Effective Immune Functions of Micronutrients against SARS-CoV-2
Abstract
:1. Introduction
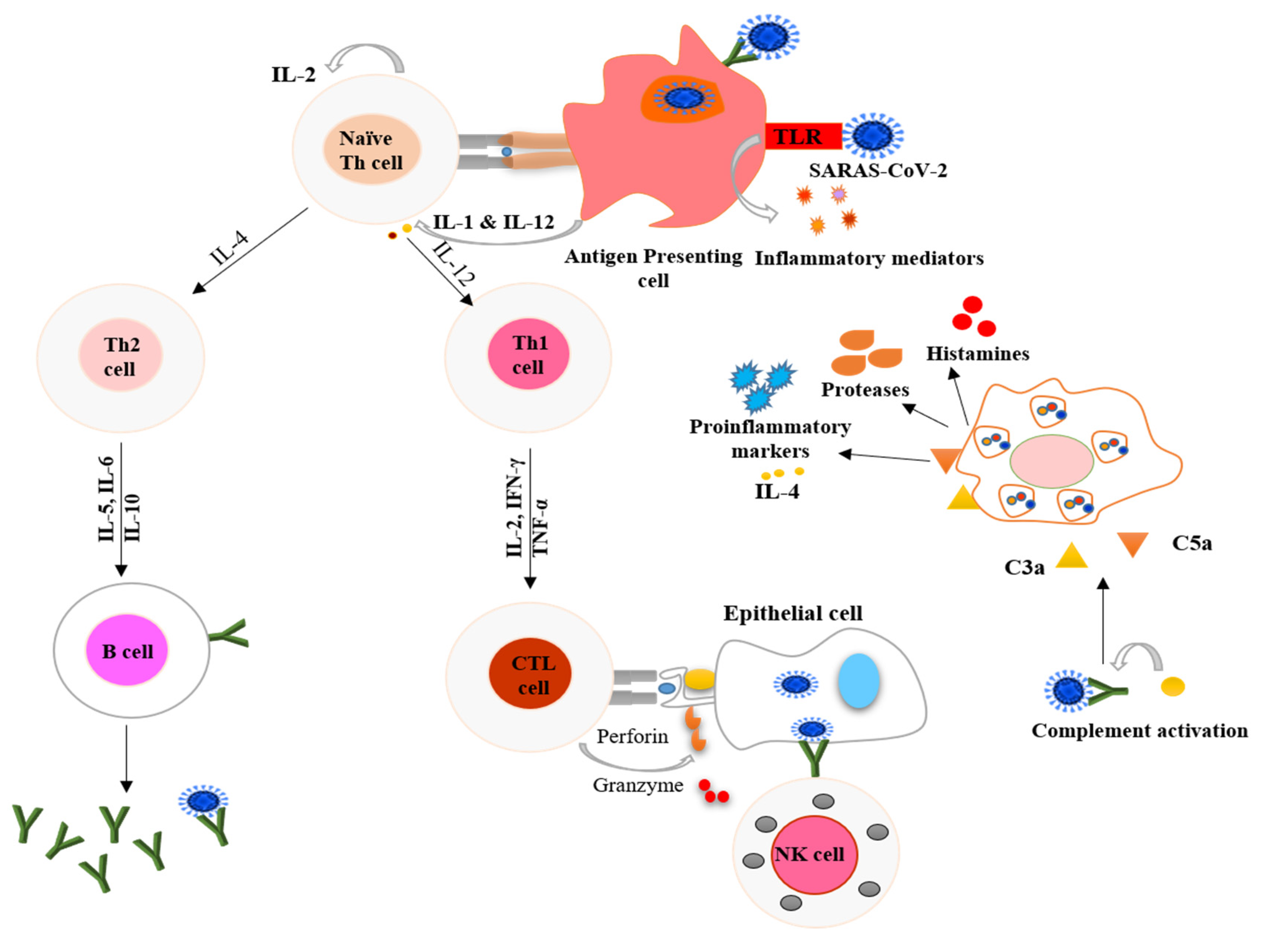
2. COVID-19 and the Immune Response
3. Nutritional Interventions for Treatment of COVID-19
4. Minerals and Immune System
4.1. Zinc
4.2. Iron
4.3. Selenium
5. Vitamins and Immune System
5.1. Vitamin A
5.2. Vitamin B
5.3. Vitamin C
5.4. Vitamin D
5.5. Vitamin E
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banerjee, A.; Kulcsar, K.; Misra, V.; Frieman, M.; Mossman, K. Bats and Coronaviruses. Viruses 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Carr, A.C.; Eggersdorfer, M.; Gombart, A.F. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]
- Karim, T.; Muhit, M.; Khandaker, G. Interventions to prevent respiratory diseases—Nutrition and the developing world. Paediatr. Respir. Rev. 2017, 22, 31–37. [Google Scholar] [CrossRef]
- WHO. COVID-19 Studies from the World Health Organization Database; WHO: Geneva, Switzerland, 2020.
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Guo, Y.; Zhou, Q.; Li, Y.; Xia, L. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Hao, G. The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hakizimana, O.; Zhang, X.; Kaushik, A.C.; Zhang, J. Orchestrated efforts on host network hijacking: Processes governing virus replication. Virulence 2020, 11, 183–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Oldridge, D.A.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. BioRxiv 2020. [Google Scholar] [CrossRef]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.; Mathew, D.; Baxter, A.E.; Vella, L.; et al. Immunologic perturbations in severe COVID-19/SARS-CoV-2 infection. BioRxiv 2020. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Pae, M.; Wu, D. Nutritional modulation of age-related changes in the immune system and risk of infection. Nutr. Res. 2017, 41, 14–35. [Google Scholar] [CrossRef] [Green Version]
- Zabetakis, I.; Lordan, R.; Tsoupras, A.; Norton, C. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Mao, R.; Liang, J.; Shen, J.; Ghosh, S.; Zhu, L.R.; Yang, H.; Wu, K.C.; Chen, M.H. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol. Hepatol. 2020, 5, 425–427. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, J.; Dai, Z.; Deng, H.; Li, X.; Huang, Q.; Wu, Y.; Sun, L.; Xu, Y. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Virol. 2020, 127, 104371. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, L.; Kuo, H.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Casas, R.; Estruch, R.; Sacanella, E. The Protective Effects of Extra Virgin Olive Oil on Immune-mediated Inflammatory Responses. Endocr. Met. B Immune. Disord. Drug Targets 2018, 18, 23–35. [Google Scholar] [CrossRef]
- Yimit, D.; Hoxur, P.; Amat, N.; Uchikawa, K.; Yamaguchi, N. Effects of soybean peptide on immune function, brain function, and neurochemistry in healthy volunteers. Nutrition 2012, 28, 154–159. [Google Scholar] [CrossRef]
- Locke, A.; Schneiderhan, J.; Zick, S.M. Diets for Health: Goals and Guidelines. Am. Fam. Physician 2018, 97, 721–728. [Google Scholar]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Awotiwon, A.A.; Oduwole, O.; Sinha, A.; Okwundu, C.I. Zinc supplementation for the treatment of measles in children. Cochrane Database Syst. Rev. 2017, 6, Cd011177. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.P.; Chiu, H.; Yang, C.F.; Lee, Y.L.; Lin, Y.L. Inhibition of Japanese encephalitis virus infection by the host zinc-finger antiviral protein. PLoS Pathog. 2018, 14, e1007166. [Google Scholar] [CrossRef] [PubMed]
- Raza, N.; Khan, D.A. Zinc deficiency in patients with persistent viral warts. J. Coll. Physicians Surg. Pak. 2010, 20, 83–86. [Google Scholar] [PubMed]
- te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID-19. Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Speth, R.; Carrera, E.; Jean-Baptiste, M.; Joachim, A.; Linares, A. Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity. FASEB J. 2014, 28, 1067.42014. [Google Scholar]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Biaggio, V.S.; Salvetti, N.R.; Pérez Chaca, M.V.; Valdez, S.R.; Ortega, H.H.; Gimenez, M.S.; Gomez, N.N. Alterations of the extracellular matrix of lung during zinc deficiency. Br. J. Nutr. 2012, 108, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Kahmann, L.; Uciechowski, P.; Warmuth, S.; Plümäkers, B.; Gressner, A.M.; Malavolta, M.; Mocchegiani, E.; Rink, L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008, 11, 227–237. [Google Scholar] [CrossRef]
- Bin, B.H.; Hojyo, S.; Seo, J.; Hara, T.; Takagishi, T.; Mishima, K.; Fukada, T. The Role of the Slc39a Family of Zinc Transporters in Zinc Homeostasis in Skin. Nutrients 2018, 10, 219. [Google Scholar] [CrossRef] [Green Version]
- Kehl-Fie, T.E.; Skaar, E.P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G. Iron and immunity: A double-edged sword. Eur. J. Clin. Investig. 2002, 32 (Suppl. 1), 70–78. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Crossing the Iron Gate: Why and How Transferrin Receptors Mediate Viral Entry. Annu. Rev. Nutr. 2018, 38, 431–458. [Google Scholar] [CrossRef]
- Jayaweera, J.; Reyes, M.; Joseph, A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci. Rep. 2019, 9, 12637. [Google Scholar] [CrossRef] [Green Version]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [Green Version]
- Bellmann-Weiler, R.; Lanser, L.; Fritsche, G.; Wöll, E.; Rangger, L.; Schapfl, A.; Schaber, M.; Weiss, G.; Barket, R. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef]
- Paikaray, S. Origin, mobilization and distribution of selenium in a soil/water/air system: A global perspective with special reference to the Indian scenario. CLEAN–Soil Air Water 2016, 44, 474–487. [Google Scholar] [CrossRef]
- Smrkolj, P.; Pograjc, L.; Hlastan-Ribič, C.; Stibilj, V. Selenium content in selected Slovenian foodstuffs and estimated daily intakes of selenium. Food Chem. 2005, 90, 691–697. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Beck, M.A.; Matthews, C.C. Micronutrients and host resistance to viral infection. Proc. Nutr. Soc. 2000, 59, 581–585. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [Green Version]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef]
- Beck, M.A.; Nelson, H.K.; Shi, Q.; Van Dael, P.; Schiffrin, E.J.; Blum, S.; Barclay, D.; Levander, O.A. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001, 15, 1481–1483. [Google Scholar] [CrossRef]
- Beck, M.A.; Shi, Q.; Morris, V.C.; Levander, O.A. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat. Med. 1995, 1, 433–436. [Google Scholar] [CrossRef]
- Fradejas-Villar, N. Consequences of mutations and inborn errors of selenoprotein biosynthesis and functions. Free Radic. Biol. Med. 2018, 127, 206–214. [Google Scholar] [CrossRef]
- Ma, X.; Bi, S.; Wang, Y.; Chi, X.; Hu, S. Combined adjuvant effect of ginseng stem-leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult. Sci. 2019, 98, 3548–3556. [Google Scholar] [CrossRef]
- Seko, T.; Imamura, S.; Ishihara, K.; Yamashita, Y.; Yamashita, M. Inhibition of angiotensin-converting enzyme by selenoneine. Fish. Sci. 2019, 85, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Kańtoch, M.; Litwińska, B.; Szkoda, M.; Siennicka, J. Importance of vitamin A deficiency in pathology and immunology of viral infections. Rocz. Panstw. Zakl. Hig. 2002, 53, 385–392. [Google Scholar]
- Villamor, E.; Mbise, R.; Spiegelman, D.; Hertzmark, E.; Fataki, M.; Peterson, K.E.; Ndossi, G.; Fawzi, W.W. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics 2002, 109, E6. [Google Scholar] [CrossRef] [Green Version]
- Jee, J.; Hoet, A.E.; Azevedo, M.P.; Vlasova, A.N.; Loerch, S.C.; Pickworth, C.L.; Hanson, J.; Saif, L.J. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am. J. Vet. Res. 2013, 74, 1353–1362. [Google Scholar] [CrossRef]
- Stachowska, E.; Folwarski, M.; Jamioł-Milc, D.; Maciejewska, D.; Skonieczna-Żydecka, K. Nutritional Support in Coronavirus 2019 Disease. Medicine 2020, 56, 289. [Google Scholar]
- West, C.E.; Sijtsma, S.R.; Kouwenhoven, B.; Rombout, J.H.; van der Zijpp, A.J. Epithelia-damaging virus infections affect vitamin A status in chickens. J. Nutr. 1992, 122, 333–339. [Google Scholar] [CrossRef]
- Trottier, C.; Colombo, M.; Mann, K.K.; Miller, W.H., Jr.; Ward, B.J. Retinoids inhibit measles virus through a type I IFN-dependent bystander effect. FASEB J. 2009, 23, 3203–3212. [Google Scholar] [CrossRef]
- Katze, M.G.; Fornek, J.L.; Palermo, R.E.; Walters, K.A.; Korth, M.J. Innate immune modulation by RNA viruses: Emerging insights from functional genomics. Nat. Rev. Immunol. 2008, 8, 644–654. [Google Scholar] [CrossRef]
- Beigmohammadi, M.T.; Bitarafan, S.; Hoseindokht, A.; Abdollahi, A.; Amoozadeh, L.; Mahmoodi Ali Abadi, M.; Foroumandi, M. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: A structured summary of a study protocol for a randomized controlled trial. Trials 2020, 21, 614. [Google Scholar] [CrossRef]
- Ross, A.C. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin. Immunol. Immunopathol. 1996, 80, S63–S72. [Google Scholar] [CrossRef]
- Morris, M.S.; Sakakeeny, L.; Jacques, P.F.; Picciano, M.F.; Selhub, J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J. Nutr. 2010, 140, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Spinas, E.; Saggini, A.; Kritas, S.K.; Cerulli, G.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Frydas, A.; Tei, M.; Speziali, A.; et al. Crosstalk between Vitamin B and Immunity. J. Biol. Regul. Homeost Agents 2015, 29, 283–288. [Google Scholar]
- Bashandy, S.A.E.; Ebaid, H.; Abdelmottaleb Moussa, S.A.; Alhazza, I.M.; Hassan, I.; Alaamer, A.; Al Tamimi, J. Potential effects of the combination of nicotinamide, vitamin B2 and vitamin C on oxidative-mediated hepatotoxicity induced by thioacetamide. Lipids Health Dis. 2018, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Kyme, P.; Thoennissen, N.H.; Tseng, C.W.; Thoennissen, G.B.; Wolf, A.J.; Shimada, K.; Krug, U.O.; Lee, K.; Müller-Tidow, C.; Berdel, W.E.; et al. C/EBPε mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice. J. Clin. Investig. 2012, 122, 3316–3329. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Narayanan, N.; Nair, D.T. Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 Virus. Preprints 2020. Available online: https://indiarxiv.org/p48fa/ (accessed on 5 September 2020). [CrossRef] [Green Version]
- Tanner, J.A.; Zheng, B.J.; Zhou, J.; Watt, R.M.; Jiang, J.Q.; Wong, K.L.; Lin, Y.P.; Lu, L.Y.; He, M.L.; Kung, H.F.; et al. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem. Biol. 2005, 12, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M. A cohort study to evaluate the effect of combination Vitamin D, Magnesium and Vitamin B12 on progression to severe outcome in older COVID-19 patients. Nutrition 2020, 111017. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and SARS coronavirus. J. Antimicrob. Chemother. 2003, 52, 1049–1050. [Google Scholar] [CrossRef] [Green Version]
- Atherton, J.G.; Kratzing, C.C.; Fisher, A. The effect of ascorbic acid on infection chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978, 56, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Field, C.J.; Johnson, I.R.; Schley, P.D. Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 2002, 71, 16–32. [Google Scholar]
- Hemilä, H. Vitamin C intake and susceptibility to the common cold. Br. J. Nutr. 1997, 77, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Matthay, M.A.; Aldrich, J.M.; Gotts, J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020, 8, 433–434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, B.; Hashimoto, K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain. Behav. Immun. 2020, 87, 59–73. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, Y.; Zhang, J.; Li, Y.; Peng, Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: Study protocol for a multicentre randomised controlled trial. BMJ Open 2020, 10, e039519. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef] [Green Version]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678s–1688s. [Google Scholar] [CrossRef] [Green Version]
- Nonnecke, B.J.; McGill, J.L.; Ridpath, J.F.; Sacco, R.E.; Lippolis, J.D.; Reinhardt, T.A. Acute phase response elicited by experimental bovine diarrhea virus (BVDV) infection is associated with decreased vitamin D and E status of vitamin-replete preruminant calves. J. Dairy Sci. 2014, 97, 5566–5579. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Ebadi, M.; Montano-Loza, A.J. Perspective: Improving vitamin D status in the management of COVID-19. Eur. J. Clin. 2020, 74, 856–859. [Google Scholar] [CrossRef]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25 [OH] D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020. [Google Scholar] [CrossRef]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. 2020, 14, 561–565. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid. Based Complement. Altern. Med. 2018, 2018, 5813095. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Komolmit, P.; Charoensuk, K.; Thanapirom, K.; Suksawatamnuay, S.; Thaimai, P.; Chirathaworn, C.; Poovorawan, Y. Correction of vitamin D deficiency facilitated suppression of IP-10 and DPP IV levels in patients with chronic hepatitis C: A randomised double-blinded, placebo-control trial. PLoS ONE 2017, 12, e0174608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Galmés, S.; Serra, F.; Palou, A. Vitamin E Metabolic Effects and Genetic Variants: A Challenge for Precision Nutrition in Obesity and Associated Disturbances. Nutrients 2018, 10, 1919. [Google Scholar] [CrossRef] [Green Version]
- Beck, M.A.; Kolbeck, P.C.; Rohr, L.H.; Shi, Q.; Morris, V.C.; Levander, O.A. Vitamin E deficiency intensifies the myocardial injury of coxsackievirus B3 infection of mice. J. Nutr. 1994, 124, 345–358. [Google Scholar] [CrossRef]

| Trial ID | Study Design | Sample Size | Settings | Intervention in COVID-19 Patients |
|---|---|---|---|---|
| ChiCTR2000032400 | Cohort | 60 | China | High dose of vitamin C. |
| RCT20200401046909N1 | Randomized clinical trial | 260 | Iran | 1000 IUs of vitamin D daily for 8 weeks. |
| IRCT20180520039738N2 | Randomized clinical trial | 140 | Iran | Vitamin A (25,000 IU/day) for 10 days. |
| DRKS00021214 | Randomized clinical trial | 1300 | Germany | Vitamin B3 (nicotinamide) 1000 mg/day for 4 weeks. |
| EUCTR2020-001602-34-FR | Randomized clinical trial | 260 | France | A high dose of vitamin D (400,000 IU) versus a standard dose (50,000 IU) once daily for 14 days. |
| TCTR20200404004 | Randomized clinical trial | 400 | Thailand | Comparison of chloroquine, (10 mg base/kg) and vitamin C (1000 mg). |
| IRCT20170117032004N3 | Randomized clinical trial | 30 | Iran | Vitamin A (50,000 IU) along with routine treatment for 2 weeks. |
| CTRI/2020/06/026189 | Randomized clinical trial | 210 | India | Vitamin D (60,000 IU) single-dose and magnesium glycinate (250 mg bi-dose) for 14 days. |
| IRCT20200319046819N1 | Randomized clinical trial | 60 | Iran | Vitamin A (25,000 IU), Vitamin D (600,000 IU) once during the intervention, Vitamin E (300 IU) twice daily, Vitamin C (500 mg) five times a day, and Vitamin B (Soluvit ampoule) daily. |
| NCT04335084 | Randomized clinical trial | 600 | USA | Hydroxychloroquine, Vitamin C, D, and Zinc. |
| NCT04264533 | Randomized clinical trial | 140 | China | Vitamin C (12 g) twice a day for 7 days. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junaid, K.; Ejaz, H.; Abdalla, A.E.; Abosalif, K.O.A.; Ullah, M.I.; Yasmeen, H.; Younas, S.; Hamam, S.S.M.; Rehman, A. Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients 2020, 12, 2992. https://doi.org/10.3390/nu12102992
Junaid K, Ejaz H, Abdalla AE, Abosalif KOA, Ullah MI, Yasmeen H, Younas S, Hamam SSM, Rehman A. Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients. 2020; 12(10):2992. https://doi.org/10.3390/nu12102992
Chicago/Turabian StyleJunaid, Kashaf, Hasan Ejaz, Abualgasim Elgaili Abdalla, Khalid O. A. Abosalif, Muhammad Ikram Ullah, Humaira Yasmeen, Sonia Younas, Sanaa S. M. Hamam, and Abdul Rehman. 2020. "Effective Immune Functions of Micronutrients against SARS-CoV-2" Nutrients 12, no. 10: 2992. https://doi.org/10.3390/nu12102992
APA StyleJunaid, K., Ejaz, H., Abdalla, A. E., Abosalif, K. O. A., Ullah, M. I., Yasmeen, H., Younas, S., Hamam, S. S. M., & Rehman, A. (2020). Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients, 12(10), 2992. https://doi.org/10.3390/nu12102992







