Influence of Nutritional Intake of Carbohydrates on Mitochondrial Structure, Dynamics, and Functions during Adipogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
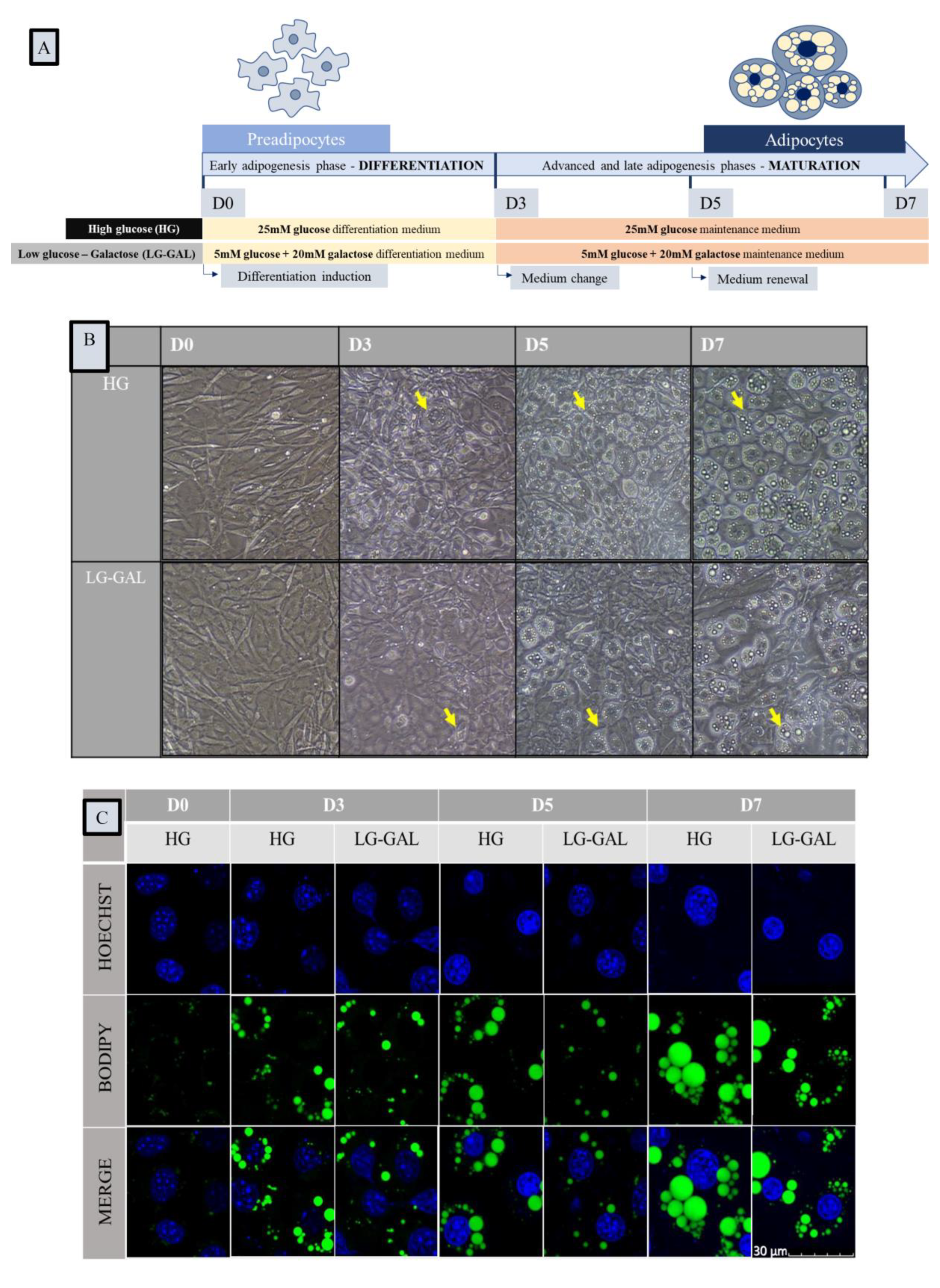
2.2. Cellular Morphology Assessment Using Bright Field Microscopy
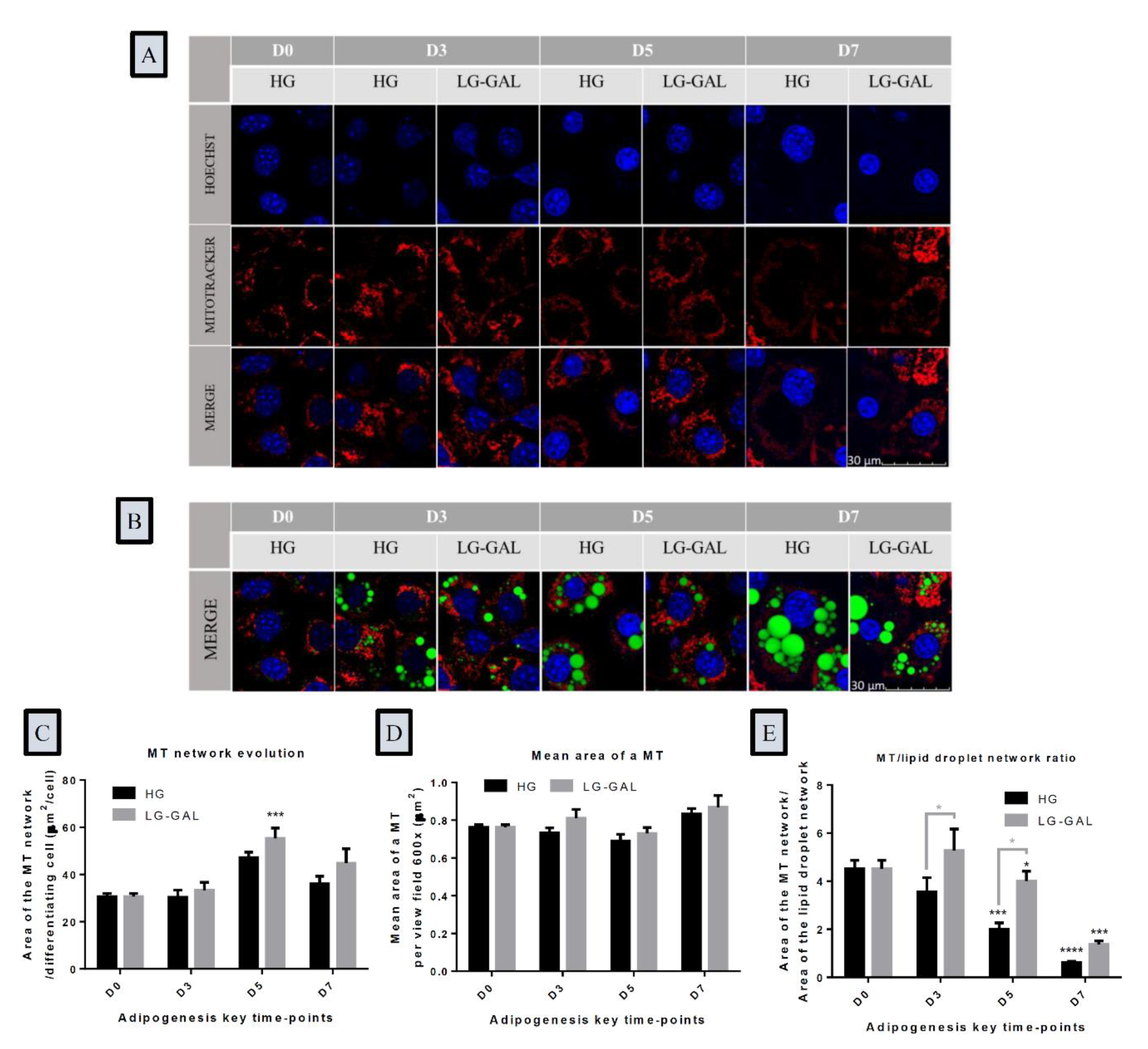
2.3. Lipid Droplets and Mitochondrial Networks Assessment Using Fluorescence Microscopy
2.4. Carbohydrates Levels Assessment
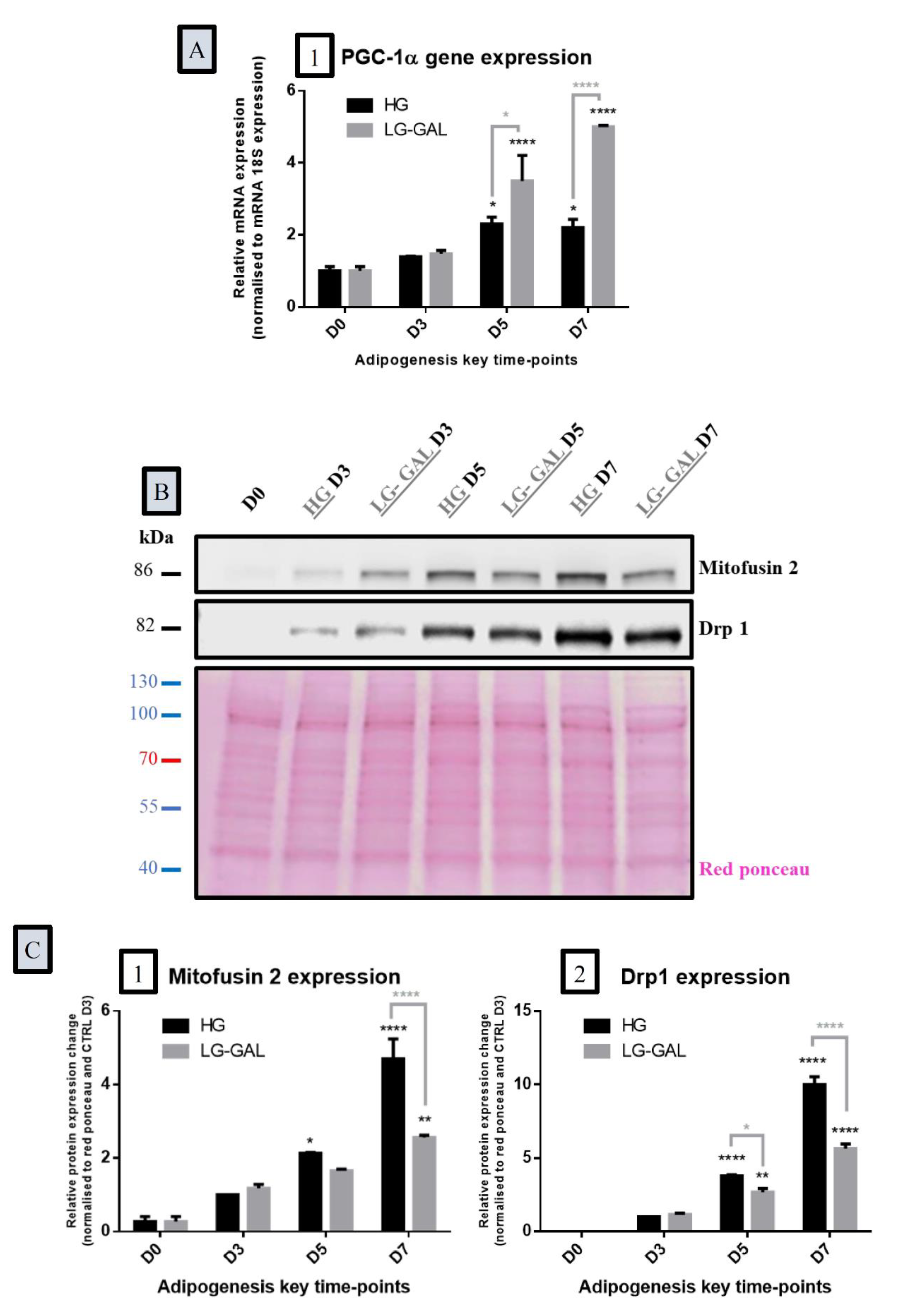
2.5. Protein Expression: Immunoblotting Analysis
2.6. Gene Expression: RT-qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Carbohydrates Intake Impact Adipocyte Differentiation and Lipid Droplet Network Evolution but Not Cellular Viability during Adipogenesis
3.2. Carbohydrates Intake Impacts the Early Adipogenesis Phase and Consequently Performances of Mature Adipocytes
3.3. Adipogenesis Progression Requires Important and Increasing Monocarbohydrates Cellular Intake
3.4. The Mitochondrial Network Evolution Depends on Carbohydrates Supplies and on the Lipid Droplet Network Evolution during Adipogenesis
3.5. Both Mitochondrial Biogenesis and Dynamics Phenomena Are Sensitive to Adipogenesis Triggering and are Impacted by Carbohydrates Supplies
3.6. Differentiating Adipocyte Mitochondria Adapt their Metabolic Function in Response to Both Carbohydrates Supplies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Church, C.; Horowitz, M.; Rodeheffer, M. WAT is a functional adipocyte? Adipocyte 2012, 1, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol. 2016, 7, 30. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4829583/ (accessed on 17 February 2020). [CrossRef] [PubMed]
- Pond, C.M. An evolutionary and functional view of mammalian adipose tissue. Proc. Nutr. Soc. 1992, 51, 367–377. [Google Scholar] [CrossRef]
- Ottaviani, E.; Malagoli, D.; Franceschi, C. The evolution of the adipose tissue: A neglected enigma. Gen. Comp. Endocrinol. 2011, 174, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Cereijo, R.; Villarroya, F. Adipokines and the Endocrine Role of Adipose Tissues. Handb. Exp. Pharmacol. 2016, 233, 265–282. [Google Scholar]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef]
- Carobbio, S.; Pellegrinelli, V.; Vidal-Puig, A. Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 161–196. [Google Scholar]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6539070/ (accessed on 18 February 2020). [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2653640/ (accessed on 18 February 2020). [CrossRef] [PubMed]
- Stenkula, K.G.; Erlanson-Albertsson, C. Adipose cell size: Importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef] [PubMed]
- White, U.; Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019, 62, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The developmental origins of adipose tissue. Dev. Camb. Engl. 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Jiang, Y.; Graff, J.M. Emerging Roles of Adipose Progenitor Cells in Tissue Development, Homeostasis, Expansion and Thermogenesis. Trends Endocrinol. Metab. TEM 2016, 27, 574–585. [Google Scholar] [CrossRef]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Morrison, S.; McGee, S.L. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte 2015, 4, 295–302. [Google Scholar] [CrossRef]
- Fève, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885. [Google Scholar] [CrossRef]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional Regulation of Adipocyte Differentiation: A Central Role for CCAAT/Enhancer-binding Protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.-X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein. Cell. 2017, 8, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and reactive oxygen species: Physiology and pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, A.; Tejerina, S.; Raes, M.; Keijer, J.; Arnould, T. Mitochondrial (Dys)function in Adipocyte (De)differentiation and Systemic Metabolic Alterations. Am. J. Pathol. 2009, 175, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gómez, G. Mitochondria and endocrine function of adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 791–804. [Google Scholar] [CrossRef]
- Mitchell, T.; Darley-Usmar, V. Metabolic syndrome and mitochondrial dysfunction: Insights from pre-clinical studies with a mitochondrially targeted antioxidant. Free Radic. Biol. Med. 2012, 52, 838–840. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Li, D.; Li, N.; Dindot, S.V.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Nutrition, Epigenetics, and Metabolic Syndrome. Antioxid. Redox Signal. 2011, 17, 282–301. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Z.; Dai, Z.; Sun, K.; Wang, J.; Wu, G. Nutritional epigenetics with a focus on amino acids: Implications for the development and treatment of metabolic syndrome. J. Nutr. Biochem. 2016, 27, 1–8. [Google Scholar] [CrossRef]
- Patel, S.; Choksi, A.; Pant, R.; Alam, A.; Chattopadhyay, S. Nutritional Programming of Metabolic Syndrome: Role of Nutrients in Shaping the Epigenetics. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V., Preedy, V., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–25. Available online: https://doi.org/10.1007/978-3-319-31143-2_42-2 (accessed on 17 February 2020).
- Langley-Evans, S.C. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009, 215, 36–51. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte Biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Harris, C.L.; Nelson, M.L.; Busboom, J.R.; Zhu, M.-J.; Du, M. Nutrigenomic regulation of adipose tissue development—Role of retinoic acid: A review. Meat Sci. 2016, 120, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tanis, R.M.; Piroli, G.G.; Day, S.D.; Frizzell, N. The effect of glucose concentration and sodium phenylbutyrate treatment on mitochondrial bioenergetics and ER stress in 3T3-L1 adipocytes. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef]
- Bravata, D.M.; Sanders, L.; Huang, J.; Krumholz, H.M.; Olkin, I.; Gardner, C.D.; Bravata, D.M. Efficacy and safety of low-carbohydrate diets: A systematic review. JAMA 2003, 289, 1837–1850. Available online: https://pubmed.ncbi.nlm.nih.gov/12684364/ (accessed on 21 September 2020). [CrossRef] [PubMed]
- Wylie-Rosett, J.; Aebersold, K.; Conlon, B.; Isasi, C.R.; Ostrovsky, N.W. Health Effects of Low-Carbohydrate Diets: Where Should New Research Go? Curr. Diab. Rep. 2013, 13, 271. [Google Scholar] [CrossRef]
- First, L. A New “Old” Method of Glycemic Control in Diabetes: The Very-Low-Carbohydrate Diet! AAP News. 2020. Available online: https://www.aappublications.org/news/2018/05/10/a-new-old-method-of-glycemic-control-in-diabetes-the-very-low-carbohydrate-diet-pediatrics-5-10-18 (accessed on 21 September 2020).
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847. [Google Scholar] [CrossRef]
- Sorisky, A. Effect of High Glucose Levels on White Adipose Cells and Adipokines—Fuel for the Fire. Int. J. Mol. Sci. 2017, 18, 944. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5454857/ (accessed on 17 February 2020). [CrossRef]
- Czech, M.P. Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol. Metab. 2020, 34, 27–42. [Google Scholar] [CrossRef]
- Jackson, R.M.; Griesel, B.A.; Gurley, J.M.; Szweda, L.I.; Olson, A.L. Glucose availability controls adipogenesis in mouse 3T3-L1 adipocytes via up-regulation of nicotinamide metabolism. J. Biol. Chem. 2017, 292, 18556–18564. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. BioTechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, L.M.S.; Fernández-Calleja, J.M.S.; van der Stelt, I.; Oosting, A.; Keijer, J.; van Schothorst, E.M. Replacing Part of Glucose with Galactose in the Postweaning Diet Protects Female but Not Male Mice from High-Fat Diet–Induced Adiposity in Later Life. J. Nutr. 2019, 149, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Hudak, C.S.; Sul, H.S. Pref-1, a Gatekeeper of Adipogenesis. Front. Endocrinol. 2013, 4, 79. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3699714/ (accessed on 15 February 2020). [CrossRef]
- Tansey, J.T.; Sztalryd, C.; Hlavin, E.M.; Kimmel, A.R.; Londos, C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 2004, 56, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wu, Y.; Zeng, R.; Liao, K. Proteomic profiling of lipid droplet-associated proteins in primary adipocytes of normal and obese mouse. Acta Biochim. Biophys. Sin. 2012, 44, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Konige, M.; Wang, H.; Sztalryd, C. Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Goodman, J.M. The collaborative work of droplet assembly. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1205–1211. [Google Scholar] [CrossRef]
- Rutter, J.; Winge, D.R.; Schiffman, J.D. Succinate Dehydrogenase—Assembly, Regulation and Role in Human Disease. Mitochondrion 2010, 10, 393–401. [Google Scholar] [CrossRef]
- Shankar, K.; Harrell, A.; Kang, P.; Singhal, R.; Ronis, M.J.J.; Badger, T.M. Carbohydrate-Responsive Gene Expression in the Adipose Tissue of Rats. Endocrinology 2010, 151, 153–164. [Google Scholar] [CrossRef]
- Fernandez, S.; Viola, J.M.; Torres, A.; Wallace, M.; Trefely, S.; Zhao, S.; Affronti, H.C.; Gengatharan, J.M.; Guertin, D.A.; Snyder, N.W.; et al. Adipocyte ACLY Facilitates Dietary Carbohydrate Handling to Maintain Metabolic Homeostasis in Females. Cell Rep. 2019, 27, 2772–2784.e6. [Google Scholar] [CrossRef]
- Krishna, M.S.; Revathy, V.M.; Jaleel, A. Adipocytes utilize sucrose as an energy source-Effect of different carbohydrates on adipocyte differentiation. J. Cell Physiol. 2020, 235, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Argmann, C.A.; Cock, T.-A.; Auwerx, J. Peroxisome proliferator-activated receptor gamma: The more the merrier? Eur. J. Clin. Investig. 2005, 35, 82–92; discussion 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, J.; Gao, X.; Ruan, H.-B. Inhibition of PPAR Gamma, Adipogenesis, and Insulin Sensitivity by MAGED1. Diabetes 2018, 67, 167–180. Available online: https://diabetes.diabetesjournals.org/content/67/Supplement_1/1750-P (accessed on 18 February 2020). [CrossRef]
- Karak, M.; Bal, N.C.; Bal, C.; Sharon, A. Targeting peroxisome proliferator-activated receptor gamma for generation of antidiabetic drug. Curr. Diabetes Rev. 2013, 9, 275–285. [Google Scholar] [CrossRef]
- Iroz, A.; Montagner, A.; Benhamed, F.; Levavasseur, F.; Polizzi, A.; Anthony, E.; Régnier, M.; Fouché, E.; Lukowicz, C.; Cauzac, M.; et al. A Specific ChREBP and PPARα Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep. 2017, 21, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Takasawa, K.; Kubota, N.; Terauchi, Y.; Kadowaki, T. Impact of increased PPARgamma activity in adipocytes in vivo on adiposity, insulin sensitivity and the effects of rosiglitazone treatment. Endocr. J. 2008, 55, 767–776. [Google Scholar] [CrossRef]
- Gumbilai, V.; Ebihara, K.; Aizawa-Abe, M.; Ebihara, C.; Zhao, M.; Yamamoto, Y.; Mashimo, T.; Hosoda, K.; Serikawa, T.; Nakao, K. Fat Mass Reduction with Adipocyte Hypertrophy and Insulin Resistance in Heterozygous PPARγ Mutant Rats. Diabetes 2016, 65, 2954–2965. [Google Scholar] [CrossRef]
- Villena, J.A.; Kim, K.H.; Sul, H.S. Pref-1 and ADSF/resistin: Two secreted factors inhibiting adipose tissue development. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2002, 34, 664–670. [Google Scholar] [CrossRef]
- Moon, Y.S.; Smas, C.M.; Lee, K.; Villena, J.A.; Kim, K.-H.; Yun, E.J.; Sul, H.S. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell Biol. 2002, 22, 5585–5592. [Google Scholar] [CrossRef]
- Mitterberger, M.C.; Lechner, S.; Mattesich, M.; Kaiser, A.; Probst, D.; Wenger, N.; Pierer, G.; Zwerschke, W. DLK1(PREF1) is a negative regulator of adipogenesis in CD105+/CD90+/CD34+/CD31−/FABP4− adipose-derived stromal cells from subcutaneous abdominal fat pats of adult women. Stem Cell Res. 2012, 9, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Lee, S.; Park, B.; Park, H. Distinct hypoxic regulation of preadipocyte factor-1 (Pref-1) in preadipocytes and mature adipocytes. Biochim. Biophys. Acta BBA Mol. Cell Res. 2018, 1865, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Khalilpourfarshbafi, M.; Gholami, K.; Murugan, D.D.; Abdul Sattar, M.Z.; Abdullah, N.A. Differential effects of dietary flavonoids on adipogenesis. Eur. J. Nutr. 2019, 58, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.A.; Choi, C.S.; Wang, Y.; Kim, S.; Hwang, Y.-J.; Kim, Y.-B.; Cline, G.; Shulman, G.I.; Sul, H.S. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): A new model of partial lipodystrophy. Diabetes 2008, 57, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.R.; Beller, M. The why, when and how of lipid droplet diversity. J. Cell Sci. 2017, 130, 315–324. [Google Scholar] [CrossRef]
- Girousse, A.; Langin, D. Adipocyte lipases and lipid droplet-associated proteins: Insight from transgenic mouse models. Int. J. Obes. 2012, 36, 581–594. [Google Scholar] [CrossRef]
- McManaman, J.L.; Bales, E.S.; Orlicky, D.J.; Jackman, M.; MacLean, P.S.; Cain, S.; Crunk, A.E.; Mansur, A.; Graham, C.E.; Bowman, T.A.; et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J. Lipid Res. 2013, 54, 1346–1359. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F. Fatty Acid Synthase (FASN) Inhibitors as Potential Treatment for Cancer, Obesity, and Liver Related Disorders. ACS Med. Chem. Lett. 2015, 6, 838–839. [Google Scholar] [CrossRef]
- López-Lluch, G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech. Ageing Dev. 2017, 162, 108–121. [Google Scholar] [CrossRef]
- Højlund, K.; Mogensen, M.; Sahlin, K.; Beck-Nielsen, H. Mitochondrial Dysfunction in Type 2 Diabetes and Obesity. Endocrinol. Metab. Clin. N. Am. 2008, 37, 713–731. [Google Scholar] [CrossRef]
- Barbour, J.A.; Turner, N. Mitochondrial Stress Signaling Promotes Cellular Adaptations. Int. J. Cell Biol. 2014, 2014, e156020. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. Overview on mitochondrial metabolite transport systems. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1979; pp. 245–252. Available online: http://www.sciencedirect.com/science/article/pii/0076687979560273 (accessed on 17 February 2020).
- Kunji, E. Structural and Mechanistic Aspects of Mitochondrial Transport Proteins. In Comprehensive Biophysics; Elsevier: Amsterdam, The Netherlands, 2012; pp. 174–205. [Google Scholar]
- Das, K.; Lewis, R.Y.; Combatsiaris, T.P.; Lin, Y.; Shapiro, L.; Charron, M.J.; Scherer, P.E. Predominant expression of the mitochondrial dicarboxylate carrier in white adipose tissue. Biochem. J. 1999, 344, 313–320. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcourt, M.; Tagliatti, V.; Delsinne, V.; Colet, J.-M.; Declèves, A.-E. Influence of Nutritional Intake of Carbohydrates on Mitochondrial Structure, Dynamics, and Functions during Adipogenesis. Nutrients 2020, 12, 2984. https://doi.org/10.3390/nu12102984
Delcourt M, Tagliatti V, Delsinne V, Colet J-M, Declèves A-E. Influence of Nutritional Intake of Carbohydrates on Mitochondrial Structure, Dynamics, and Functions during Adipogenesis. Nutrients. 2020; 12(10):2984. https://doi.org/10.3390/nu12102984
Chicago/Turabian StyleDelcourt, Manon, Vanessa Tagliatti, Virginie Delsinne, Jean-Marie Colet, and Anne-Emilie Declèves. 2020. "Influence of Nutritional Intake of Carbohydrates on Mitochondrial Structure, Dynamics, and Functions during Adipogenesis" Nutrients 12, no. 10: 2984. https://doi.org/10.3390/nu12102984
APA StyleDelcourt, M., Tagliatti, V., Delsinne, V., Colet, J.-M., & Declèves, A.-E. (2020). Influence of Nutritional Intake of Carbohydrates on Mitochondrial Structure, Dynamics, and Functions during Adipogenesis. Nutrients, 12(10), 2984. https://doi.org/10.3390/nu12102984





