Low Selenium Levels in Amniotic Fluid Correlate with Small-For-Gestational Age Newborns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Analysis and Biological Sample Processing
2.3. Statistical Analysis
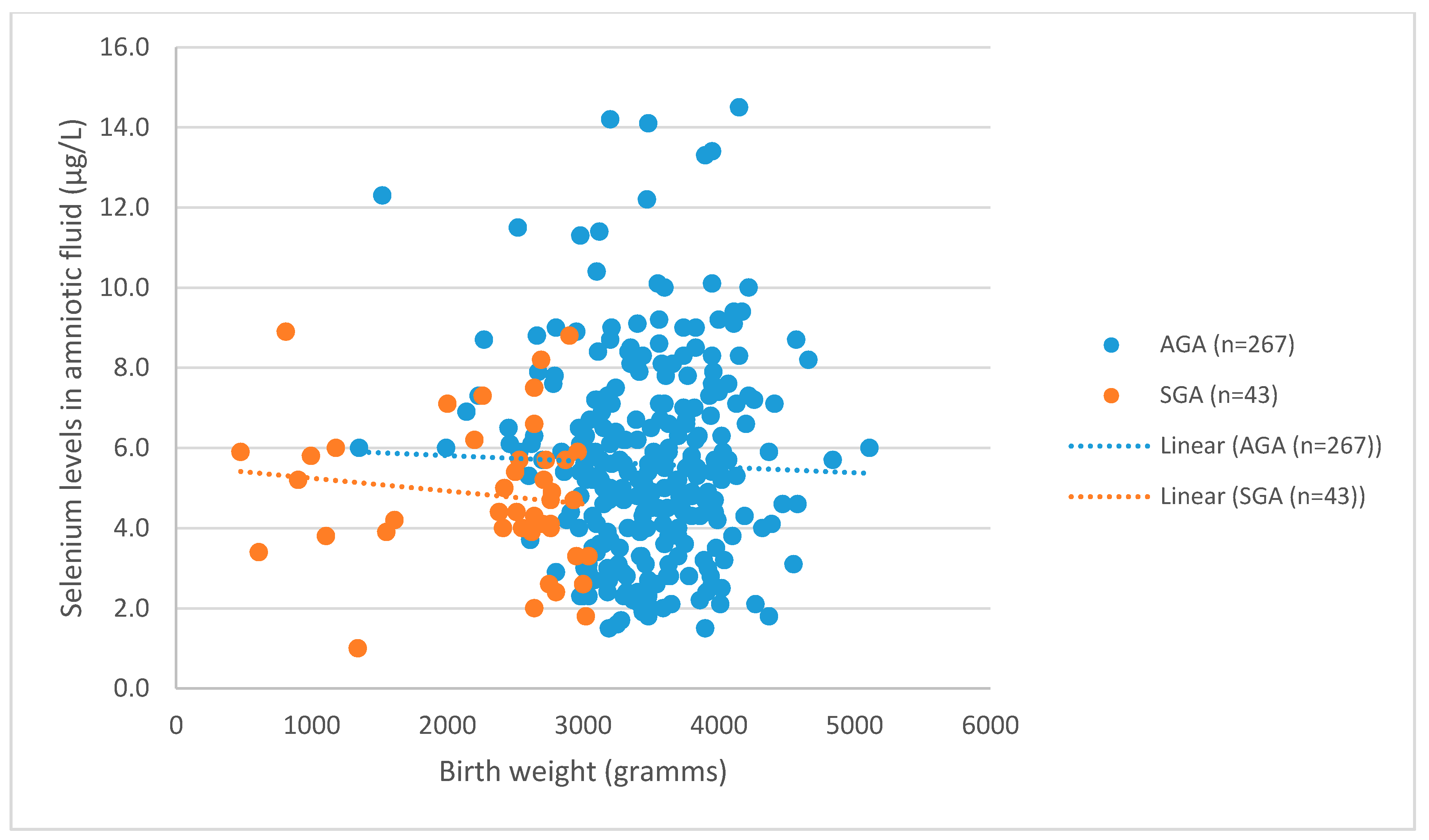
3. Results
3.1. Levels of Se in AGA and SGA
3.2. Pre-Eclampsia and Selenium Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Royal College of Obstetricians and Gynaecologists. The Investigation and Manangement of the Small-for-Gestational-Age Fetus; RCOG Green-top Guideline No. 31; Royal College of Obstetricians and Gynaecologists: London, UK, 2013; pp. 1–34. [Google Scholar]
- Gordijn, S.J.; Beune, I.M.; Ganzevoort, W. Building consensus and standards in fetal growth restriction studies. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, V.; Castorani, V.; Guidone, P.; Derraik, J.G.B.; Liberati, M.; Chiarelli, F.; Mohn, A. Incidence of infants born small- and large-for-gestational-age in an Italian cohort over a 20-year period and associated risk factors. Ital. J. Pediatr. 2016, 42, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. The role of early pregnancy maternal selenium levels on the risk for small-for-gestational age newborns. Nutrients 2019, 11, 2298. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; Syngelaki, A.; Maiz, N.; Zinevich, Y.; Nicolaides, K.H. Maternal age and adverse pregnancy outcome: A cohort study. Ultrasound Obstet. Gynecol. 2013, 42, 634–643. [Google Scholar] [CrossRef]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Malinowski, W.; Szymański, S.; Mularczyk, M.; Tomska, N.; Rotter, I. Interactions between 14 elements in the human placenta, fetal membrane and umbilical cord. Int. J. Environ. Res. Public Health 2019, 16, 1615. [Google Scholar] [CrossRef] [Green Version]
- Lewicka, I.; Kocyłowski, R.; Grzesiak, M.; Gaj, Z.; Oszukowski, P.; Suliburska, J. Selected trace elements concentrations in pregnancy and their possible role—Literature review. Ginekol. Pol. 2017, 88, 509–514. [Google Scholar] [CrossRef]
- Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Grzeskowiak, L.E.; Dekker, G.A.; Roberts, C.T. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J. Trace Elem. Med. Biol. 2018, 46, 103–109. [Google Scholar] [CrossRef]
- Hofstee, P.; Cuffe, J.S.M.; Perkins, A.V. Analysis of selenoprotein expression in response to dietary selenium deficiency during pregnancy indicates tissue specific differential expression in mothers and sex specific changes in the fetus and offspring. Int. J. Mol. Sci. 2020, 21, 2210. [Google Scholar] [CrossRef] [Green Version]
- Na, J.Y.; Seok, J.; Park, S.; Kim, J.S.; Kim, G.J. Effects of selenium on the survival and invasion of trophoblasts. Clin. Exp. Reprod. Med. 2018, 45, 10–16. [Google Scholar] [CrossRef]
- Liu, P.J.; Yao, A.; Ma, L.; Chen, X.Y.; Yu, S.L.; Liu, Y.; Hou, Y.X. Associations of Serum Selenium Levels in the First Trimester of Pregnancy with the Risk of Gestational Diabetes Mellitus and Preterm Birth: A Preliminary Cohort Study. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.G.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogden, J.D.; Kemp, F.W.; Chen, X.; Stagnaro-Green, A.; Stein, T.P.; Scholl, T.O. Low-normal serum selenium early in human pregnancy predicts lower birth weight. Nutr. Res. 2006, 26, 497–502. [Google Scholar] [CrossRef]
- Mihailovič, M.; Cvetkovč, M.; Ljubič, A.; Kosanovič, M.; Nedeljkovič, S.; Jovanovič, I.; Pešut, O. Selenium and Malondialdehyde Content and Glutathione Peroxidase Activity in Maternal and Umbilical Cord Blood and Amniotic Fluid. Biol. Trace Elem. Res. 2000, 73, 47–54. [Google Scholar] [CrossRef]
- Rayman, M.P.; Bode, P.; Redman, C.W. Low selenium status is associated with the occurrence of the pregnancy disease preeclampsia in women from the United Kingdom. Am. J. Obstet. Gynecol. 2003, 189, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Mistry, H.D.; Wilson, V.; Ramsay, M.M.; Symonds, M.E.; Pipkin, F.B. Reduced Selenium Concentrations and Glutathione Peroxidase Activity in Preeclamptic Pregnancies. Hypertension 2008, 52, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Everson, T.M.; Kappil, M.; Hao, K.; Jackson, B.P.; Punshon, T.; Karagas, M.R.; Chen, J.; Marsit, C.J. Maternal exposure to selenium and cadmium, fetal growth, and placental expression of steroidogenic and apoptotic genes. Environ. Res. 2017, 158, 233–244. [Google Scholar] [CrossRef]
- Hofstee, P.; Bartho, L.A.; McKeating, D.R.; Radenkovic, F.; McEnroe, G.; Fisher, J.J.; Holland, O.J.; Vanderlelie, J.J.; Perkins, A.V.; Cuffe, J.S.M. Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J. Physiol. 2019, 597, 5597–5617. [Google Scholar] [CrossRef]
- Richard, K.; Holland, O.; Landers, K.; Vanderlelie, J.J.; Hofstee, P.; Cuffe, J.S.M.; Perkins, A.V. Review: Effects of maternal micronutrient supplementation on placental function. Placenta 2017, 54, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Tindell, R.; Tipple, T. Selenium: Implications for outcomes in extremely preterm infants. J. Perinatol. 2018, 38, 197–202. [Google Scholar] [CrossRef]
- Zachara, B.A. Selenium in Complicated Pregnancy. A Review. Adv. Clin. Chem. 2018, 86, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Hubel, C.A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 1999, 222, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Šikić Pogačar, M.; Mičetić-Turk, D. Vitamin D in Human Health. Acta Medico-Biotech. 2017, 10, 12–24. [Google Scholar]
- Fujimoto, V.Y.; Bloom, M.S.; Huddleston, H.G.; Shelley, W.B.; Ocque, A.J.; Browne, R.W. Correlations of follicular fluid oxidative stress biomarkers and enzyme activities with embryo morphology parameters during in vitro fertilization. Fertil. Steril. 2011, 96, 1357–1361. [Google Scholar] [CrossRef]
- Asemi, Z.; Jazayeri, S.; Najafi, M.; Samimi, M.; Mofid, V.; Shidfar, F.; Shakeri, H.; Esmaillzadeh, A. Effect of Daily Consumption of Probiotic Yogurt on Oxidative Stress in Pregnant Women: A Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2012, 60, 62–68. [Google Scholar] [CrossRef]
- Toblli, J.E.; Cao, G.; Oliveri, L.; Angerosa, M. Effects of iron deficiency anemia and its treatment with iron polymaltose complex in pregnant rats, their fetuses and placentas: Oxidative stress markers and pregnancy outcome. Placenta 2012, 33, 81–87. [Google Scholar] [CrossRef]
- Yust-Katz, S.; Fisher-Shoval, Y.; Barhum, Y.; Ben-Zur, T.; Barzilay, R.; Lev, N.; Hod, M.; Melamed, E.; Offen, D. Placental mesenchymal stromal cells induced into neurotrophic factor-producing cells protect neuronal cells from hypoxia and oxidative stress. Cytotherapy 2012, 14, 45–55. [Google Scholar] [CrossRef]
- Perkins, A.V. Placental oxidative stress, selenium and preeclampsia. Pregnancy Hypertens. 2011, 1, 95–99. [Google Scholar] [CrossRef]
- Zachara, B.A.; Dobrzynski, W.; Trafikowska, U.; Szymanski, W. Blood selenium and glutathione peroxidases in miscarriage. BJOG 2001, 108, 244–247. [Google Scholar] [CrossRef]
- Punshon, T.; Li, Z.; Jackson, B.P.; Parks, W.T.; Romano, M.; Conway, D.; Baker, E.R.; Karagas, M.R. Placental metal concentrations in relation to placental growth, efficiency and birth weight. Environ. Int. 2019, 126, 533–542. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Carreras, O.; Díaz-Castro, J.; Murillo, M.L.; Nogales, F. High- and low- selenium diets affect endocrine energy balance during early programming. Toxicol. Appl. Pharmacol. 2019, 382, 114744. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Nogales, F.; Membrilla, A.; Carreras, O. Maternal selenium status is profoundly involved in metabolic fetal programming by modulating insulin resistance, oxidative balance and energy homeostasis. Eur. J. Nutr. 2019, 58, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Nogales, F.; Serrano, A.; Murillo, M.L.; Carreras, O. Maternal metabolic syndrome and selenium: Endocrine energy balance during early programming. Life Sci. 2019, 233, 116689. [Google Scholar] [CrossRef] [PubMed]
- Jalali, L.M.; Koski, K.G. Amniotic fluid minerals, trace elements, and prenatal supplement use in humans emerge as determinants of fetal growth. J. Trace Elem. Med. Biol. 2018, 50, 139–145. [Google Scholar] [CrossRef]
- Yıldırım, E.; Derici, M.K.; Demir, E.; Apaydın, H.; Koçak, Ö.; Kan, Ö.; Görkem, Ü. Is the Concentration of Cadmium, Lead, Mercury, and Selenium Related to Preterm Birth? Biol. Trace Elem. Res. 2019, 191, 306–312. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.H.; Yoon, B.S.; Jun, E.K.; Lee, G.; Kim, I.Y.; You, S. Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells 06 Biological Sciences 0601 Biochemistry and Cell Biology. Stem Cell Res. Ther. 2018, 9, 293. [Google Scholar] [CrossRef]
- Verdenik, I. Slovene reference standards for weight, length and head circumference at birth for given gestational age of population born in years 1987–96. Zdravniski Vestnik 2000, 69, 153–156. [Google Scholar]
- Kramer, M.S.; McLean, F.H.; Olivier, M.; Willis, D.M.; Usher, R.H. Body proportionality and head and length “sparing” in growth-retarded neonates: A critical reappraisal. Pediatrics 1989, 84, 717–723. [Google Scholar]
- Bizerea, T.; Dezsi, S.; Marginean, O.; Stroescu, R.; Rogobete, A.; Bizerea-Spiridon, O.; Ilie, C. The Link Between Selenium, Oxidative Stress and Pregnancy Induced Hypertensive Disorders. Clin. Lab. 2018, 64, 1593–1610. [Google Scholar] [CrossRef]
- Klapec, T.; Ćavar, S.; Kasač, Z.; Ručević, S.; Popinjač, A. Selenium in placenta predicts birth weight in normal but not intrauterine growth restriction pregnancy. J. Trace Elem. Med. Biol. 2008, 22, 54–58. [Google Scholar] [CrossRef]
- Fall, C.H.D.; Yajnik, C.S.; Rao, S.; Davies, A.A.; Brown, N.; Farrant, H.J.W. Micronutrients and Fetal Growth. J. Nutr. 2003, 133, 1747S–1756S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadrożna, M.; Gawlik, M.; Nowak, B.; Marcinek, A.; Mrowiec, H.; Walas, S.; Wietecha-Posłuszny, R.; Zagrodzki, P. Antioxidants activities and concentration of selenium, zinc and copper in preterm and IUGR human placentas. J. Trace Elem. Med. Biol. 2009, 23, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Llanos, M.N.; Ronco, A.M. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod. Toxicol. 2009, 27, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Strambi, M.; Longini, M.; Vezzosi, P.; Berni, S.; Buoni, S. Selenium status, birth weight, and breast-feeding: Pattern in the first month. Biol. Trace Elem. Res. 2004, 99, 71–81. [Google Scholar] [CrossRef]
- Atamer, Y.; Koçyigit, Y.; Yokus, B.; Atamer, A.; Erden, A.C. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 119, 60–66. [Google Scholar] [CrossRef]
- Maleki, A.; Fard, M.K.; Zadeh, D.H.; Mamegani, M.A.; Abasaizadeh, S.; Mazloomzadeh, S. The Relationship between Plasma Level of Se and Preeclampsia. Hypertens. Pregnancy 2011, 30, 180–187. [Google Scholar] [CrossRef]
- Dawson, E.B.; Evans, D.R.; Nosovitch, J. Third-Trimester Amniotic Fluid Metal Levels Associated with Preeclampsia. Arch. Environ. Health An Int. J. 1999, 54, 412–415. [Google Scholar] [CrossRef]
- Vanderlelie, J.; Venardos, K.; Clifton, V.L.; Gude, N.M.; Clarke, F.M.; Perkins, A.V. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005, 26, 53–58. [Google Scholar] [CrossRef]
- Roy, A.C.; Ratnam, S.S.; Karunanithy, R. Amniotic Fluid Selenium Status in Pre-Eclampsia. Gynecol. Obstet. Invest. 1989, 28, 161–162. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Martins-Costa, S.H.; Valério, E.G.; Lopes Ramos, J.G. Comparison of serum selenium levels among hypertensive and normotensive pregnant women. Hypertens. Pregnancy 2017, 36, 64–69. [Google Scholar] [CrossRef]
- Xu, M.; Guo, D.; Gu, H.; Zhang, L.; Lv, S. Selenium and Preeclampsia: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2016, 171, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, R.; Zandian, A.; Mohammadizadeh, M.; Mohammadzadeh, A.; Balali-Mood, M.; Hajiheydari, M. Comparison of maternal and umbilical cord blood selenium levels in term and preterm infants. Zhongguo Dang Dai Er Ke Za Zhi 2009, 11, 513–516. [Google Scholar] [PubMed]
- Tsuji, M.; Shibata, E.; Morokuma, S.; Tanaka, R.; Senju, A.; Araki, S.; Sanefuji, M.; Koriyama, C.; Yamamoto, M.; Ishihara, Y.; et al. The association between whole blood concentrations of heavy metals in pregnant women and premature births: The Japan Environment and Children’s Study (JECS). Environ. Res. 2018, 166, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, I.; Kocyłowski, R.; Grzesiak, M.; Gaj, Z.; Sajnóg, A.; Barałkiewicz, D.; von Kaisenberg, C.; Suliburska, J. Relationship between pre-pregnancy body mass index and mineral concentrations in serum and amniotic fluid in pregnant women during labor. J. Trace Elem. Med. Biol. 2019, 52, 136–142. [Google Scholar] [CrossRef]
- Caserta, D.; Mantovani, A.; Ciardo, F.; Fazi, A.; Baldi, M.; Sessa, M.T.; la Rocca, C.; Ronchi, A.; Moscarini, M.; Minoia, C. Heavy metals in human amniotic fluid: A pilot study. Prenat. Diagn. 2011, 31, 792–796. [Google Scholar] [CrossRef]
- Han, L.; Zhou, S.M. Selenium supplement in the prevention of pregnancy induced hypertension. Chin. Med. J. 1994, 107, 870–871. [Google Scholar]
- Mesdaghinia, E.; Rahavi, A.; Bahmani, F.; Sharifi, N.; Asemi, Z. Clinical and Metabolic Response to Selenium Supplementation in Pregnant Women at Risk for Intrauterine Growth Restriction: Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2017, 178, 14–21. [Google Scholar] [CrossRef]
- Tara, F.; Rayman, M.P.; Boskabadi, H.; Ghayour-Mobarhan, M.; Sahebkar, A.; Yazarlu, O.; Ouladan, S.; Tavallaie, S.; Azimi-Nezhad, M.; Shakeri, M.T.; et al. Selenium supplementation and premature (pre-labour) rupture of membranes: A randomised double-blind placebo-controlled trial. J. Obstet. Gynaecol. 2010, 30, 30–34. [Google Scholar] [CrossRef]
- Mistry, H.D.; Kurlak, L.O.; Young, S.D.; Briley, A.L.; Broughton Pipkin, F.; Baker, P.N.; Poston, L. Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Matern. Child Nutr. 2014, 10, 327–334. [Google Scholar] [CrossRef]
- Mičetić-Turk, D.; Rossipal, E.; Krachler, M.; Li, F. Maternal selenium status in Slovenia and its impact on the selenium concentration of umbilical cord serum and colostrum. Eur. J. Clin. Nutr. 2000, 54, 522–524. [Google Scholar] [CrossRef] [Green Version]
- Heazell, A.E.; Whitworth, M.; Duley, L.; Thornton, J.G. Use of biochemical tests of placental function for improving pregnancy outcome. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeating, D.R.; Clifton, V.L.; Hurst, C.P.; Fisher, J.J.; Bennett, W.W.; Perkins, A.V. Elemental Metabolomics for Prediction of Term Gestational Outcomes Utilising 18-Week Maternal Plasma and Urine Samples. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.M.; Keil, A.P.; van ’t Erve, T.J.; Deterding, L.J.; Williams, J.G.; Lih, F.B.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Longitudinal profiles of plasma eicosanoids during pregnancy and size for gestational age at delivery: A nested case-control study. PLoS Med. 2020, 17, e1003271. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Galiano, J.M.; Amezcua-Prieto, C.; Cano-Ibañez, N.; Olmedo-Requena, R.; Jiménez-Moleón, J.J.; Bueno-Cavanillas, A.; Delgado-Rodríguez, M. Diet as a counteracting agent of the effect of some well-known risk factors for small for gestational age. Nutrition 2020, 72, 110665. [Google Scholar] [CrossRef]


| Characteristics | AGA (n = 267) | SGA (n = 43) | p-Value |
|---|---|---|---|
| Maternal age (year) | 35.9 ± 4.2 | 37.1 ± 4.5 | p = 0.11 (t = 1.59) |
| Nulliparity | 24.3% (n = 65) | 30.2% (n = 13) | p = 0.411 (t = 0.779) |
| Preterm birth | 6.3% (n = 17) | 27.9% (n = 12) | p = 0.006 (t = 1.65) |
| Boys Girls | 55.4% (n = 148) 44.6% (n = 119) | 34.8% (n = 15) 65.2% (n = 28) | p = 0.011 (t = 2.63) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogrizek-Pelkič, K.; Sobočan, M.; Takač, I. Low Selenium Levels in Amniotic Fluid Correlate with Small-For-Gestational Age Newborns. Nutrients 2020, 12, 3046. https://doi.org/10.3390/nu12103046
Ogrizek-Pelkič K, Sobočan M, Takač I. Low Selenium Levels in Amniotic Fluid Correlate with Small-For-Gestational Age Newborns. Nutrients. 2020; 12(10):3046. https://doi.org/10.3390/nu12103046
Chicago/Turabian StyleOgrizek-Pelkič, Ksenija, Monika Sobočan, and Iztok Takač. 2020. "Low Selenium Levels in Amniotic Fluid Correlate with Small-For-Gestational Age Newborns" Nutrients 12, no. 10: 3046. https://doi.org/10.3390/nu12103046
APA StyleOgrizek-Pelkič, K., Sobočan, M., & Takač, I. (2020). Low Selenium Levels in Amniotic Fluid Correlate with Small-For-Gestational Age Newborns. Nutrients, 12(10), 3046. https://doi.org/10.3390/nu12103046







