Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Approval
2.2. Stock and Cultivation of the Strain
2.3. Bacterial Culture
2.4. Induction of Experimental Diabetes
2.5. Biochemical Analysis of Plasma
2.6. Oral Glucose Tolerance Test
2.7. In Vitro Glucose Uptake by Isolated Rat Hemi-Diaphragm
2.8. In Vivo Intestinal Permeability Assay
2.9. Determination of Plasma Lipopolysaccharide (LPS)
2.10. Determination of Triglyceride Accumulation in Liver and Skeletal Muscle
2.11. DNA Extraction from Fecal Samples
2.12. q-PCR Assay Conditions and Cycle Threshold
2.13. Measurement of Organic Acid Contents in Cecal Samples
2.14. Western Blot Analysis
2.15. Statistical Analysis
3. Results
3.1. Effects of L. paracasei HII01 on Body Weight (BW), Visceral Fat (VF) Weight and Visceral Fat/100 g BW
3.2. Effects of L. paracasei HII01 on Glycemic Control and Plasma Adipokine Hormones
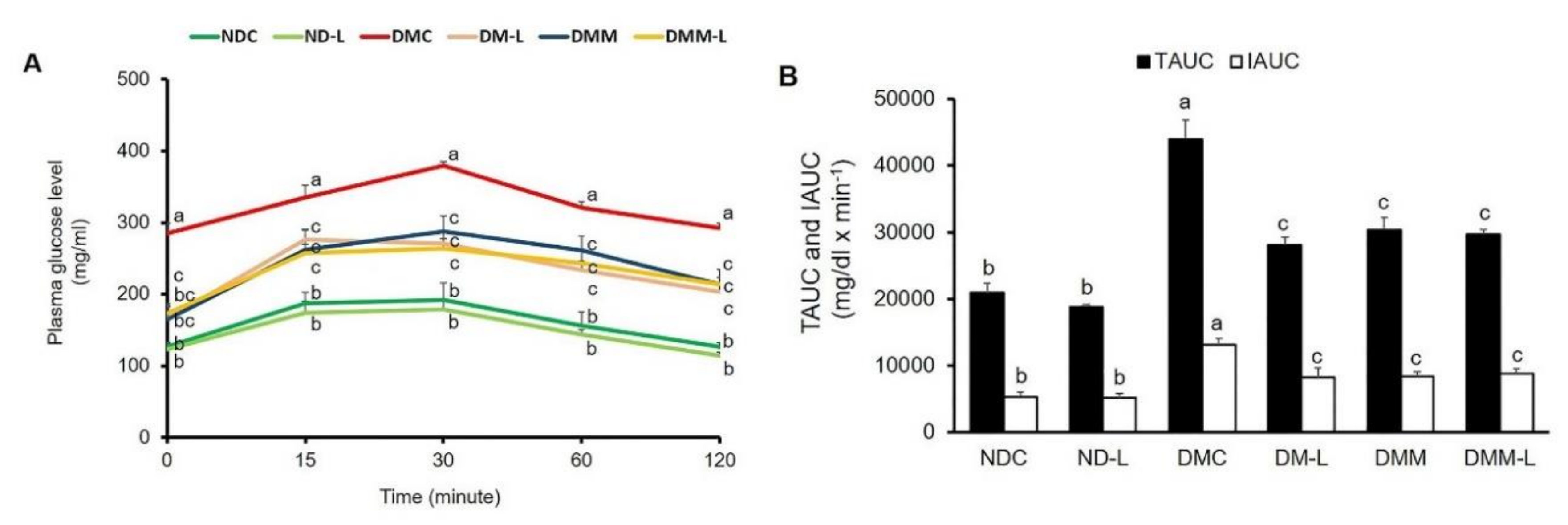
3.3. Effects of L. paracasei HII01 on the Glucose Tolerance Test
3.4. Effects of L. paracasei HII01 on Lipid Parameters
3.5. Effects of L. paracasei HII01 on Tissue Triglyceride Accumulation
3.6. Effects of L. paracasei HII01 on In Vitro Skeletal Muscle Glucose Uptake
3.7. Effects of L. paracasei HII01 on Protein Expressions of GLUT4, pAktSer473, pAMPKThr172, NF-kB and TNF-α in Soleus Muscle
3.8. Effects of L. paracasei HII01 on Plasma Endotoxemia
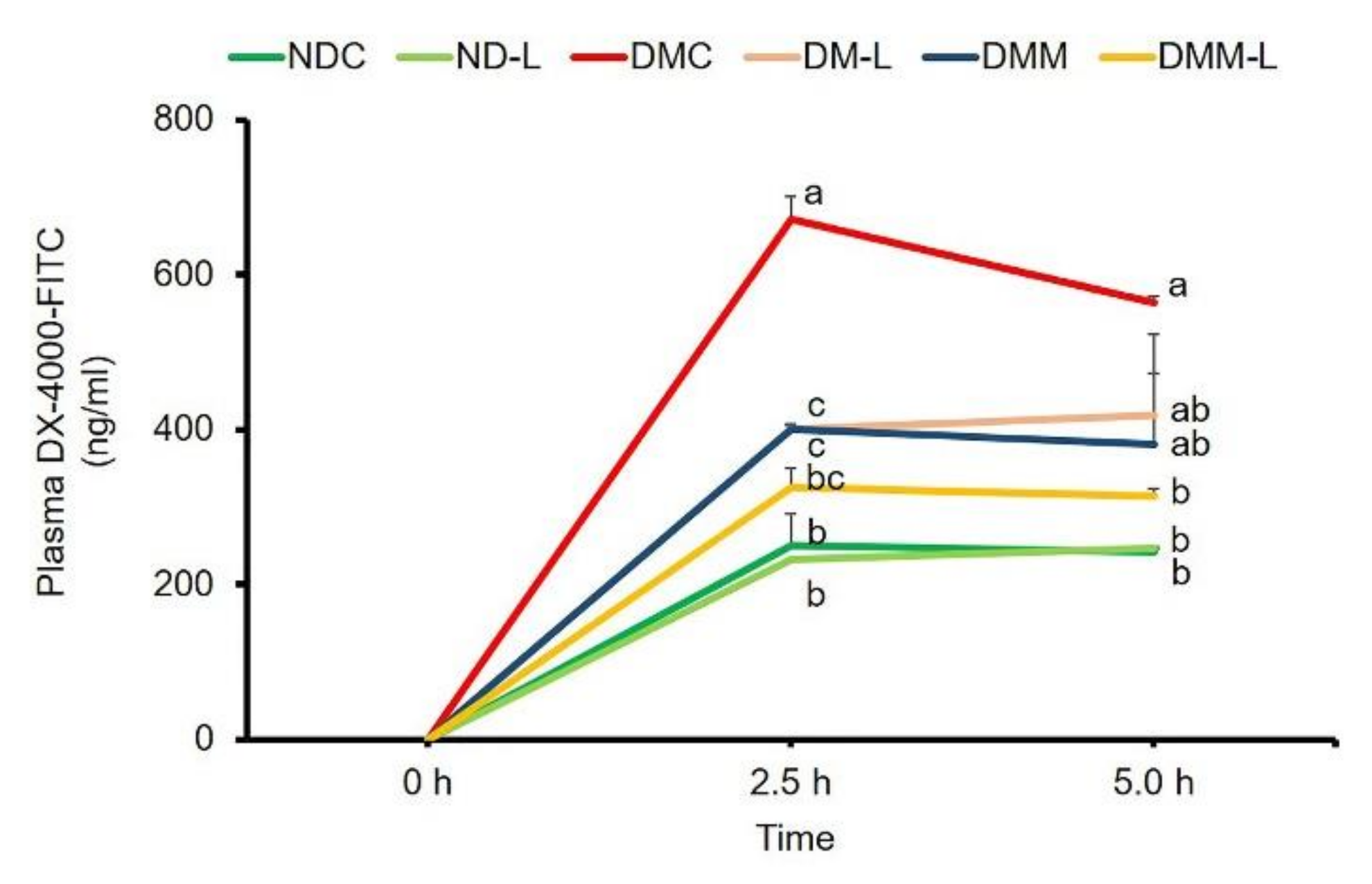
3.9. Effects of L. paracasei HII01 on Intestinal Permeability
3.10. Effects of L. paracasei HII01 on Short-Chain Fatty Acids in Cecal Content
3.11. Effects of L. paracasei HII01 on the Bacterial DNA in Feces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015, 172, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, B.M.; Saad, M.J.A. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediat. Inflamm. 2013, 2013, 986734. [Google Scholar] [CrossRef] [Green Version]
- Panwar, H.; Rashmi, H.M.; Batish, V.K.; Grover, S. Probiotics as potential biotherapeutics in the management of type 2 diabetes-prospects and perspectives. Diabetes Metab. Res. Rev. 2013, 29, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.; Reynoso, M.; Geddis, A.V.; Mitrophanov, A.Y.; Matheny, R.W. LPS-stimulated NF-κB p65 dynamic response marks the initiation of TNF expression and transition to IL-10 expression in RAW 264.7 macrophages. Physiol. Rep. 2018, 6, e13914. [Google Scholar] [CrossRef] [Green Version]
- Khorami, S.A.H.; Movahedi, A.; Huzwah, K.; Sokhini, A.M.M. PI3K/AKT pathway in modulating glucose homeostasis and its alteration in Diabetes. Ann. Med Biomed. Sci. 2015, 1, 46–55. [Google Scholar]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas) 2016, 52, 28–34. [Google Scholar] [CrossRef]
- Kocsis, T.; Molnár, B.; Németh, D.; Hegyi, P.; Szakács, Z.; Bálint, A.; Garami, A.; Soós, A.; Márta, K.; Solymár, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Vidal, H. Impact of Gut Microbiota on Host Glycemic Control. Front. Endocrinol. 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Swami, O.C. Role of Probiotics in Diabetes: A Review of Their Rationale and Efficacy. EMJ Diabetes 2017, 5, 104–110. [Google Scholar]
- Hsieh, F.C.; Lee, C.L.; Chai, C.Y.; Chen, W.T.; Lu, Y.C.; Wu, C.S. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr. Metab. (Lond.) 2013, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.M.; Jeong, J.J.; Woo, K.H.; Han, M.J.; Kim, D.H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016, 36, 337–348. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Thiennimitr, P.; Yasom, S.; Tunapong, W.; Chunchai, T.; Wanchai, K.; Pongchaidecha, A.; Lungkaphin, A.; Sirilun, S.; Chaiyasut, C.; Chattipakorn, N.; et al. Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition 2018, 54, 40–47. [Google Scholar] [CrossRef]
- Wanchai, K.; Yasom, S.; Tunapong, W.; Chunchai, T.; Eaimworawuthikul, S.; Thiennimitr, P.; Chaiyasut, C.; Pongchaidecha, A.; Chatsudthipong, V.; Chattipakorn, S.; et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin. Sci. (Lond.) 2018, 132, 1545–1563. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharm. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. BMJ 1990, 300, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabet, H.S.; Saleh, N.K.; Thabet, S.S.; Abdel-Aziz, M.; Kalleny, N.K. Decreased basal non-insulin-stimulated glucose uptake by diaphragm in streptozotocin-induced diabetic mice infected with Schistosoma mansoni. Parasitol. Res. 2008, 103, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Joly Condette, C.; Khorsi-Cauet, H.; Morlière, P.; Zabijak, L.; Reygner, J.; Bach, V.; Gay-Quéheillard, J. Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS ONE 2014, 14, e102217. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.G.; Licht, T.R.; Leser, T.D.; Bahl, M.I. Dietary xylo-oligosaccharide stimulates intestinal bifidobacteria and lactobacilli but has limited effect on intestinal integrity in rats. BMC Res. Notes 2014, 19, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Maycock, P.F. Skeletal muscle triacylglycerol in the rat: Methods for sampling and measurement, and studies of biological variability. J. Lipid Res. 1980, 21, 139–144. [Google Scholar]
- Khat-udomkiri, N.; Toejing, P.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Antihyperglycemic effect of rice husk derived xylooligosaccharides in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rat mod. Food Sci. Nutr. 2019, 8, 428–444. [Google Scholar] [CrossRef]
- Kohei, K. Pathophysiology of Type 2 Diabetes and Its Treatment Policy. JMAJ 2010, 53, 41–46. [Google Scholar]
- Wiwanitkit, V. Outbreak of Escherichia coli and diabetes mellitus. Indian J. Endocrinol. Metab. 2011, 15, 70–71. [Google Scholar] [CrossRef]
- Ghonim, E.M.; Hindawy, G.R.E.; Motelb, T.M.A.E.; Labib, A.Z.; Ahmady, I.; Salem, E.H.H. Study of bacteremia in diabetic patients. Menoufia Med. J. 2016, 29, 846. [Google Scholar]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.J.; Renata, F.; Carol, F.E. The PI3K signaling pathway mediates the biological effects of leptin. Arq. Bras Endocrinol. Metab. 2010, 54, 591–602. [Google Scholar]
- Li, X.; Wang, E.; Yin, B.; Fang, D.; Chen, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef. Microbes 2017, 8, 421–432. [Google Scholar] [CrossRef]
- Petrone, A.; Zavarella, S.; Caiazzo, A.; Leto, G.; Spoletini, M.; Potenziani, S.; Osborn, J.; Vania, A.; Buzzetti, R. The promoter region of the adiponectin gene is a determinant in modulating insulin sensitivity in childhood obesity. Obesity 2006, 14, 1498–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.I.; Goodyear, L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Amati, F.; Dubé, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.S.; et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.; Sears, D.D. TLR4 and Insulin Resistance. Gastroenterol. Res. Pr. 2010, 2010, 212563. [Google Scholar] [CrossRef] [Green Version]
- Summers, S.A.; Garza, L.A.; Zhou, H.; Birnbaum, M.J. Regulation of Insulin-Stimulated Glucose Transporter GLUT4 Translocation and Akt Kinase Activity by Ceramide. Mol. Cell Biol. 1998, 18, 5457–5464. [Google Scholar] [CrossRef] [Green Version]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Huh, C.S.; Choi, I.D.; Jeong, J.W.; Ku, H.K.; Ra, J.H.; Kim, T.Y.; Kim, G.B.; Sim, J.H.; Ahn, Y.T. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. J. Appl. Microbiol. 2014, 117, 834–845. [Google Scholar] [CrossRef]
- Kurth-Kraczek, E.J.; Hirshman, M.F.; Goodyear, L.J.; Winder, W.W. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 1999, 48, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef]
- Ang, Z.; Xiong, D.; Wu, M.; Ding, J.L. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. Faseb. J. 2018, 32, 289–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.; Chen, G.; Xu, H.; Ge, R.; Lin, J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypotheses 2010, 74, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]






| Parameters | NDC | ND-L | DMC | DM-L | DMM | DMM-L |
|---|---|---|---|---|---|---|
| BW (g) | 553.33 ± 18.60 b | 568.33 ± 26.13 b | 682.50 ± 35.25 a | 575.00 ± 13.78 b | 586.25 ± 11.43 b | 589.00 ± 4.00 b |
| VF (g) | 45.17 ± 2.36 b | 41.00 ± 3.20 b | 86.33 ± 7.84 a | 62.8 ± 2.99 c | 61.75 ± 2.46 c | 60.40 ± 5.62 c |
| VF/100g BW | 8.13 ± 0.22 b | 7.23 ± 0.53 b | 12.62 ± 0.51 a | 10.89 ± 0.27 c | 10.54 ± 0.41 c | 10.27 ± 1.00 c |
| Parameters (mg/dL) | NDC | ND-L | DMC | DM-L | DMM | DMM-L |
|---|---|---|---|---|---|---|
| Triglyceride | 38.35 ± 1.26 b | 31.82 ± 1.67 b | 83.57 ± 7.18 a | 35.76 ± 2.78 b | 33.88 ± 1.02 b | 38.15 ± 3.55 b |
| Cholesterol | 44.35 ± 1.04 b | 40.53 ± 2.48 b | 72.92 ± 6.02 a | 42.85 ± 1.81b | 35.13 ± 2.63 c | 33.85 ± 3.21c |
| HDL | 59.75 ± 1.55 b | 64.00 ± 3.67 ab | 69.00 ± 5.98 ab | 72.33 ± 1.11a | 71.00 ± 2.64 a | 72.33 ± 0.76 a |
| LDL | 10.00 ± 1.22 b | 10.08 ± 1.32 b | 23.75 ± 3.50 a | 16.67 ± 3.28 c | 14.00 ± 1.30 b | 13.00 ± 1.14 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toejing, P.; Khat-Udomkiri, N.; Intakhad, J.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats. Nutrients 2020, 12, 3015. https://doi.org/10.3390/nu12103015
Toejing P, Khat-Udomkiri N, Intakhad J, Sirilun S, Chaiyasut C, Lailerd N. Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats. Nutrients. 2020; 12(10):3015. https://doi.org/10.3390/nu12103015
Chicago/Turabian StyleToejing, Parichart, Nuntawat Khat-Udomkiri, Jannarong Intakhad, Sasithorn Sirilun, Chaiyavat Chaiyasut, and Narissara Lailerd. 2020. "Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats" Nutrients 12, no. 10: 3015. https://doi.org/10.3390/nu12103015
APA StyleToejing, P., Khat-Udomkiri, N., Intakhad, J., Sirilun, S., Chaiyasut, C., & Lailerd, N. (2020). Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats. Nutrients, 12(10), 3015. https://doi.org/10.3390/nu12103015






