The Relevance of Selenium Status in Rheumatoid Arthritis
Abstract
:1. Introduction
2. Selenium and Selenoproteins
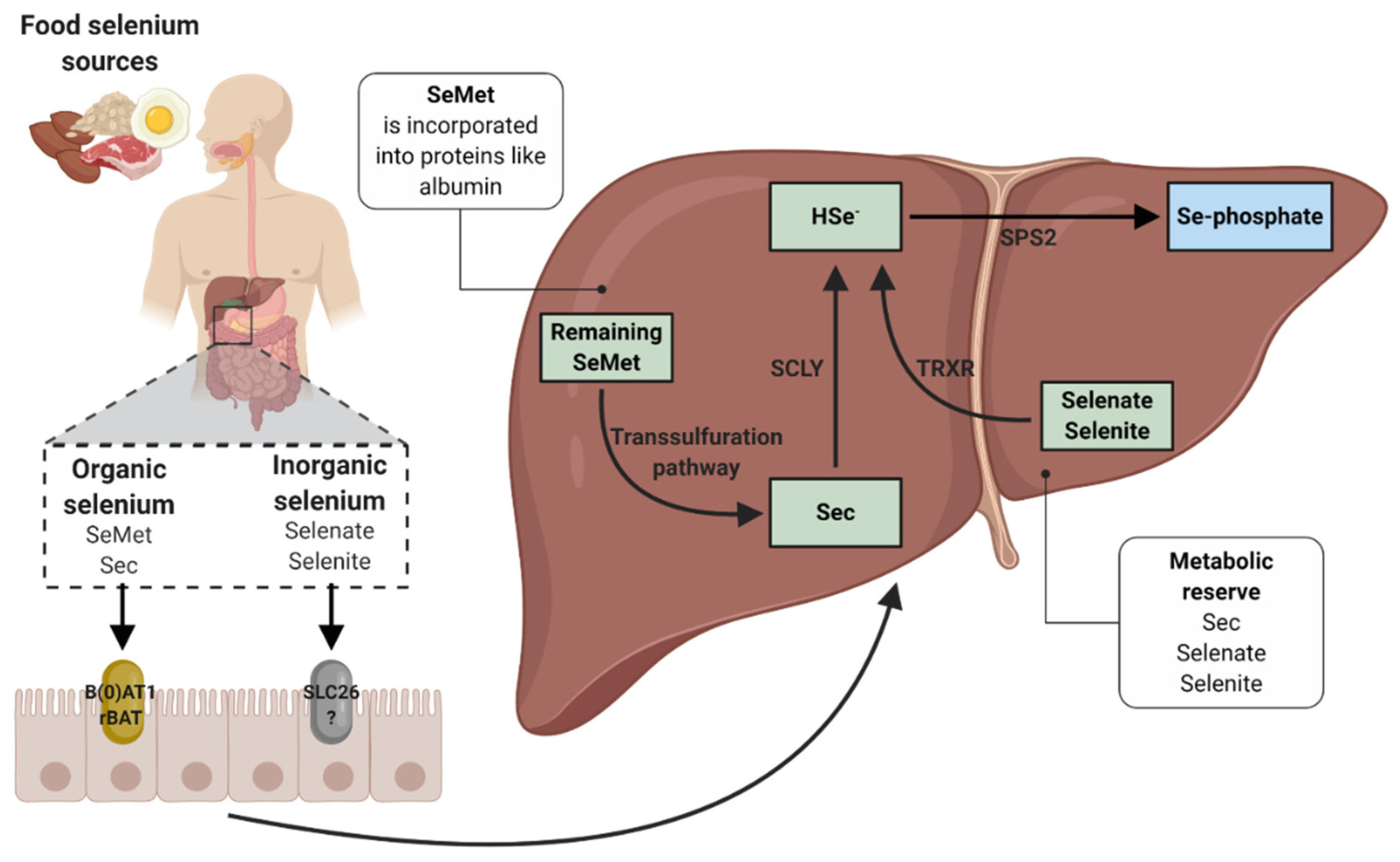
3. Biochemistry, Absorption and Metabolism of Selenium
3.1. Interactions with Selenium Absorption
3.2. Incorporation of Dietary Selenium into Selenoproteins
4. Nutritional Selenium Status in Rheumatoid Arthritis
4.1. Clinical Trials with Selenium in the Treatment of Rheumatoid Arthritis
4.2. Preclinical Studies with Selenium Nanoparticles in the Treatment of Rheumatoid Arthritis-Induced Models
4.3. Polymorphisms Related to the Availability of Selenium and Selenoproteins in Humans
5. Serum Selenium Status in Rheumatoid Arthritis
5.1. Effect of Rheumatoid Arthritis Medication on Selenium Status
5.2. Current Hypotheses that Explain the Decrease in Serum Selenium in Rheumatoid Arthritis
5.3. Serum Selenium as a Biomarker for Rheumatoid Arthritis
6. Antioxidant and Anti-Inflammatory Effect of Selenium
7. Selenium Intake Recommendation and its Rich Sources
Brief Overview of Current Nutritional Approaches in Rheumatoid Arthritis
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OH | hydroxyl |
| ACE2 | angiotensin converting enzyme 2 |
| ACPAs | autoantibodies against citrullinated peptides |
| B(0)AT1 | sodium-dependent neutral amino acid transporter |
| CRP | C-reactive protein |
| DMARDs | disease-modifying anti-rheumatic drugs |
| DRIs | Dietary Reference Intakes |
| EFSec | specific elongation factor |
| ESR | erythrocyte sedimentation rate |
| GPx | glutathione peroxidase |
| H2O2 | hydrogen peroxide |
| HEI | Healthy Eating Index |
| HO2 | perhydroxyl |
| HSe− | selenide |
| HUVECs | human umbilical vein endothelial cells |
| iNOS | nitric oxide synthase |
| MsrB1 | methionine-R-sulfoxide reductase B1 |
| NIK | NF-κB inducing kinase |
| NO | nitric oxide |
| NO2 | nitrogen dioxide |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| O2− | superoxide |
| OONO− | peroxynitrite |
| PSTK | phosphoseryl-tRNA[Ser]Sec kinase |
| PUFAs | polyunsaturated fatty acids |
| RA | rheumatoid arthritis |
| rBAT | neutral and basic amino acid transport protein |
| RDA | Recommended Dietary Allowance |
| RF | rheumatoid factor |
| RNS | reactive nitrogen species |
| ROO | peroxyl |
| ROS | reactive oxygen species |
| SARS | seryl-tRNA synthetase |
| SBP2 | selenocysteine insertion sequence binding protein 2 |
| SCFAs | short chain fatty acids |
| SCLY | selenocysteine β-lyase |
| Se | selenium |
| Sec | selenocysteine |
| SECIS | selenocysteine insertion sequence |
| Sec-tRNA[Ser]Sec | selenocysteine tRNA |
| SeMet | selenomethionine |
| SeNPS | selenium nanoparticles |
| SeP | selenoprotein P |
| Se-phosphate | selenophosphate |
| SEPSECS | O-phosphoseril-tRNA[Ser]Sec selenium transferase |
| SLC26 | solute carrier 26 |
| SNPs | single nucleotide polymorphisms |
| TRXR | thioredoxin reductase |
| UL | upper intake level |
| UTR | untranslated region |
| WHO | World Health Organization |
References
- Ramiro, S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Ramiro, S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Firestein, G.; McInnes, I.B. Immunopathogenesis of rheumatoid arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, D.A.; Pluchino, N.; Canny, G.; Gabay, C.; Straub, R.H. The role of female hormonal factors in the development of rheumatoid arthritis. Rheumatology 2016, 56, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Lin, J. Characteristics and risk factors of rheumatoid arthritis in the United States: An NHANES analysis. PeerJ 2017, 5, e4035. [Google Scholar] [CrossRef]
- Skoczyńska, M.; Świerkot, J. The role of diet in rheumatoid arthritis. Reumatologia 2018, 56, 259–267. [Google Scholar] [CrossRef]
- Zapatera, B.; Prados, A.; Gómez-Martínez, S.; Marcos, A. Immunonutrition: Methodology and applications. Nutr. Hosp. 2015, 31, 145–154. [Google Scholar] [CrossRef]
- Suchner, U.; Kuhn, K.S.; Fürst, P. The scientific basis of immunonutrition. Proc. Nutr. Soc. 2000, 59, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Vetvicka, V.; Vetvickova, J. Concept of Immuno-Nutrition. J. Nutr. Food Sci. 2016, 6, 500. [Google Scholar] [CrossRef]
- Khanna, S.; Jaiswal, K.S.; Gupta, B. Managing Rheumatoid Arthritis with Dietary Interventions. Front. Nutr. 2017, 4, 52. [Google Scholar] [CrossRef]
- García-González, A.; Gaxiola-Robles, R.; Zenteno-Savín, T. Oxidative stress in patients with rheumatoid arthritis. Rev. Investig. Clin. 2015, 67, 46–53. [Google Scholar]
- Veselinovic, M.; Barudzic, N.; Vuletic, M.; Zivkovic, V.; Tomic-Lucic, A.; Djuric, D.; Jakovljevic, V. Oxidative stress in rheumatoid arthritis patients: Relationship to diseases activity. Mol. Cell. Biochem. 2014, 391, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Nájera, D.D.R.; Pacheco-Tena, C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. Biomed. Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, M.; Konieczka, P.; Namieśnik, J. The properties, functions, and use of selenium compounds in living organisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 225–252. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Chemistry. In Selenium in Nutrition, Revised ed.; The National Academies Press: Washington, DC, USA, 1983; pp. 3–9. [Google Scholar]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Labunskyy, V.M.; Hatfield, L.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [Green Version]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017, 9, 21–37. [Google Scholar] [CrossRef]
- Oropeza-Moe, M.; Wisløff, H.; Bernhoft, A. Selenium deficiency associated porcine and human cardiomyopathies. J. Trace Elem. Med. Biol. 2015, 31, 148–156. [Google Scholar] [CrossRef]
- Thomson, C.D. Selenium|Physiology. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Elsevier: Dunedin, New Zeland, 2003; pp. 5117–5124. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid-base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather-Tait, S.; Hurrell, R.F. Bioavailability of minerals and trace elements. Nutr. Res. Rev. 1996, 9, 295–324. [Google Scholar] [CrossRef] [Green Version]
- Nickel, A.; Kottra, G.; Schmidt, G.; Danier, J.; Hofmann, T.; Daniel, H. Characteristics of transport of selenoamino acids by epithelial amino acid transporters. Chem. Biol. Interact. 2009, 177, 234–241. [Google Scholar] [CrossRef]
- Drug Bank. Available online: https://www.drugbank.ca/drugs/DB11135 (accessed on 24 April 2020).
- Fung, E.B. Nutritional deficiencies in patients with thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 188–196. [Google Scholar] [CrossRef]
- Sherief, L.M.; El-Salam, S.M.A.; Kamal, N.M.; El Safy, O.; Almalky, M.A.; Azab, S.F.; Morsy, H.M.; Gharieb, A.F. Nutritional biomarkers in children and adolescents with Beta-thalassemia-major: An Egyptian center experience. Biomed. Res. Int. 2014, 2014, 261761. [Google Scholar] [CrossRef] [Green Version]
- Pliakou, X.I.; Koutsouka, F.P.; Damigos, D.; Bourantas, K.L.; Briasoulis, E.C.; Voulgari, P.V. Rheumatoid arthritis in patients with hemoglobinopathies. Rheumatol. Int. 2012, 32, 2889–2892. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Roberts, B.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228. [Google Scholar] [CrossRef] [Green Version]
- Seale, L.A. Selenocysteine β-Lyase: Biochemistry, Regulation and Physiological Role of the Selenocysteine Decomposition Enzyme. Antioxidants 2019, 8, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, J.E.; Berry, M.J. Eukaryotic selenoprotein synthesis: Mechanistic insight incorporating new factors and new functions for old factors. IUBMB Life 2008, 60, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, F.-S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Comee, L.; Taylor, C.A.; Nahikian-Nelms, M.; Ganesan, L.P.; Krok-Schoen, J.L. Dietary patterns and nutrient intake of individuals with rheumatoid arthritis and osteoarthritis in the United States. Nutrition 2019, 67–68, 110533. [Google Scholar] [CrossRef] [Green Version]
- Grimstvedt, M.E.; Woolf, K.; Milliron, B.-J.; Manore, M.M. Lower Healthy Eating Index-2005 dietary quality scores in older women with rheumatoid arthritis v. healthy controls. Public Health Nutr. 2010, 13, 1170–1177. [Google Scholar] [CrossRef] [Green Version]
- Berube, L.T.; Kiely, M.; Woolf, K.; Yazici, Y. Diet quality of individuals with rheumatoid arthritis using the Healthy Eating Index (HEI)-2010. Nutr. Health 2017, 23, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bärebring, L.; Winkvist, A.; Gjertsson, I.; Lindqvist, H.M. Poor Dietary Quality Is Associated with Increased Inflammation in Swedish Patients with Rheumatoid Arthritis. Nutrients 2018, 10, 1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune function and micronutrient requirements change over the life course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [Green Version]
- Duntas, L.H. Selenium and inflammation: Underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009, 41, 443–447. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Mohtadinia, J.; Kolahi, S.; Bakhtiyari, M.; Delpisheh, A. Nutritional status of Iranian women with rheumatoid arthritis: An assessment of dietary intake and disease activity. Womens Health 2011, 7, 599–605. [Google Scholar] [CrossRef]
- Arablou, T.; Aryaeian, N.; Djalali, M.; Shahram, F.; Rasouli, L. Association between dietary intake of some antioxidant micronutrients with some inflammatory and antioxidant markers in active Rheumatoid Arthritis patients. Int. J. Vitam. Nutr. Res. 2019, 89, 238–245. [Google Scholar] [CrossRef]
- Stone, J.; Doube, A.; Dudson, D.; Wallace, J. Inadequate calcium, folic acid, vitamin E, zinc, and selenium intake in rheumatoid arthritis patients: Results of a dietary survey. Semin. Arthritis Rheum. 1997, 27, 180–185. [Google Scholar] [CrossRef]
- Silva, B.N.S.; De Araújo Ísis, L.S.B.; Queiroz, P.M.A.; Duarte Ângela, L.B.P.; Burgos, M.G.P.D.A. Intake of antioxidants in patients with rheumatoid arthritis. Rev. Assoc. Med. Bras. (1992) 2014, 60, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Hagfors, L.; Leandersson, P.; Sköldstam, L.; Andersson, J.; Johansson, G. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr. J. 2003, 2, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.; Stripp, C.; Klarlund, M.; Olsen, S.F.; Tjønneland, A.; Frisch, M. Diet and risk of rheumatoid arthritis in a prospective cohort. J. Rheumatol. 2005, 32, 1249–1252. [Google Scholar] [PubMed]
- Knekt, P.; Heliövaara, M.; Aho, K.; Alfthan, G.; Marniemi, J.; Aromaa, A. Serum selenium, serum alpha-tocopherol, and the risk of rheumatoid arthritis. Epidemiology 2000, 11, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Saag, K.G.; Merlino, L.A.; Mikuls, T.R.; Criswell, L.A. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am. J. Epidemiol. 2003, 157, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Tarp, U.; Overvad, K.; Thorling, E.B.; Graudal, H.; Hansen, J.C. Selenium treatment in rheumatoid arthritis. Scand. J. Rheumatol. 1985, 14, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Jäntti, J.; Vapaatalo, H.; Seppala, E.; Ruutsalo, H.M.; Isomaki, H. Treatment of rheumatoid arthritis with fish oil, selenium, Vitamins A and E, and placebo. Scand. J. Rheumatol. 1991, 20, 225. [Google Scholar]
- Peretz, A.; Neve, J.; Duchateau, J.; Famaey, J.P. Adjuvant treatment of recent onset rheumatoid arthritis by selenium supplementation: Preliminary observations. Br. J. Rheumatol. 1992, 31, 281–282. [Google Scholar] [CrossRef]
- Heinle, K.; Adam, A.; Gradl, M.; Wiseman, M.; Adam, O. Selenkonzentration in den Erythrozyten bei Patienten mit rheumatoider Arthritis. Med. Klin. 1997, 92, 29–31. [Google Scholar] [CrossRef]
- Peretz, A.; Siderova, V.; Nève, J. Selenium supplementation in rheumatoid arthritis investigated in a double blind, placebo-controlled trial. Scand. J. Rheumatol. 2001, 30, 208–212. [Google Scholar] [CrossRef]
- Canter, P.; Wider, B.; Ernst, E. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: A systematic review of randomized clinical trials. Rheumatology 2007, 46, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, S.; Welling, M.N.; Mantri, S.B.; Desai, K. In vitro and in vivo antioxidant, cytotoxic, and anti-chronic inflammatory arthritic effect of selenium nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 993–1003. [Google Scholar] [CrossRef]
- Ren, S.-X.; Zhang, B.; Lin, Y.; Ma, D.-S.; Yan, H. Selenium Nanoparticles Dispersed in Phytochemical Exert Anti-Inflammatory Activity by Modulating Catalase, GPx1, and COX-2 Gene Expression in a Rheumatoid Arthritis Rat Model. Med. Sci. Monit. 2019, 25, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Liua, J.; Ma, L.; Zhou, H.; Zhu, X.; Yu, Q.; Chen, X.; Zhao, Y.; Liu, J. Polypeptide nano-Se targeting inflammation and theranostic rheumatoid arthritis by anti-angiogenic and NO activating AMPKα signaling pathway. J. Mater. Chem. B 2018, 6, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Hitchon, C.A.; El-Gabalawy, H.S. Oxidation in rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Cejka, D.; Hayer, S.; Niederreiter, B.; Sieghart, W.; Fuereder, T.; Zwerina, J.; Schett, G. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2294–2302. [Google Scholar] [CrossRef]
- Camargo, S.M.R.; Singer, D.; Makrides, V.; Huggel, K.; Pos, K.M.; Wagner, C.A.; Kuba, K.; Danilczyk, U.; Skovby, F.; Kleta, R.; et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology 2009, 136, 872–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seow, H.-F.; Bröer, S.; Broer, A.; Bailey, C.G.; Potter, S.J.; Cavanaugh, J.A.; Rasko, J.E.; Br, S. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat. Genet. 2004, 36, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Popovska-Jankovic, K.; Tasic, V.; Bogdanovic, R.; Miljkovic, P.; Golubovic, E.; Soylu, A.; Saraga, M.; Pavicevic, S.; Baskin, E.; Akil, I.; et al. Molecular characterization of cystinuria in south-eastern European countries. Urolithiasis 2013, 41, 21–30. [Google Scholar] [CrossRef]
- Calonge, M.J.; Gasparini, P.; Chillarón, J.; Chillón, M.; Gallucci, M.; Rousaud, F.; Zelante, L.; Testar, X.; Dallapiccola, B.; Di Silverio, F.; et al. Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat. Genet. 1994, 6, 420–425. [Google Scholar] [CrossRef]
- Karunasinghe, N.; Han, D.Y.; Zhu, S.; Yu, J.; Lange, K.; Duan, H.; Medhora, R.; Singh, N.; Kan, J.; Alzaher, W.; et al. Serum selenium and single-nucleotide polymorphisms in genes for selenoproteins: Relationship to markers of oxidative stress in men from Auckland, New Zealand. Genes Nutr. 2012, 7, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Donadio, J.L.S.; Guerra-Shinohara, E.M.; Rogero, M.M.; Cozzolino, S.M.F. Influence of Gender and SNPs in GPX1 Gene on Biomarkers of Selenium Status in Healthy Brazilians. Nutrients 2016, 8, 81. [Google Scholar] [CrossRef]
- Donadio, J.L.S.; Rogero, M.M.; Guerra-Shinohara, E.M.; Barbosa, F., Jr.; Desmarchelier, C.; Borel, P.; Sneddon, A.A.; Hesketh, J.E.; Cozzolino, S.M.F. Genetic variants in selenoprotein genes modulate biomarkers of selenium status in response to Brazil nut supplementation (the SU.BRA.NUT study). Clin. Nutr. 2019, 38, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meplan, C.; Crosley, L.K.; Nicol, F.; Beckett, G.J.; Howie, A.F.; Hill, K.E.; Horgan, G.; Mathers, J.C.; Arthur, J.R.; Hesketh, J.E. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study). FASEB J. 2007, 21, 3063–3074. [Google Scholar] [CrossRef] [Green Version]
- Kopp, T.I.; Outzen, M.; Olsen, A.; Vogel, U.B.; Ravn-Haren, G. Genetic polymorphism in selenoprotein P modifies the response to selenium-rich foods on blood levels of selenium and selenoprotein P in a randomized dietary intervention study in Danes. Genes Nutr. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfthan, G.; Neve, J. Reference values for serum selenium in various areas-evaluated according to the TRACY protocol. J. Trace Elem. Med. Biol. 1996, 10, 77–87. [Google Scholar] [CrossRef]
- National Research Council. Distribution. In Selenium in Nutrition, Revised ed.; The National Academies Press: Washington, DC, USA, 1983; pp. 10–39. [Google Scholar]
- Xia, Y.; E Hill, K.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Müller, S.M.; Dawczynski, C.; Wiest, J.; Lorkowski, S.; Kipp, A.P.; Schwerdtle, T. Functional Biomarkers for the Selenium Status in a Human Nutritional Intervention Study. Nutrients 2020, 12, 676. [Google Scholar] [CrossRef] [Green Version]
- Bredholt, M.; Frederiksen, J.L. Zinc in Multiple Sclerosis: A Systematic Review and Meta-Analysis. ASN Neuro 2016, 8. [Google Scholar] [CrossRef]
- Sahebari, M.; Abrishami-Moghaddam, M.; Moezzi, A.; Avan, A.; Mirfeizi, Z.; Esmaily, H.; Ferns, G. Association between serum trace element concentrations and the disease activity of systemic lupus erythematosus. Lupus 2014, 23, 793–801. [Google Scholar] [CrossRef]
- Sanna, A.; Davide, F.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Sahebari, M.; Rezaieyazdi, Z.; Khodashahi, M. Selenium and Autoimmune Diseases: A Review Article. Curr. Rheumatol. Rev. 2019, 15, 123–134. [Google Scholar] [CrossRef]
- Aaseth, J.; Munthe, E.; Førre, Ø.; Steinnes, E. Trace elements in serum and urine of patients with rheumatoid arthritis. Scand. J. Rheumatol. 1978, 7, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Hannonen, P.; Möttönen, T.; Oka, M. Serum selenium and rheumatoid arthritis. Scand. J. Rheumatol. 1985, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Borglund, M.; Akesson, A.; Akesson, B. Distribution of selenium and glutathione peroxidase in plasma compared in healthy subjects and rheumatoid arthritis patients. Scand. J. Clin. Lab. Investig. 1988, 48, 27–32. [Google Scholar] [CrossRef]
- Bacon, M.C.; White, P.H.; Raiten, D.J.; Craft, N.; Margolis, S.; Levander, O.A.; Taylor, M.L.; Lipnick, R.N.; Sami, S. Nutritional status and growth in juvenile rheumatoid arthritis. Semin. Arthritis Rheum. 1990, 20, 97–106. [Google Scholar] [CrossRef]
- Jacobsson, L.; Lindgärde, F.; Manthorpe, R.; Akesson, B. Correlation of fatty acid composition of adipose tissue lipids and serum phosphatidylcholine and serum concentrations of micronutrients with disease duration in rheumatoid arthritis. Ann. Rheum. Dis. 1990, 49, 901–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Dell, J.R.; Lemley-Gillespie, S.; Palmer, W.R.; Weaver, A.L.; Moore, G.F.; Klassen, L.W. Serum selenium concentrations in rheumatoid arthritis. Ann. Rheum. Dis. 1991, 50, 376–378. [Google Scholar] [CrossRef] [Green Version]
- Heliövaara, M.; Knekt, P.; Aho, K.; Aaran, R.K.; Alfthan, G.; Aromaa, A. Serum antioxidants and risk of rheumatoid arthritis. Ann. Rheum. Dis. 1994, 53, 51–53. [Google Scholar] [CrossRef]
- Köse, K.; Doĝan, P.; Kardaş, Y.; Saraymen, R. Plasma selenium levels in rheumatoid arthritis. Biol. Trace Elem. Res. 1996, 53, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M.; Kuryliszyn-Moskal, A.; Borawska, M.H.; Hukałowicz, K.; Markiewicz, R. A study on soluble intercellular adhesion molecule-1 and selenium in patients with rheumatoid arthritis complicated by vasculitis. Clin. Rheumatol. 2003, 22, 414–419. [Google Scholar] [CrossRef]
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, U.E. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol. Trace Elem. Res. 2005, 106, 123–132. [Google Scholar] [CrossRef]
- Pemberton, P.W.; Ahmad, Y.; Bodill, H.; Lokko, D.; Hider, S.L.; Yates, A.P.; Walker, M.G.; Laing, I.; Bruce, I. Biomarkers of oxidant stress, insulin sensitivity and endothelial activation in rheumatoid arthritis: A cross-sectional study of their association with accelerated atherosclerosis. BMC Res. Notes 2009, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Onal, S.; Nazıroğlu, M.; Çolak, M.; Bulut, V.; Flores-Arce, M.F. Effects of different medical treatments on serum copper, selenium and zinc levels in patients with rheumatoid arthritis. Biol. Trace Elem. Res. 2011, 142, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, Y.; Mao, H.; Deng, W.; Zhang, J. Effects of B-lymphocyte dysfunction on the serum copper, selenium and zinc levels of rheumatoid arthritis patients. Pak. J. Med. Sci. 2014, 30, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.I.; Talpur, F.N.; Kazi, T.G.; Brabazon, D. Estimation of toxic elements in the samples of different cigarettes and their effect on the essential elemental status in the biological samples of Irish smoker rheumatoid arthritis consumers. Environ. Monit. Assess. 2015, 187, 157. [Google Scholar] [CrossRef] [Green Version]
- Sahebari, M.; Ayati, R.; Mirzaei, H.; Sahebkar, A.; Hejazi, S.; Saghafi, M.; Saadati, N.; Ferns, G.A.; Avan, A. Serum Trace Element Concentrations in Rheumatoid Arthritis. Biol. Trace Elem. Res. 2016, 171, 237–245. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Fan, D.; Xia, Q.; Wang, M.; Pan, F. Common trace metals in rheumatoid arthritis: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2019, 56, 81–89. [Google Scholar] [CrossRef]
- Mian, A.N.; Ibrahim, F.; Scott, D.L. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol. 2019, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Cheung, J.M.; Scarsbrook, D.; Klinkhoff, A.V. Characterization of patients with arthritis referred for gold therapy in the era of biologics. J. Rheumatol. 2012, 39, 716–719. [Google Scholar] [CrossRef]
- Jackson-Rosario, S.; Cowart, D.; Myers, A.; Tarrien, R.; Levine, R.L.; Scott, R.A.; Self, W.T. Auranofin disrupts selenium metabolism in Clostridium difficile by forming a stable Au-Se adduct. J. Biol. Inorg. Chem. 2009, 14, 507–519. [Google Scholar] [CrossRef] [Green Version]
- Radenkovic, F.; Holland, O.; Vanderlelie, J.J.; Perkins, A. Selective inhibition of endogenous antioxidants with Auranofin causes mitochondrial oxidative stress which can be countered by selenium supplementation. Biochem. Pharmacol 2017, 146, 42–52. [Google Scholar] [CrossRef]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an old drug for a golden new age. Drugs R D 2015, 15, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Chaudiere, J.; Wilhelmsen, E.C.; Tappel, A.L. Mechanism of selenium-glutathione peroxidase and its inhibition by mercaptocarboxylic acids and other mercaptans. J. Biol. Chem. 1984, 259, 1043–1050. [Google Scholar]
- Grażyna, G.; Agata, K.; Adam, P.; Tomasz, L.; Agata, W.-C.; Karolina, D.; Grzegorz, C.; Anna, C.; Gromadzka, G.; Karpińska, A.; et al. Treatment with D-penicillamine or zinc sulphate affects copper metabolism and improves but not normalizes antioxidant capacity parameters in Wilson disease. Biometals 2014, 27, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peretz, A.; Neve, J.; Vertongen, F.; Famaey, J.P.; Molle, L. Selenium status in relation to clinical variables and corticosteroid treatment in rheumatoid arthritis. J. Rheumatol. 1987, 14, 1104–1107. [Google Scholar] [PubMed]
- Marano, G.; Fischioni, P.; Graziano, C.; Iannone, M.; Morisi, G. Increased serum selenium levels in patients under corticosteroid treatment. Pharmacol. Toxicol. 1990, 67, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, V.E. The factors affecting plasma glutathione peroxidase and selenium in rheumatoid arthritis: A multiple linear regression analysis. Scand. J. Rheumatol. 1991, 20, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Deyab, G.; Hokstad, I.; Aaseth, J.; Småstuen, M.C.; Whist, J.E.; Agewall, S.; Lyberg, T.; Tveiten, D.; Hjeltnes, G.; Ghanbari, A.; et al. Effect of anti-rheumatic treatment on selenium levels in inflammatory arthritis. J. Trace Elem. Med. Biol. 2018, 49, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ghashut, R.A.; McMillan, D.C.; Kinsella, J.; Vasilaki, A.T.; Talwar, D.; Duncan, A. The effect of the systemic inflammatory response on plasma zinc and selenium adjusted for albumin. Clin. Nutr. 2016, 35, 381–387. [Google Scholar] [CrossRef]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S.J.; O’Reilly, D.S.J. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Braunstein, M.; Kusmenkov, T.; Zuck, C.; Angstwurm, M.; Becker, N.-P.; Böcker, W.; Schomburg, L.; Bogner, V. Selenium and Selenoprotein P Deficiency Correlates with Complications and Adverse Outcome after Major Trauma. Shock 2020, 53, 63–70. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Day, A.G.; Muscedere, J.; Drover, J.; Suchner, U.; Cook, D.; Canadian Critical Care Trials Group. REducing Deaths due to OXidative Stress (The REDOXS Study): Rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc. Nutr. Soc. 2006, 65, 250–263. [Google Scholar] [CrossRef]
- Mateo, G.F.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch. Intern. Med. 2008, 168, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Post, C.; Aaseth, J. Relatively high mortality risk in elderly Swedish subjects with low selenium status. Eur. J. Clin. Nutr. 2016, 70, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suadicani, P.; Hein, H.; Gyntelberg, F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis 1992, 96, 33–42. [Google Scholar] [CrossRef]
- Alehagen, U.; Alexander, J.; Aaseth, J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS ONE 2016, 11, e0157541. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxid. Med. Cell. Longev. 2019, 2019, 7536805. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Aoki, Y.; Shibata, Y.; Sonobe, M.; Terajima, F.; Takahashi, H.; Saito, M.; Taniguchi, S.; Yamada, M.; Nakagawa, K. Identification of clinical parameters associated with serum oxidative stress in patients with rheumatoid arthritis. Mod. Rheumatol. 2014, 24, 926–930. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Hamza, A.-B. Reactive oxygen and nitrogen species in patients with rheumatoid arthritis as potential biomarkers for disease activity and the role of antioxidants. Free Radic. Biol. Med. 2016, 97, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-kB in Oxidative Stress. Curr. Opin. Toxicol. 2017, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Shahzad, S.; Khan, A.Q. Level of inflammatory cytokines in rheumatoid arthritis patients: Correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS ONE 2017, 12, e0178879. [Google Scholar] [CrossRef]
- Bala, A.; Mondal, C.; Haldar, P.K.; Khandelwal, B. Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: Clinical efficacy of dietary antioxidants. Inflammopharmacology 2017, 25, 595–607. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.C.; Sivakumar, B. Hypoxia and angiogenesis in rheumatoid arthritis. Curr. Opin. Rheumatol. 2005, 17, 293–298. [Google Scholar] [CrossRef]
- Kretz-Remy, C.; Arrigo, A.P. Selenium: A key element that controls NF-kappa B activation and I kappa B alpha half life. Biofactors 2001, 14, 117–125. [Google Scholar] [CrossRef]
- Maehira, F.; Miyagi, I.; Eguchi, Y. Selenium regulates transcription factor NF-kappaB activation during the acute phase reaction. Clin. Chim. Acta 2003, 334, 163–171. [Google Scholar] [CrossRef]
- Alhasan, R.; Kharma, A.; Leroy, P.; Jacob, C.; Gaucher, C. Selenium Donors at the Junction of Inflammatory Diseases. Curr. Pharm. Des. 2019, 25, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Agricultural Library. Selenium. Available online: https://www.nal.usda.gov/fnic/selenium (accessed on 10 May 2020).
- National Agricultural Library. Food Composition. Available online: https://www.nal.usda.gov/fnic/food-composition (accessed on 10 May 2020).
- Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; Dort, S.; Calcagno, M.; Burgess, N.; Crosby, L.; Barnard, N.D. Nutrition Interventions in Rheumatoid Arthritis: The Potential Use of Plant-Based Diets. A Review. Front. Nutr. 2019, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Kreps, D.J.; Halperin, F.; Desai, S.P.; Zhang, Z.Z.; Losina, E.; Olson, A.T.; Karlson, E.W.; Bermas, B.L.; Sparks, J.A. Association of weight loss with improved disease activity in patients with rheumatoid arthritis: A retrospective analysis using electronic medical record data. Int. J. Clin. Rheumtol. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Petersson, S.; Philippou, E.; Rodomar, C.; Nikiphorou, E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: Evidence from clinical trials. Autoimmun. Rev. 2018, 17, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Hafstrom, I.; Ringertz, B.; Spångberg, A.; Von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef] [Green Version]
- Caja, S.; Mäki, M.; Kaukinen, K.; Lindfors, K. Antibodies in celiac disease: Implications beyond diagnostics. Cell. Mol. Immunol. 2011, 8, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef]
- Badsha, H. Role of Diet in Influencing Rheumatoid Arthritis Disease Activity. Open Rheumatol. J. 2018, 12, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gang, D.; Yu, X.; Hu, Y.; Yue, Y.; Cheng, W.; Pan, X.; Zhang, P. Genistein: The potential for efficacy in rheumatoid arthritis. Clin. Rheumatol. 2013, 32, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Kolahi, S.; Aref-Hosseini, S.-R.; Mamegani, M.E.; Hekmatdoost, A. Beneficial role of antioxidants on clinical outcomes and erythrocyte antioxidant parameters in rheumatoid arthritis patients. Int. J. Prev. Med. 2014, 5, 835–840. [Google Scholar] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell. Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, B.; Park, S.-K. Selenocysteine mimics the effect of dietary restriction on lifespan via SKN-1 and retards age-associated pathophysiological changes in Caenorhabditis elegans. Mol. Med. Rep. 2018, 18, 5389–5398. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Selenoprotein (Abbreviation) | Well-Known Function |
| Glutathione peroxidases (GPx1, GPx2, GPx3, GPx4, GPx6) | Antioxidant enzymes that reduce H2O2 and organic peroxides to water and alcohols, respectively |
| Iodothyronine deiodinase (D1, D2, D3) | Their role is to produce and regulate the level of active thyroid hormone, T3, from thyroxine, T4 |
| Thioredoxin reductases (TRXR1, TRXR2, TRXR3) | They are involved in the regulation of redox reactions in mammalian cells. They are responsible for maintaining the correct intracellular redox potential |
| Selenoprotein P (SeP) | It is responsible for the transport of Se through plasma to certain tissues |
| Methionine-R-sulfoxide reductase B1 (MsrB1) | Functions as a methionine sulfoxide reductase |
| Selenophosphate synthetase 2 (SPS2) | It is required for the synthesis of selenoproteins (including itself) because it produces selenophosphate, the precursor to selenocysteine |
| Selenoprotein (Abbreviation) | Potential Function |
| Selenoprotein H (SELH) | In D. melanogaster, it may be involved in antioxidant defense |
| Selenoprotein I (SELI) | Potential role in phospholipid biosynthesis |
| Selenoprotein K (SELK) | Involved in calcium flux in immune cells |
| Selenoprotein M (SELM) | Potential role in protein-folding |
| Selenoprotein N (SELN) | Possibly involved in early muscle formation |
| Selenoprotein T (SELT) | Involved in calcium mobilization |
| Selenoprotein W (SELW) | It may be involved in muscle growth |
| Author, Year [Reference] | Sample Size | Design | Results |
|---|---|---|---|
| Tarp, 1985 [57] | SG: n = 20 PG: n = 20 | 6-month follow-up SG: 256 µg/day enriched yeast PG: selenium-deficient yeast | No significant differences between groups |
| Jäntti, 1991 [58] Abstract | n = 28 | 2-month follow-up SG: 150 µg/day PG: not described | ‘No clear effect in RA’ |
| Peretz, 1992 [59] | SG: n = 8 PG: n = 7 | 3-month follow-up SG: 200 µg/day enriched yeast PG: selenium-free yeast | No between-group comparisons reported |
| Heinle, 1997 [60] | SG: n = 35 PG: n = 30 | 3-month follow-up SG: 200 µg/day sodium selenite PG: not described Concomitant supplementation with fish oil fatty acids (30 mg/kg body) in both groups | No significant differences between groups and not analysis performed in some parameters |
| Peretz, 2001 [61] | SG: n = 28 PG: n = 27 | 3-month follow-up SG: 200 µg/day enriched yeast PG: selenium-free yeast | Significant difference only in two items of a quality of life questionnaire (arm movements and health perception) |
| Author, Year [Reference] | RA Patients | Healthy Controls | p Value | ||
|---|---|---|---|---|---|
| n | µg/L | n | µg/L | ||
| Aaseth, 1978 [85] | 23 | 93.7 ± 25.2 | 30 | 129.13 ± 8.66 | - |
| Hannonen, 1985 [86] | 20 | 75.2 ± 9.2 | 20 | 89.6 ± 13.1 | - |
| Borglund, 1988 [87] | 7 | 66.14 ± 8.66 | 5 | 77.17 ± 2.36 | 0.02 |
| Bacon, 1990 [88] | 34 | 99 ± 19 | 9 | 109 ± 11 | NS |
| Jacobsson, 1990 [89] | 41 | 76.38 ± 15.75 | 57 | 85 ± 13.39 | <0.05 |
| O’Dell, 1991 [90] | 122 | 148 ± 42 | 29 | 160 ± 25 | 0.05 |
| Heliovaara, 1994 [91] | 14 | 60 ± 12.8 | 27 | 61.2 ± 12.2 | 0.78 |
| Köse, 1996 [92] | 60 | 107.5 ± 23.76 | 60 | 168.45 ± 46.44 | <0.001 |
| Knekt, 2000 [55] | 122 | 49.4 ± 12.9 | 357 | 50.7 ± 10.2 | NS |
| Witkowska, 2003 [93] | 37 | 64.5 ± 12.18 | 18 | 83.9 ± 11 | <0.05 |
| Yazar, 2005 [94] | 25 | 64.41 ± 28 | 25 | 111.76 ± 67.73 | <0.05 |
| Pemberton, 2009 [95] | 46 | 84.55 ± 10.3 | 58 | 91.14 ± 12.74 | 0.003 |
| Önal, 2011 [96] | 32 | 140 ± 37.7 | 52 | 166.2 ± 44.3 | <0.01 |
| Li, 2014 [97] | 60 | 157.48 ± 49.61 | 60 | 192.91 ± 54.33 | <0.05 |
| Afridi, 2015 [98] | 53 | 119.45 ± 7.1 | 52 | 212.12 ± 8.46 | <0.001 |
| Sahebari, 2015 [99] | 110 | 90.92 ± 22.77 | 100 | 110.11 ± 18.59 | <0.0001 |
| SMD = −1.04 (95% CI = −1.58 to −0.50, Z = −3.77, p =< 0.001), I2 = 95.6% | |||||
| Age | Male | Female | Pregnancy | Lactation | UL |
|---|---|---|---|---|---|
| 14–18 years | 55 | 55 | 60 | 70 | 400 |
| 19–50 years | 55 | 55 | 60 | 70 | 400 |
| ≥51 years | 70–100 | 70–100 | 400 |
| Food | Serving | Selenium (µg) | Selenium Compound |
|---|---|---|---|
| Brazil nuts | 1 ounce | 543.5 | SeMet |
| Fish | 3 ounces | 92 | SeMet/selenite/selenate |
| Pork | 3 ounces | 32.5 | SeMet/selenate |
| Chicken | 3 ounces | 22 | SeMet/Sec |
| Rice | 1 cup | 19.1 | SeMet |
| Beef | 3 ounces | 18 | SeMet |
| Whole-wheat bread | 2 slices | 16.4 | SeMet/selenate |
| Egg | 1 large | 15 | SeMet/Sec |
| Milk (fat free or skim) | 1 cup | 7.6 | Sec/selenite |
| Lentils | 1 cup | 6 | SeMet/selenate |
| Broccoli | 1 cup | 4.4 | SeMet/selenate |
| Potatoes | 1 piece | 1.5 | SeMet |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrubiates-Hernández, F.J.; Márquez-Sandoval, Y.F.; González-Estevez, G.; Reyes-Castillo, Z.; Muñoz-Valle, J.F. The Relevance of Selenium Status in Rheumatoid Arthritis. Nutrients 2020, 12, 3007. https://doi.org/10.3390/nu12103007
Turrubiates-Hernández FJ, Márquez-Sandoval YF, González-Estevez G, Reyes-Castillo Z, Muñoz-Valle JF. The Relevance of Selenium Status in Rheumatoid Arthritis. Nutrients. 2020; 12(10):3007. https://doi.org/10.3390/nu12103007
Chicago/Turabian StyleTurrubiates-Hernández, Francisco Javier, Yolanda Fabiola Márquez-Sandoval, Guillermo González-Estevez, Zyanya Reyes-Castillo, and José Francisco Muñoz-Valle. 2020. "The Relevance of Selenium Status in Rheumatoid Arthritis" Nutrients 12, no. 10: 3007. https://doi.org/10.3390/nu12103007
APA StyleTurrubiates-Hernández, F. J., Márquez-Sandoval, Y. F., González-Estevez, G., Reyes-Castillo, Z., & Muñoz-Valle, J. F. (2020). The Relevance of Selenium Status in Rheumatoid Arthritis. Nutrients, 12(10), 3007. https://doi.org/10.3390/nu12103007







