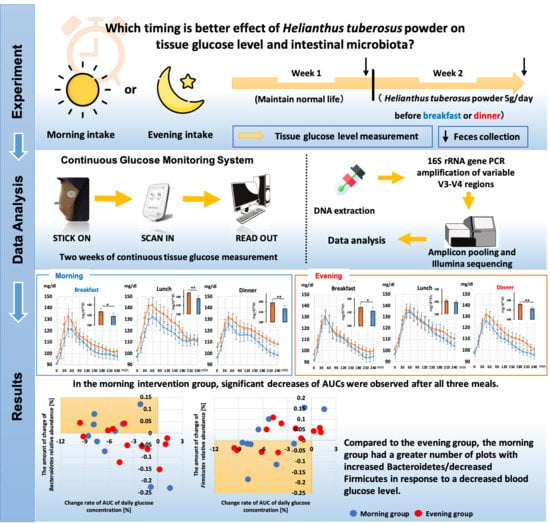
Ingestion of Helianthus tuberosus at Breakfast Rather Than at Dinner is More Effective for Suppressing Glucose Levels and Improving the Intestinal Microbiota in Older Adults
Abstract
1. Introduction
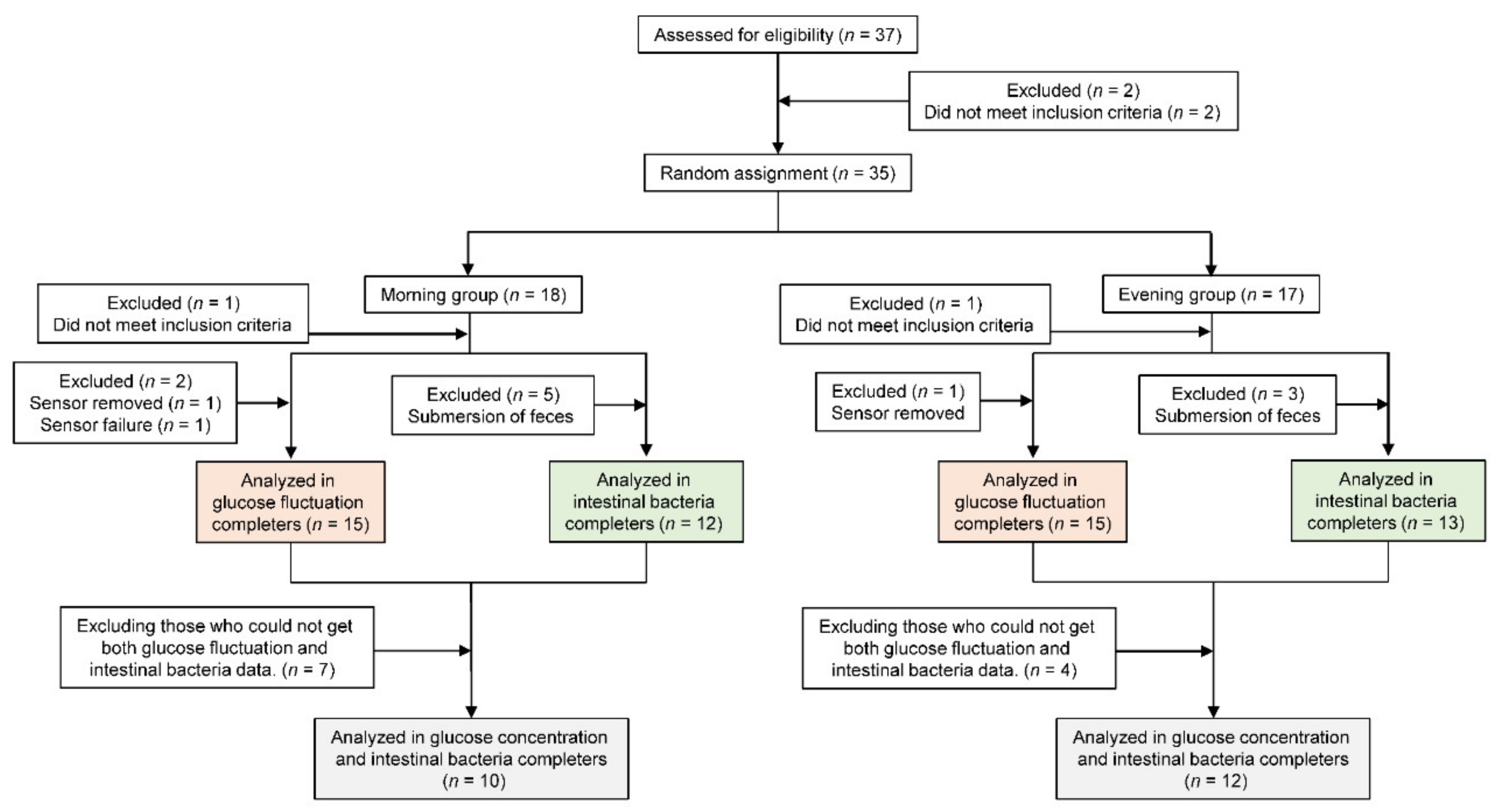
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Helianthus tuberosus Contents
2.4. Measurements
2.4.1. Anthropometry
2.4.2. Tissue Glucose Level and Analysis
2.4.3. Physical Activity Assessment
2.4.4. Chronotype Assessment
2.4.5. Dietary Assessment
2.4.6. Fecal pH Measurement
2.4.7. Short-Chain Fatty Acid (SCFA) Measurement
2.4.8. Fecal DNA Extraction and 16S rRNA Gene Sequencing
2.4.9. Analysis of 16S rRNA Gene Sequences
2.4.10. Constipation Assessment scale (CAS) Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Physical Activity Level and Mealtime
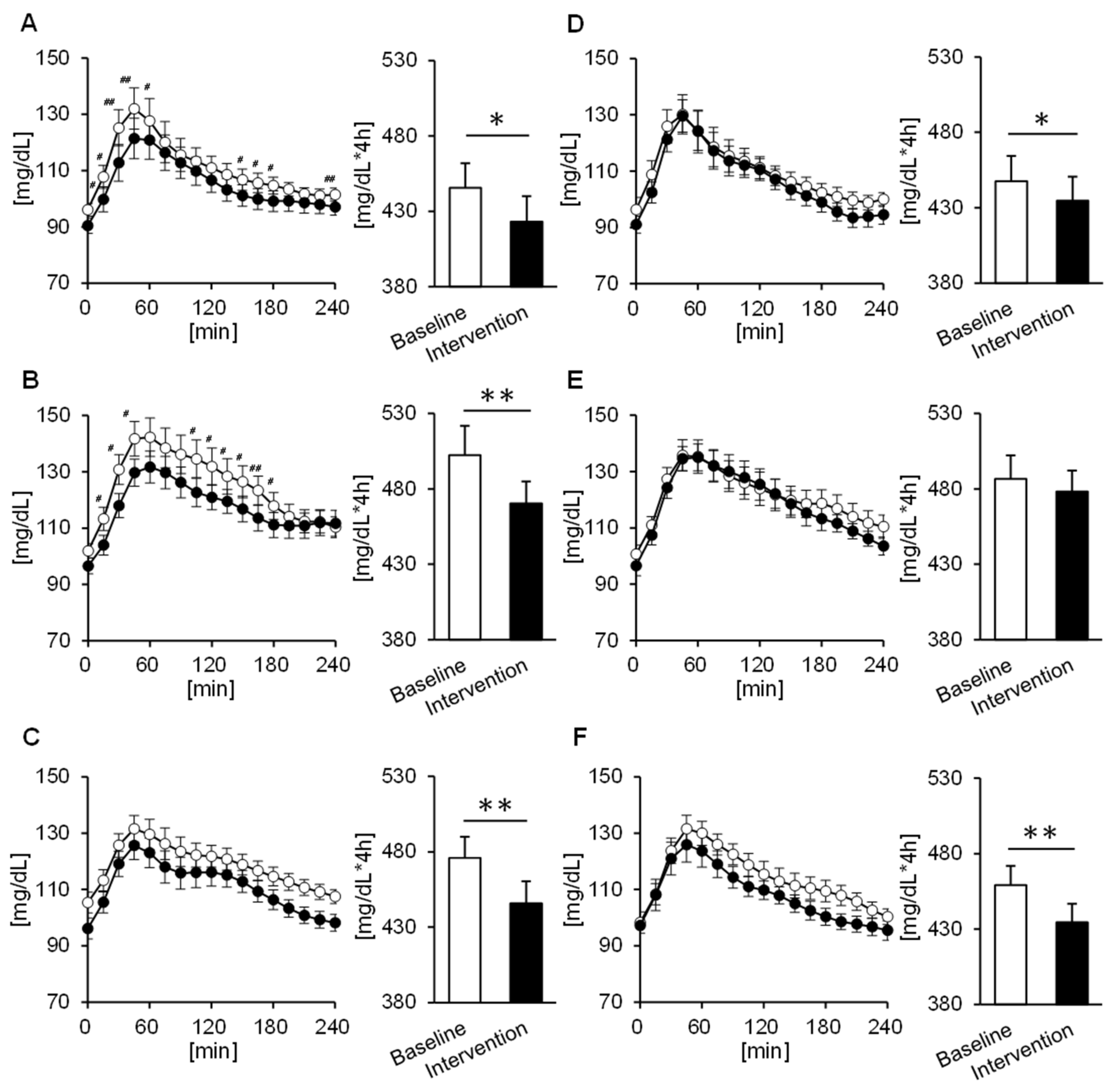
3.3. Comparison of the 24 h Tissue Glucose Level
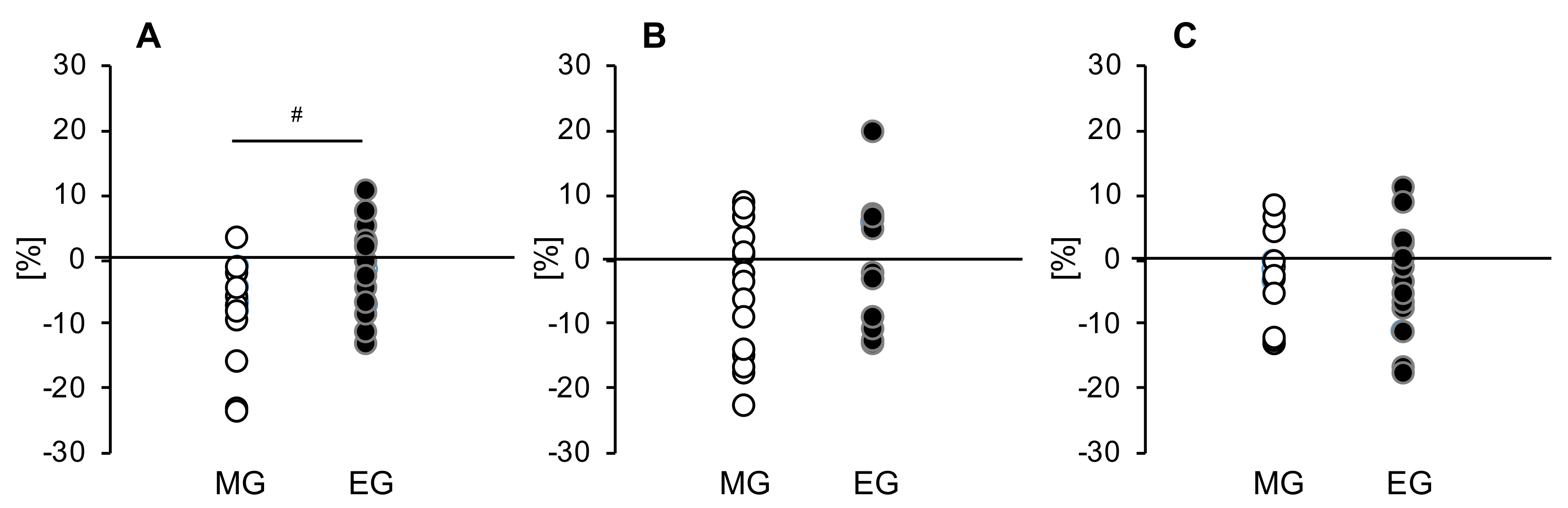
3.4. Comparison of the Tissue Glucose Level 4 h Postprandial
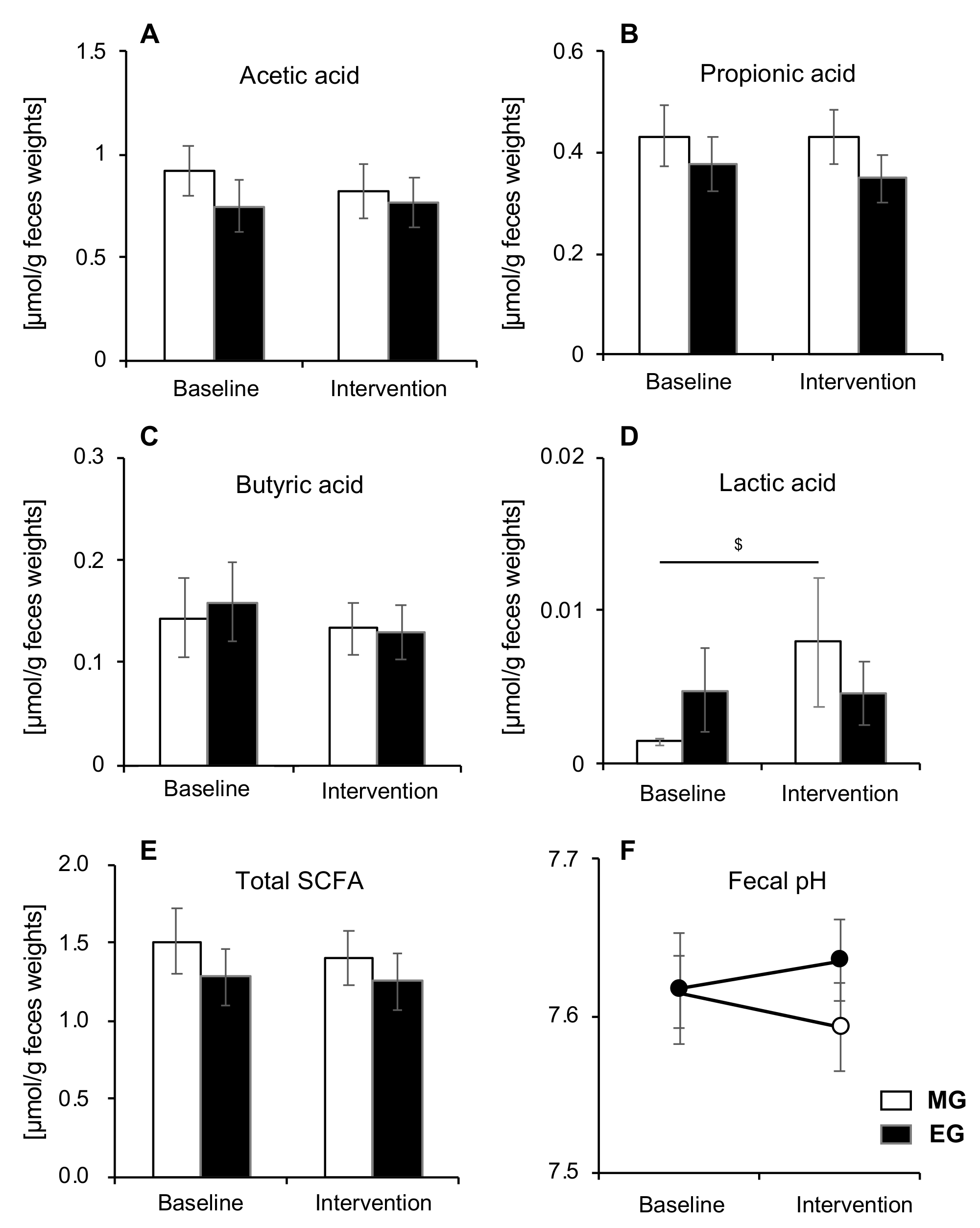
3.5. Comparison of SCFA and pH
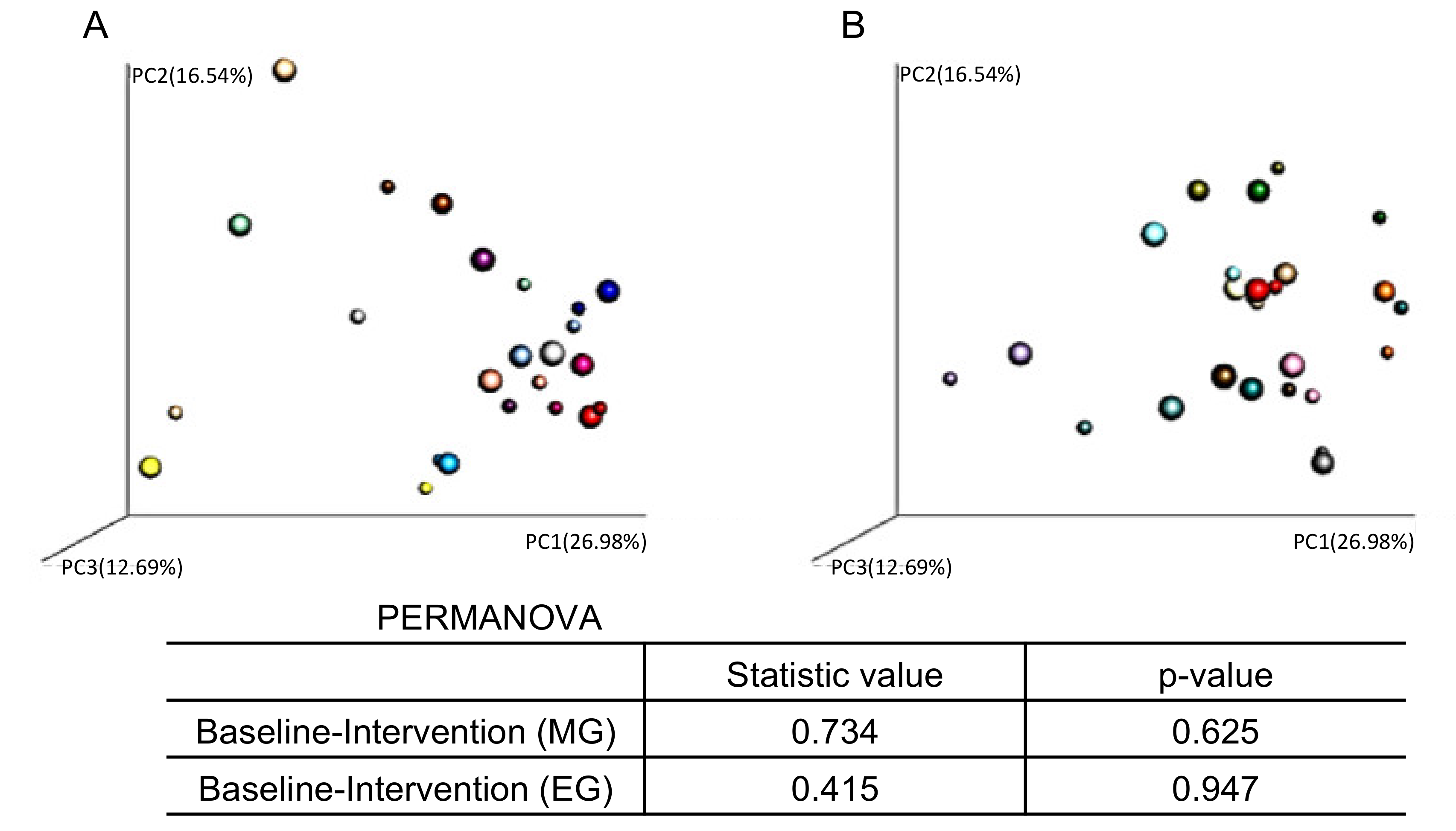
3.6. Relationship between Changes in Tissue Glucose Levels and Changes in Intestinal Microbiota
3.7. Comparison of the Relative Abundance of some Bacteria
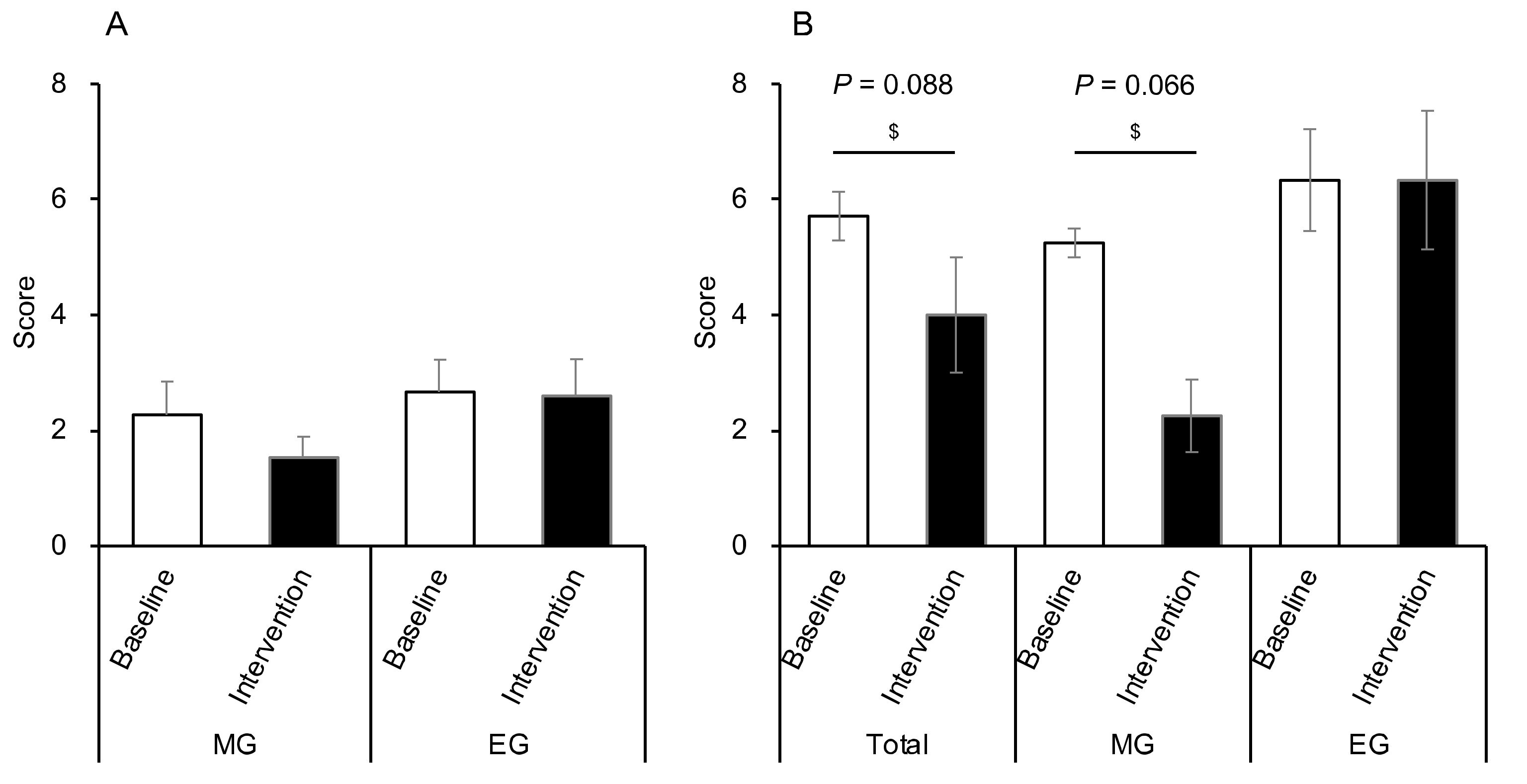
3.8. Comparison of the CAS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; pp. 1–87. [Google Scholar]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Breda, E.; Oberg, A.L.; Powell, C.C.; Dalla Man, C.; Basu, A.; Vittone, J.L.; Klee, G.G.; Arora, P.; Jensen, M.D.; et al. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003, 52, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, T.; Group, D.S. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004, 47, 385–394. [Google Scholar] [CrossRef]
- Decode Study Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- DECODE Study Group; European Diabetes Epidemiology Group. Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. Diabetes epidemiology: Collaborative analysis of diagnostic criteria in Europe. Lancet 1999, 354, 617–621. [Google Scholar] [CrossRef]
- Pan, X.; Hussain, M.M. Clock is important for food and circadian regulation of macronutrient absorption in mice. J. Lipid Res. 2009, 50, 1800–1813. [Google Scholar] [CrossRef]
- Tavakkolizadeh, A.; Ramsanahie, A.; Levitsky, L.L.; Zinner, M.J.; Whang, E.E.; Ashley, S.W.; Rhoads, D.B. Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J. Surg. Res. 2005, 129, 73–78. [Google Scholar] [CrossRef]
- Lindgren, O.; Mari, A.; Deacon, C.F.; Carr, R.D.; Winzell, M.S.; Vikman, J.; Ahrén, B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J. Clin. Endocrinol. Metab. 2009, 94, 2887–2892. [Google Scholar] [CrossRef]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef]
- Bandín, C.; Scheer, F.A.; Luque, A.J.; Ávila-Gandía, V.; Zamora, S.; Madrid, J.A.; Gómez-Abellán, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. (London) 2015, 39, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ozaki, M.; Kang, M.I.; Sasaki, H.; Fukazawa, M.; Iwakami, T.; Lim, P.J.; Kim, H.K.; Aoyama, S.; Shibata, S. Effects of meal timing on postprandial glucose metabolism and blood metabolites in healthy adults. Nutrients 2018, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New horizons for the study of dietary fiber and health: A review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V. Dose-response effects of inulin and oligofructose on intestinal bifidogenesis effects. J. Nutr. 1999, 129, 1442s–1445s. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergstrom, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Backhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabet. Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93 (Suppl 1), S13–S25. [Google Scholar] [CrossRef]
- Brighenti, F.; Casiraghi, M.C.; Canzi, E.; Ferrari, A. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. Eur. J. Clin. Nutr. 1999, 53, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ozaki, M.; Miyashita, M.; Fukazawa, M.; Nakaoka, T.; Wakisaka, T.; Matsui, Y.; Hibi, M.; Osaki, N.; Shibata, S. Effects of timing of acute catechin-rich green tea ingestion on postprandial glucose metabolism in healthy men. J. Nutr. Biochem. 2019, 73, 108221. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ozaki, M.; Tsubosaka, M.; Kim, H.K.; Sasaki, H.; Matsui, Y.; Hibi, M.; Osaki, N.; Miyashita, M.; Shibata, S. Effects of timing of acute and consecutive catechin ingestion on postprandial glucose metabolism in mice and humans. Nutrients 2020, 12, 565. [Google Scholar] [CrossRef]
- Sasaki, H.; Miyakawa, H.; Watanabe, A.; Nakayama, Y.; Lyu, Y.; Hama, K.; Shibata, S. Mice microbiota composition changes by inulin feeding with a long fasting period under a two-meals-per-day schedule. Nutrients 2019, 11, 2802. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Raskine, L.; Simoneau, G.; Paineau, D.; Bornet, F. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: A dose-response relationship study in healthy humans. Nutr. J. 2006, 5, 8. [Google Scholar] [CrossRef]
- Kelly, G. Inulin-type prebiotics: A review. (Part 2). Altern. Med. Rev. 2009, 14, 36–55. [Google Scholar]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Wakai, K. A review of food frequency questionnaires developed and validated in Japan. J. Epidemiol. 2009, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huazano-Garcia, A.; Lopez, G.M. Metabolism of short chain fatty acids in the colon and faeces of mice after a supplementation of diets with agave fructans. In Lipid Metabolism; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- McMillan, S.C.; Williams, F.A. Validity and reliability of the constipation assessment scale. Cancer Nurs. 1989, 12, 183–188. [Google Scholar] [CrossRef]
- Kuwahara, M.; Kim, H.K.; Ozaki, M.; Nanba, T.; Chijiki, H.; Fukazawa, M.; Okubo, J.; Mineshita, Y.; Takahashi, M.; Shibata, S. Consumption of biscuits with a beverage of mulberry or barley leaves in the afternoon prevents dinner-induced high, but not low, increases in blood glucose among young adults. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- A Fletcher, J. The second meal effect and its influence on glycemia. J. Nutr. Dis. Ther. 2012, 2. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Griffiths, C.; Krzeminska, K.; Lawrie, J.A.; Bennett, C.M.; Goff, D.V.; Sarson, D.L.; Bloom, S.R. Slow release dietary carbohydrate improves second meal tolerance. Am. J. Clin. Nutr. 1982, 35, 1339–1346. [Google Scholar] [CrossRef]
- Wolever, T.M.; Bentum-Williams, A.; Jenkins, D.J. Physiological modulation of plasma free fatty acid concentrations by diet. Metabolic implications in nondiabetic subjects. Diabet. Care 1995, 18, 962–970. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-meal effect: Low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Madsbad, S. Exenatide and liraglutide: Different approaches to develop GLP-1 receptor agonists (incretin mimetics)-preclinical and clinical results. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Dukas, L.; Willett, W.C.; Giovannucci, E.L. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am. J. Gastroenterol. 2003, 98, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Betti, C.; Imbimbo, B.P.; Pelli, M.A.; Morelli, A. Colonic motor response to eating: A manometric investigation in proximal and distal portions of the viscus in man. Am. J. Gastroenterol. 1989, 84, 118–122. [Google Scholar] [PubMed]
- Malone, J.C.; Thavamani, A. Physiology, Gastrocolic Reflex (Gastrocolic Response); StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]


| Physical Characteristics and Dietary Intake | All Participants (n = 30) | MG (n = 15) | EG (n = 15) |
|---|---|---|---|
| Age (years) | 74.4 ± 1.0 | 74.3 ± 0.9 | 74.6 ± 1.7 |
| Height (cm) | 158.4 ± 1.6 | 158.1 ± 2.0 | 158.7 ± 2.6 |
| Body Mass (kg) | 55.3 ± 1.9 | 54.9 ± 2.8 | 55.7 ± 2.6 |
| BMI (kg/m2) | 21.8 ± 0.4 | 21.7 ± 0.7 | 21.9 ± 0.6 |
| Muscle Mass (kg) | 21.6 ± 0.9 | 21.3 ± 1.2 | 21.8 ± 1.3 |
| MEQ | 54.2 ± 1.3 | 53.0 ± 2.2 | 55.4 ± 1.5 |
| Energy Intake (kcal/day) | 2379.7 ± 134.7 | 2292.6 ± 112.9 | 2466.9 ± 247.7 |
| Carbohydrate Intake (g/day) | 289.2 ± 14.4 | 288.7 ± 14.5 | 289.7 ± 25.6 |
| Fat Intake (g/day) | 85.4 ± 7.2 | 81.6 ± 5.0 | 89.3 ± 13.6 |
| Protein Intake (g/day) | 87.8 ± 5.0 | 85.7 ± 4.3 | 89.9 ± 9.1 |
| Total Dietary Fiber Quantity (g/day) | 18.0 ± 1.3 | 17.9 ± 1.3 | 18.1 ± 2.3 |
| Water Soluble Dietary Fiber Quantity (g/day) | 4.0 ± 0.2 | 4.0 ± 0.3 | 4.1 ± 0.4 |
| Insoluble Dietary Fiber Quantity (g/day) | 12.6 ± 1.0 | 12.5 ± 0.9 | 12.7 ± 1.8 |
| Glucose Parameters | MG (n = 15) | EG (n = 15) | ||
|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | |
| SD (mg/dL) | 15.9 ± 1.6 | 15.3 ± 1.5 | 16.3 ± 1.3 | 15.2 ± 1.2 |
| CV (%) | 14.6 ± 1.2 | 15.0 ± 1.3 | 15.8 ± 1.3 | 15.3 ± 1.3 |
| Max (mg/dL) | 141.4 ± 5.7 | 134.5 ± 5.5 | 143.9 ± 5.1 | 135.0 ± 5.1 |
| Min (mg/dL) | 84.7 ± 1.8 | 80.1 ± 2.0 | 83.0 ± 2.7 | 81.7 ± 2.5 |
| MAGE (mg/dL) | 56.6 ± 5.2 | 54.4 ± 5.3 | 60.9 ± 5.0 | 53.3 ± 4.6 |
| Bacterial | MG (n = 10) | EG (n = 12) | ||
|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | |
| Bifidobacterium | 0.0592 ± 0.0189 | 0.0790 ± 0.0280 | 0.0902 ± 0.0255 | 0.0871 ± 0.0209 |
| Bacteroides | 0.1302 ± 0.0377 | 0.1287 ± 0.0385 | 0.1778 ± 0.0293 | 0.1267 ± 0.0198 |
| Parabacteroides | 0.0162 ± 0.0028 | 0.0169 ± 0.0045 | 0.0153 ± 0.0046 | 0.0159 ± 0.0064 |
| Lactobacillus | 0.0108 ± 0.0047 | 0.0152 ± 0.0076 | 0.0361 ± 0.0239 | 0.0296 ± 0.0160 |
| Streptococcus | 0.0299 ± 0.0116 | 0.0537 ± 0.0245 | 0.0497 ± 0.0202 | 0.0414 ± 0.0140 |
| Coprococcus | 0.0161 ± 0.0053 | 0.0137 ± 0.0062 | 0.0141 ± 0.0041 | 0.0158 ± 0.0043 |
| Dorea | 0.0098 ± 0.0022 | 0.0121 ± 0.0027 | 0.0129 ± 0.0029 | 0.0154 ± 0.0045 |
| Roseburia | 0.0086 ± 0.0029 | 0.0084 ± 0.0047 | 0.0087 ± 0.0033 | 0.0121 ± 0.0034 |
| [Ruminococcus] | 0.0068 ± 0.0022 | 0.0095 ± 0.0024 | 0.0127 ± 0.0044 | 0.0106 ± 0.0041 |
| Oscillospira | 0.0097 ± 0.0020 | 0.0081 ± 0.0020 | 0.0138 ± 0.0028 | 0.0087 ± 0.0018 |
| Ruminococcus | 0.0592 ± 0.0189 # | 0.0113 ± 0.0045 # | 0.0462 ± 0.0098 | 0.0523 ± 0.0115 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K.; Chijiki, H.; Nanba, T.; Ozaki, M.; Sasaki, H.; Takahashi, M.; Shibata, S. Ingestion of Helianthus tuberosus at Breakfast Rather Than at Dinner is More Effective for Suppressing Glucose Levels and Improving the Intestinal Microbiota in Older Adults. Nutrients 2020, 12, 3035. https://doi.org/10.3390/nu12103035
Kim H-K, Chijiki H, Nanba T, Ozaki M, Sasaki H, Takahashi M, Shibata S. Ingestion of Helianthus tuberosus at Breakfast Rather Than at Dinner is More Effective for Suppressing Glucose Levels and Improving the Intestinal Microbiota in Older Adults. Nutrients. 2020; 12(10):3035. https://doi.org/10.3390/nu12103035
Chicago/Turabian StyleKim, Hyeon-Ki, Hanako Chijiki, Takuya Nanba, Mamiho Ozaki, Hiroyuki Sasaki, Masaki Takahashi, and Shigenobu Shibata. 2020. "Ingestion of Helianthus tuberosus at Breakfast Rather Than at Dinner is More Effective for Suppressing Glucose Levels and Improving the Intestinal Microbiota in Older Adults" Nutrients 12, no. 10: 3035. https://doi.org/10.3390/nu12103035
APA StyleKim, H.-K., Chijiki, H., Nanba, T., Ozaki, M., Sasaki, H., Takahashi, M., & Shibata, S. (2020). Ingestion of Helianthus tuberosus at Breakfast Rather Than at Dinner is More Effective for Suppressing Glucose Levels and Improving the Intestinal Microbiota in Older Adults. Nutrients, 12(10), 3035. https://doi.org/10.3390/nu12103035