | - | 58.7 ± 5.8 b | 65.0 ± 6.6 b | 44.3 ± 4.3 a |
| 72 | - | 63.7 ± 6.3 b | 78.7 ± 7.9 b | 47.1 ± 4.6 a |
| Antioxidant capacity (umol TE/umol) | ||||
| Control | 5.65 ± 0.56 a | - | - | - |
| 24 | - | 4.56 ± 0.55 a | 3.13 ± 0.32 b | 5.75 ± 0.57 a |
| 48 | - | 4.11 ± 0.51 a | 2.75 ± 0.27 b | 5.36 ± 0.54 a |
| 72 | - | 3.99 ± 0.51 a | 2.00 ± 0.22 b | 5.10 ± 0.50 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tereshchuk, L.; Starovoytova, K.; Babich, O.; Dyshlyuk, L.; Sergeeva, I.; Pavsky, V.; Ivanova, S.; Prosekov, A. Sea Buckthorn and Rosehip Oils with Chokeberry Extract to Prevent Hypercholesterolemia in Mice Caused by a High-Fat Diet In Vivo. Nutrients 2020, 12, 2941. https://doi.org/10.3390/nu12102941
Tereshchuk L, Starovoytova K, Babich O, Dyshlyuk L, Sergeeva I, Pavsky V, Ivanova S, Prosekov A. Sea Buckthorn and Rosehip Oils with Chokeberry Extract to Prevent Hypercholesterolemia in Mice Caused by a High-Fat Diet In Vivo. Nutrients. 2020; 12(10):2941. https://doi.org/10.3390/nu12102941
Chicago/Turabian StyleTereshchuk, Lubov, Kseniya Starovoytova, Olga Babich, Lyubov Dyshlyuk, Irina Sergeeva, Valery Pavsky, Svetlana Ivanova, and Alexander Prosekov. 2020. "Sea Buckthorn and Rosehip Oils with Chokeberry Extract to Prevent Hypercholesterolemia in Mice Caused by a High-Fat Diet In Vivo" Nutrients 12, no. 10: 2941. https://doi.org/10.3390/nu12102941
APA StyleTereshchuk, L., Starovoytova, K., Babich, O., Dyshlyuk, L., Sergeeva, I., Pavsky, V., Ivanova, S., & Prosekov, A. (2020). Sea Buckthorn and Rosehip Oils with Chokeberry Extract to Prevent Hypercholesterolemia in Mice Caused by a High-Fat Diet In Vivo. Nutrients, 12(10), 2941. https://doi.org/10.3390/nu12102941