Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Energy Requirements
2.1. Optimal Weight Gain
2.2. Energy Requirements for Normal or Underweight Women
2.3. Energy Requirements for Women with Overweight or with Excessive Gestational Weight Gain
2.4. Summary, Energy Requirements
3. Carbohydrates
3.1. Low-Carbohydrate Diets
3.2. Dietary Fibres
3.3. Low Glycaemic Index Diets
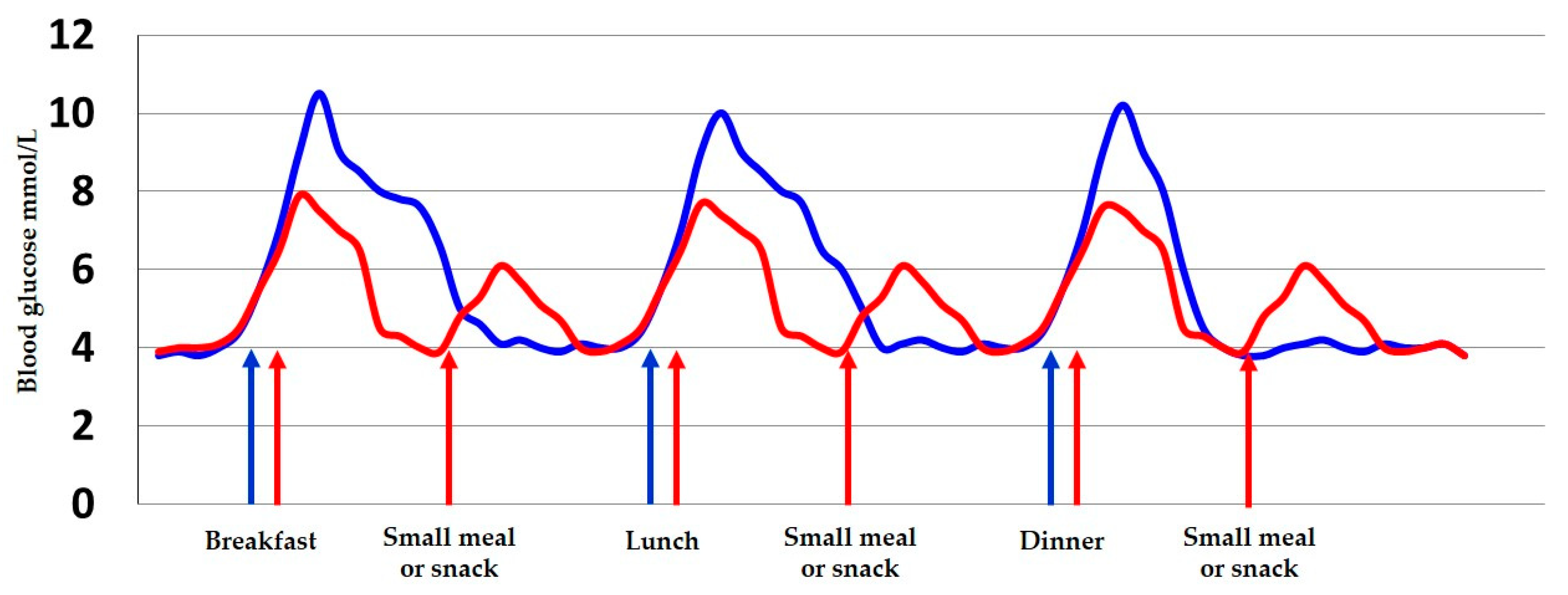
3.4. Meal Frequency and Carbohydrate Distribution
3.5. Artificial Sweeteners
3.6. Summary, Carbohydrates
4. Protein
4.1. Protein Metabolism in GDM
4.2. Protein, the Placenta and GDM
4.3. Plant vs. Animal Protein
4.4. High Protein Supplementation
4.5. Pre-Meals and GDM
4.6. Summary, Protein
5. Fat
5.1. Saturated Fatty Acids
5.2. Monounsaturated Fatty Acids
5.3. Polyunsaturated Fatty Acids
5.4. Summary, Fat
6. Vitamins, Minerals and Tracers
6.1. Vitamin B9/Folic Acid
6.2. 25-Hydroxyvitamin D
6.3. Calcium
6.4. Iron
7. Probiotics
Summary, Probiotics
8. Nutrition Counselling
9. Physical Activity
9.1. Short Term Effects of Physical Activity in Pregnancy on Maternal Blood Glucose Levels
9.2. Longer-Term Effects of Physical Activity
9.3. Recommendations for Exercise in GDM Pregnancy
9.4. Societal Interventions
9.5. Hindrances to Exercise in Pregnancy
9.6. Summary, Physical Activity
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Amino acids |
| AS | Artificial sweeteners |
| ASB | Artificially sweetened beverages |
| BCAA | Branch chained amino acids |
| BMI | Body mass index |
| E% | Energy percent |
| FPG | Fasting plasma glucose |
| GDM | Gestational diabetes mellitus |
| GI | Glycaemic index |
| GL | Glycaemic load |
| GWG | Gestational weight gain |
| HOMA-IR | Homeostatic Model Assessment for insulin Resistance |
| IOM | Institute of Medicine |
| LGA | Large for gestational age |
| MUFA | Monounsaturated fatty acid |
| NGTP | Normal glucose tolerance pregnancies |
| NNR | Nordic Nutrition Recommendations |
| NNS | Non-Nutritive sweeteners |
| PA | Physical activity coefficient |
| PAL | Physical activity level |
| PUFA | Polyunsaturated fatty acid |
| T2DM | Type 2 diabetes mellitus |
References
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K.; Jayaprakash Murthy, D.S. Normal Pregnancy—A State of Insulin Resistance. J. Clin. Diagn. Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Hernández, M.; Bergua, M.; Alvarez, M.C.; Arce, M.A.; Rodriguez, K.; Martinez-Alonso, M.; Iglesias, M.; Mateu, M.; Santos, M.D.; et al. Low-Carbohydrate Diet for the Treatment of Gestational Diabetes Mellitus. Diabetes Care 2013, 36, 2233–2238. [Google Scholar] [CrossRef]
- Ovesen, P.; Fuglsang, J.; Andersen, M.B.; Wolff, C.; Petersen, O.B.; McIntyre, H.D. Temporal Trends in Gestational Diabetes Prevalence, Treatment, and Outcomes at Aarhus University Hospital, Skejby, between 2004 and 2016. J. Diabetes Res. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kurtzhals, L.L.; Nørgaard, S.K.; Secher, A.L.; Nichum, V.L.; Ronneby, H.; Tabor, A.; Simmons, D.; Damm, P.; Mathiesen, E.R. The impact of restricted gestational weight gain by dietary intervention on fetal growth in women with gestational diabetes mellitus. Diabetologia 2018, 61, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; Van Der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational Diabetes Mellitus and Diet: A Systematic Review and Meta-analysis of Randomized Controlled Trials Examining the Impact of Modified Dietary Interventions on Maternal Glucose Control and Neonatal Birth Weight. Diabetes Care 2018, 41, 1346–1361. [Google Scholar] [CrossRef]
- American Diabetes Association 13. Management of Diabetes in Pregnancy. Diabetes Care 2017, 40, S114–S119. [Google Scholar] [CrossRef]
- Yaktine, A.L.; Rasmussen, K.M.; Youth, F.; National Research Council; Institute of Medicine; Board on Children; Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines (2009); Rasmussen, K.M., Yaktine, A.L., Eds.; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group; Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.-A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; et al. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [CrossRef]
- American Diabetes Association 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2020. Diabetes Care 2019, 43, S183–S192. [Google Scholar] [CrossRef]
- Nordic Nutrition of Ministers. Nordic Nutrition Recommendations 2012, 5th ed.; Norden: Copenhagen, Denmark, 2014; pp. 1–629. [Google Scholar]
- Butte, N.F.; King, J.C. Energy requirements during pregnancy and lactation. Public Healt Nutr. 2005, 8, 1010–1027. [Google Scholar] [CrossRef]
- Mottola, M.F.; Artal, R. Fetal and maternal metabolic responses to exercise during pregnancy. Early Hum. Dev. 2016, 94, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Viecceli, C.; Remonti, L.; Hirakata, V.; Mastella, L.; Gnielka, V.; Oppermann, M.; Silveiro, S.; Reichelt, A. Weight gain adequacy and pregnancy outcomes in gestational diabetes: A meta-analysis. Obes. Rev. 2017, 18, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J. Lifestyle modifications for diabetes management. Endocrinol. Metab. Clin. N. Am. 1997, 26, 499–510. [Google Scholar] [CrossRef]
- Peterson, C.M.; Jovanovic-Peterson, L. Percentage of Carbohydrate and glycemic Response to Breakfast, Lunch, and Dinner in Women with Gestational Diabetes. Diabetes 1991, 40, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W. Placental-Fetal Glucose Exchange and Fetal Glucose Metabolism. Trans. Am. Clin. Clim. Assoc. 2006, 117, 321–340. [Google Scholar]
- American Diabetes Association; Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; et al. Nutrition Recommendations and Interventions for Diabetes: A position statement of the American Diabetes Association. Diabetes Care 2007, 31 (Suppl. 1), S61–S78. [Google Scholar] [CrossRef]
- Nutrition Subcommittee of the Diabetes Care Advisory Committee of Diabetes UK. The implementation of nutritional advice for people with diabetes. Diabet. Med. 2003, 20 (Suppl. 2), 786–807. [Google Scholar] [CrossRef] [PubMed]
- Tamás, G.; Kerényi, Z. Gestational diabetes: Current aspects on pathogenesis and treatment. Exp. Clin. Endocrinol. Diabetes 2001, 109, 400–411. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Van Pelt, R.E.; Anderson, M.A.; Daniels, L.J.; West, N.A.; Donahoo, W.T.; Friedman, J.E.; Barbour, L.A. A Higher-Complex Carbohydrate Diet in Gestational Diabetes Mellitus Achieves Glucose Targets and Lowers Postprandial Lipids: A Randomized Crossover Study. Diabetes Care 2014, 37, 1254–1262. [Google Scholar] [CrossRef]
- Augustin, L.S.; Kendall, C.; Jenkins, D.; Willett, W.; Astrup, A.; Barclay, A.; Björck, I.; Brand-Miller, J.; Brighenti, F.; Buyken, A.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Moses, R.G.; Barker, M.; Winter, M.; Petocz, P.; Brand-Miller, J.C. Can a Low–Glycemic Index Diet Reduce the Need for Insulin in Gestational Diabetes Mellitus? Diabetes Care 2009, 32, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ye, S. Influence of low-glycemic index diet for gestational diabetes: A meta-analysis of randomized controlled trials. J. Matern. Neonatal Med. 2018, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Middleton, P.; Shepherd, E.; Van Ryswyk, E.; Crowther, A.C. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst. Rev. 2017, 2017, CD009275. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Vuksan, V.; Faulkner, D.; Augustin, L.S.; Mitchell, S.; Ireland, C.; Srichaikul, K.; Mirrahimi, A.; Chiavaroli, L.; et al. Effect of Lowering the Glycemic Load With Canola Oil on Glycemic Control and Cardiovascular Risk Factors: A Randomized Controlled Trial. Diabetes Care 2014, 37, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.P.; Herath, R.P.; Wickremasinghe, R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women—A community based retrospective cohort study. PLoS ONE 2017, 12, e0179647. [Google Scholar] [CrossRef]
- Bao, J.; Atkinson, F.; Petocz, P.; Willett, W.C.; Brand-Miller, J.C. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: Glycemic load compared with carbohydrate content alone. Am. J. Clin. Nutr. 2011, 93, 984–996. [Google Scholar] [CrossRef]
- Lv, S.; Yu, S.; Chi, R.; Wang, D. Effects of nutritional nursing intervention based on glycemic load for patient with gestational diabetes mellitus. Ginekol. Polska 2019, 90, 46–49. [Google Scholar] [CrossRef]
- Ovesen, P.; Damm, P.; Renault, K.; Holm, A.M.; Wolff, C.; Knold, B.; Pagh Jensen, B.; Møller, M.; Svare, J.; Bødker, B.; et al. Sandbjerg 2007—GUIDELINE. Behandling af Gestationel Diabetes Mellitus. 2007. Available online: http://gynobsguideline.dk/wp/wp-content/uploads/2013/02/GDM-Sandbjerg-2014-godkendt-2014.pdf (accessed on 17 July 2020).
- Rasmussen, L.; Christensen, M.L.; Poulsen, C.W.; Rud, C.; Christensen, A.S.; Andersen, J.; Kampmann, U.; Ovesen, P. Effect of High Versus Low Carbohydrate Intake in the Morning on Glycemic Variability and Glycemic Control Measured by Continuous Blood Glucose Monitoring in Women with Gestational Diabetes Mellitus—A Randomized Crossover Study. Nutritiens 2020, 12, 475. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Figueroa, J.; Rother, I.K.; I Goran, M.; Welsh, J.A. Trends in Low-Calorie Sweetener Consumption Among Pregnant Women in the United States. Curr. Dev. Nutr. 2019, 3, nzz004. [Google Scholar] [CrossRef]
- Skreden, M.; Bere, E.; Sagedal, L.R.; Vistad, I.; Øverby, N.C. Changes in beverage consumption from pre-pregnancy to early pregnancy in the Norwegian Fit for Delivery study. Public Health Nutr. 2014, 18, 1187–1196. [Google Scholar] [CrossRef]
- Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States. Available online: https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states (accessed on 20 August 2020).
- Food Additives. Available online: http://www.efsa.europa.eu/en/topics/topic/food-additives (accessed on 20 August 2020).
- Palatnik, A.; Moosreiner, A.; Stichelen, S.O.-V. Consumption of non-nutritive sweeteners during pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Statement of EFSA on the scientific evaluation of two studies related to the safety of artificial sweeteners. EFSA J. 2011, 9, 2089. [CrossRef]
- Zhu, Y.; Olsen, S.F.; Mendola, P.; Halldorsson, T.I.; Rawal, S.; Hinkle, S.N.; Yeung, E.; Chavarro, J.E.; Grunnet, L.G.; Granström, C.; et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. Int. J. Epidemiol. 2017, 46, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Tserng, K.-Y.; Gilfillan, C.; Dierker, L.J. Metabolism of urea and glucose in normal and diabetic pregnancy. Metabolism 1982, 31, 824–833. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Denne, S.C.; Patel, D.M.; Nuamah, I.F.; Savin, S.M. Leucine kinetics during a brief fast in diabetes in pregnancy. Metabolism 1994, 43, 378–384. [Google Scholar] [CrossRef]
- Zimmer, D.M.; Golichowski, A.M.; Karn, C.A.; Brechtel, G.; Baron, A.D.; Denne, S.C. Glucose and Amino Acid Turnover in Untreated Gestational Diabetes. Diabetes Care 1996, 19, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-C.; Ho, L.-F.; Lao, T. Nutritional intake and placental size in gestational diabetic pregnancies—A preliminary observation. Placenta 2003, 24, 985–988. [Google Scholar] [CrossRef]
- Ghadimi, H.; Pecora, P. Free Amino Acids of Cord Plasma as Compared with Maternal Plasma During Pregnancy. Pediatrics 1964, 33, 500–506. [Google Scholar]
- Kuruvilla, A.G.; D’Souza, S.W.; Glazier, J.D.; Mahendran, D.; Maresh, M.J.; Sibley, C.P. Altered activity of the system a amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J. Clin. Investig. 1994, 94, 689–695. [Google Scholar] [CrossRef]
- Dicke, J.M.; Henderson, G.I. Placental Amino Acid Uptake in Normal and Complicated Pregnancies. Am. J. Med. Sci. 1988, 295, 223–227. [Google Scholar] [CrossRef]
- Jansson, T.; Ekstrand, Y.; Björn, C.; Wennergren, M.; Powell, T.L. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 2002, 51, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Phelps, R.L.; Freinkel, N.; Navickas, I.A. Effects of Gestational Diabetes on Diurnal Profiles of Plasma Glucose, Lipids, and Individual Amino Acids. Diabetes Care 1980, 3, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoff, R.; Kandaraki, E.; Morrow, P.; Mitchell, T.; Kelber, S.; Borkowf, H. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic pregnant women. Metabolism 1988, 37, 234–239. [Google Scholar] [CrossRef]
- Kadakia, R.; Talbot, O.; Kuang, A.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. Cord Blood Metabolomics: Association with Newborn Anthropometrics and C-Peptide Across Ancestries. J. Clin. Endocrinol. Metab. 2019, 104, 4459–4472. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Rifas-Shiman, S.L.; McCulloch, S.; Chatzi, L.; Mantzoros, C.; Hivert, M.-F.; Oken, E. Associations of cord blood metabolites with perinatal characteristics, newborn anthropometry, and cord blood hormones in project viva. Metabolism 2017, 76, 11–22. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Clari, R.; Vigotti, F.; Leone, F.; Attini, R.; Cabiddu, G.; Mauro, G.; Castelluccia, N.; Colombi, N.; Capizzi, I.; et al. Vegan-vegetarian diets in pregnancy: Danger or panacea? A systematic narrative review. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 623–633. [Google Scholar] [CrossRef]
- Sebastiani, G.; Barbero, A.H.; Borràs-Novell, C.; Casanova, M.A.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Tutusaus, M.P.; Ferrero, S.; Gómez-Roig, M.D.; García-Algar, Ó. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef]
- Croxford, S.; Gupta, D.; Bandyopadhyay, M.; Itsiopoulos, C. An evaluation of dietary intakes of a selected group of South Asian migrant women with gestational diabetes mellitus. Ethn. Health 2018, 1–17. [Google Scholar] [CrossRef]
- Tan, C.; Zhao, Y.; Wang, S. Is a vegetarian diet safe to follow during pregnancy? A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 2586–2596. [Google Scholar] [CrossRef]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B12deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Asemi, Z. The Effect of Soy Intake on Metabolic Profiles of Women with Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4654–4661. [Google Scholar] [CrossRef] [PubMed]
- Sarathi, V.; Kolly, A.; Chaithanya, H.B.; Dwarakanath, C.S. Effect of Soya based Protein Rich Diet on Glycaemic Parameters and Thyroid Function Tests in Women with Gestational Diabetes Mellitus. Rom. J. Diabetes Nutr. Metab. Dis. 2016, 23. [Google Scholar] [CrossRef]
- Rush, D.; Stein, Z.; Susser, M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 1980, 65, 683–697. [Google Scholar]
- Akhavan, T.; Luhovyy, B.L.; Brown, P.H.; Cho, C.E.; Anderson, G.H. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 2010, 91, 966–975. [Google Scholar] [CrossRef]
- Clifton, P.M.; Galbraith, C.E.; Coles, L. Effect of a low dose whey/guar preload on glycemic control in people with type 2 diabetes—A randomised controlled trial. Nutr. J. 2014, 13, 103. [Google Scholar] [CrossRef]
- Saleh, L.; Schrier, N.L.; Bruins, M.J.; Steegers, E.A.; Meiracker, A.H.V.D.; Visser, W. Effect of oral protein hydrolysate on glucose control in patients with gestational diabetes. Clin. Nutr. 2018, 37, 878–883. [Google Scholar] [CrossRef]
- Mignone, L.E.; Wu, T.; Horowitz, M.; Rayner, C.K. Whey protein: The “whey” forward for treatment of type 2 diabetes? World J. Diabetes 2015, 6, 1274–1284. [Google Scholar] [CrossRef]
- Bjørnshave, A.; Holst, J.J.; Hermansen, K. A pre-meal of whey proteins induces differential effects on glucose and lipid metabolism in subjects with the metabolic syndrome: A randomised cross-over trial. Eur. J. Nutr. 2018, 58, 755–764. [Google Scholar] [CrossRef]
- Saldana, T.M.; Siega-Riz, A.M.; Adair, L.S. Effect of macronutrient intake on the development of glucose intolerance during pregnancy. Am. J. Clin. Nutr. 2004, 79, 479–486. [Google Scholar] [CrossRef]
- Bo, S.; Menato, G.; Lezo, A.; Signorile, A.; Bardelli, C.; De Michieli, F.; Massobrio, M.; Pagano, G. Dietary fat and gestational hyperglycaemia. Diabetologia 2001, 44, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Lauszus, F.F.; Rasmussen, O.W.; Henriksen, J.E.; Klebe, J.G.; Jensen, L.; Lauszus, K.S.; Hermansen, K. Effect of a high monounsaturated fatty acid diet on blood pressure and glucose metabolism in women with gestational diabetes mellitus. Eur. J. Clin. Nutr. 2001, 55, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Innis, S. Essential fatty acid transfer and fetal development. Placenta 2005, 26, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Périnat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef]
- Haggarty, P. Fatty Acid Supply to the Human Fetus. Annu. Rev. Nutr. 2010, 21, 237–255. [Google Scholar] [CrossRef]
- Cunningham, P.; McDermott, L.C. Long Chain PUFA Transport in Human Term Placenta. J. Nutr. 2009, 139, 636–639. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [CrossRef]
- Cetin, I.; Giovannini, N.; Alvino, G.; Agostoni, C.; Riva, E.; Giovannini, M.; Pardi, G. Intrauterine Growth Restriction Is Associated with Changes in Polyunsaturated Fatty Acid Fetal-Maternal Relationships. Pediatr. Res. 2002, 52, 750–755. [Google Scholar] [CrossRef]
- Råd om Mad Når du er Gravid. Available online: https://altomkost.dk/raad-og-anbefalinger/saerlige-grupper/raad-om-mad-naar-du-er-gravid/ (accessed on 15 August 2020).
- Jamilian, M.; Dizaji, S.H.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Karamali, M.; Akbari, M.; Asemi, Z. A Randomized Controlled Clinical Trial Investigating the Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Biomarkers of Oxidative Stress, Inflammation and Pregnancy Outcomes in Gestational Diabetes. Can. J. Diabetes 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Ebrahimi, F.A.; Aghadavod, E.; Taghizadeh, M.; Asemi, Z. A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes. Nutrients 2018, 10, 163. [Google Scholar] [CrossRef]
- Samimi, M.; Jamilian, M.; Asemi, Z.; Esmaillzadeh, A. Effects of omega-3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2015, 34, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M.; Yaghoubi, S.; Shahrisa, E.; Farshbaf-Khalili, A. Effects of Fish Oil Supplementation on Gestational Diabetes Mellitus (GDM): A Systematic Review. Iran. Red Crescent Med. J. 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. 2016, 100, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Nutrition During Pregnancy; The National Academies Press: Washington, DC, USA, 1990. [Google Scholar]
- Lucock, M. Folic Acid: Nutritional Biochemistry, Molecular Biology, and Role in Disease Processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Lillycrop, K.A. Nutrition, Epigenetics, and Developmental Plasticity: Implications for Understanding Human Disease. Annu. Rev. Nutr. 2010, 30, 315–339. [Google Scholar] [CrossRef]
- Farkas, A.S.; Böttiger, A.K.; Isaksson, H.S.; Finnell, R.H.; Ren, A.; Nilsson, T.K. Epigenetic alterations in folate transport genes in placental tissue from fetuses with neural tube defects and in leukocytes from subjects with hyperhomocysteinemia. Epigenetics 2013, 8, 303–316. [Google Scholar] [CrossRef][Green Version]
- Greenberg, A.J.; Bell, S.J.; Guan, Y.; Yu, Y.-H. Folic Acid Supplementation and Pregnancy: More Than Just Neural Tube Defect Prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Guven, M.A.; Kilinc, M.; Batukan, C.; Ekerbicer, H.C.; Aksu, T. Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch. Gynecol. Obstet. 2006, 274, 333–337. [Google Scholar] [CrossRef]
- Mitri, J.; Pittas, A.G. Vitamin D and Diabetes. Endocrinol. Metab. Clin. North Am. 2014, 43, 205–232. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ashraf, A.P. Role of Vitamin D in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int. J. Endocrinol. 2009, 2010, 1–18. [Google Scholar] [CrossRef]
- Kampmann, U.; Mosekilde, L.; Juhl, C.; Moller, N.; Christensen, B.; Rejnmark, L.; Wamberg, L.; Orskov, L. Effects of 12weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency—A double-blind, randomized, placebo-controlled trial. Metabolism 2014, 63, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Poel, Y.; Hummel, P.; Lips, P.; Stam, F.; Van Der Ploeg, T.; Simsek, S. Vitamin D and gestational diabetes: A systematic review and meta-analysis. Eur. J. Intern. Med. 2012, 23, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Hashemi, T.; Karamali, M.; Samimi, M.; Esmaillzadeh, A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: A double-blind randomized controlled clinical trial. Am. J. Clin. Nutr. 2013, 98, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, Y.; He, M.; Li, T.; Ma, Z.; Cheng, H. Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: A randomized controlled trial. Exp. Ther. Med. 2016, 12, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Chamani, M.; Moosazadeh, M.; Tabrizi, R.; Samimi, M.; Karamali, M.; Jamilian, M.; Kolahdooz, F.; Lankarani, K.B.; Asemi, Z. The Effects of Vitamin D Supplementation on Glucose Metabolism and Lipid Profiles in Patients with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2017, 49, 647–653. [Google Scholar] [CrossRef]
- Asemi, Z.; Karamali, M.; Esmaillzadeh, A. Effects of calcium–vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: A randomised placebo-controlled trial. Diabetologia 2014, 57, 1798–1806. [Google Scholar] [CrossRef]
- Kost og Kosttilskud. Available online: https://www.sst.dk/da/viden/graviditet-og-foedsel/information-til-gravide/kost-og-kosttilskud (accessed on 15 August 2020).
- Bo, S.; Menato, G.; Villois, P.; Gambino, R.; Cassader, M.; Cotrino, I.; Cavallo-Perin, P. Iron supplementation and gestational diabetes in midpregnancy. Am. J. Obstet. Gynecol. 2009, 201, e1–e6. [Google Scholar] [CrossRef]
- Bouter, K.E.; Van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; González, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.S.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.A.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-blind Randomized Controlled Trial. Diabetes Care 2019, 42, dc182248. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.; Maguire, O.; Smith, T.; Curran, S.; Coffey, M.; Hatunic, M.; Foley, M.; Shanahan, F.; et al. 32: Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, S22. [Google Scholar] [CrossRef]
- Peng, T.R.; Wu, T.-W.; Chao, Y.-C. Effect of Probiotics on the Glucose Levels of Pregnant Women: A Meta-Analysis of Randomized Controlled Trials. Medicina 2018, 54, 77. [Google Scholar] [CrossRef]
- Han, M.-M.; Sun, J.-F.; Su, X.-H.; Peng, Y.-F.; Goyal, H.; Wu, C.-H.; Zhu, X.-Y.; Li, L. Probiotics improve glucose and lipid metabolism in pregnant women: A meta-analysis. Ann. Transl. Med. 2019, 7, 99. [Google Scholar] [CrossRef]
- Pan, J.; Pan, Q.; Chen, Y.; Zhang, H.; Zheng, X. Efficacy of probiotic supplement for gestational diabetes mellitus: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2017, 32, 317–323. [Google Scholar] [CrossRef]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2018, 10, 163–170. [Google Scholar] [CrossRef]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef]
- King, D.S.; Dalsky, G.P.; Clutter, W.E.; Young, D.A.; Staten, M.A.; Cryer, P.E.; Holloszy, J.O. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J. Appl. Physiol. 1988, 64, 1942–1946. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 5320156. [Google Scholar] [CrossRef] [PubMed]
- Bessinger, R.C.; McMurray, R.G.; Hackney, A.C. Substrate utilization and hormonal responses to moderate intensity exercise during pregnancy and after delivery. Am. J. Obstet. Gynecol. 2002, 186, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.B.; Ovesen, P.G.; Daugaard, M.; Ostenfeld, E.B.; Fuglsang, J. Cycling reduces blood glucose excursions after an oral glucose tolerance test in pregnant women: A randomized crossover trial. Appl. Physiol. Nutr. Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ruchat, S.-M.; Davenport, M.H.; Giroux, I.; Hillier, M.; Batada, A.; Sopper, M.M.; McManus, R.; Hammond, J.-A.; Mottola, M.F. Effect of exercise intensity and duration on capillary glucose responses in pregnant women at low and high risk for gestational diabetes. Diabetes Metab. Res. Rev. 2012, 28, 669–678. [Google Scholar] [CrossRef]
- García-Robles, R.; Martín, E.; Ubeda, J.; María, M.A.; De Leiva, A.; Corcoy, R. Evaluation of light exercise in the treatment of gestational diabetes. Diabetes Care 2001, 24, 2006–2007. [Google Scholar] [CrossRef]
- Coe, D.P.; Conger, S.A.; Kendrick, J.M.; Howard, B.C.; Thompson, D.L.; Bassett, D.R.; White, J.D. Postprandial walking reduces glucose levels in women with gestational diabetes mellitus. Appl. Physiol. Nutr. Metab. 2018, 43, 531–534. [Google Scholar] [CrossRef]
- Avery, M.D.; Walker, A.J. Acute effect of exercise on blood glucose and insulin levels in women with gestational diabetes. J. Matern. Fetal Med. 2001, 10, 52–58. [Google Scholar] [CrossRef]
- De Barros, M.C.; Lopes, M.A.; Francisco, R.P.; Sapienza, A.D.; Zugaib, M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010, 203, e1–e6. [Google Scholar] [CrossRef]
- Bung, P.; Artal, R.; Khodiguian, N.; Kjos, S. Exercise in Gestational Diabetes: An Optional Therapeutic Approach? Diabetes 1991, 40 (Suppl. 2), 182–185. [Google Scholar] [CrossRef]
- Avery, M.D.; Leon, A.S.; Kopher, R.A. Effects of a Partially Home-Based Exercise Program for Women with Gestational Diabetes. Obstet. Gynecol. 1997, 89, 10–15. [Google Scholar] [CrossRef]
- Martis, R.; Crowther, C.A.; Shepherd, E.; Alsweiler, J.M.; Downie, M.R.; Brown, J. Treatments for women with gestational diabetes mellitus: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2018, 8, CD012327. [Google Scholar] [CrossRef] [PubMed]
- Anbefalinger for Svangreomsorgen (Recommendations for the Care of Pregnant Women); Committee for Health Information: Copenhagen, Denmark, 2013; Available online: https://www.sst.dk/-/media/Udgivelser/2015/Anbefalinger-svangreomsorgen/Anbefalinger-for-svangreomsorgen.ashx?la=da&hash=757F1953C4B437A70A44024B32D7DD2E1B0A9F5B (accessed on 30 August 2020).
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.-M.; Davies, G.A.; Poitras, V.; Gray, C.E.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. No. 367-2019 Canadian Guideline for Physical Activity throughout Pregnancy. J. Obstet. Gynaecol. Can. 2018, 40, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Cremona, A.; O’Gorman, C.; Cotter, A.; Saunders, J.; Donnelly, A. Effect of exercise modality on markers of insulin sensitivity and blood glucose control in pregnancies complicated with gestational diabetes mellitus: A systematic review. Obes. Sci. Pr. 2018, 4, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Booth, G.L.; Creatore, M.I.; Luo, J.; Fazli, G.S.; Johns, A.; Rosella, L.C.; Glazier, R.H.; Moineddin, R.; Gozdyra, P.; Austin, P.C. Neighbourhood walkability and the incidence of diabetes: An inverse probability of treatment weighting analysis. J. Epidemiol. Community Health 2019, 73, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Connolly, C.P.; Conger, S.A.; Montoye, A.H.; Marshall, M.R.; Schlaff, R.A.; Badon, S.E.; Pivarnik, J.M. Walking for health during pregnancy: A literature review and considerations for future research. J. Sport Health Sci. 2019, 8, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Kew, S.; Ye, C.; Mehmood, S.; Hanley, A.; Sermer, M.; Zinman, B.; Retnakaran, R. Neighborhood walkability and risk of gestational diabetes. BMJ Open Diabetes Res. Care 2020, 8, e000938. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, E.R.; Christensen, A.-B.L.; Hellmuth, E.; Hornnes, P.; Stage, E.; Damm, P. Insulin dose during glucocorticoid treatment for fetal lung maturation in diabetic pregnancy: Test of an algorithm [correction of analgoritm]. Acta Obstet. Gynecol. Scand. 2002, 81, 835–839. [Google Scholar] [CrossRef]
| Pre-Pregnancy BMI | Total Weight Gain (Range in kg) |
|---|---|
| Underweight (<18.5 kg/m2) | 12.5–C18 |
| Normal weight (18.5–24.9 kg/m2) | 11.5–16 |
| Overweight (25.0–29.9 kg/m2) | 7–11.5 |
| Obese(≥30 kg/m2) | 5–9 |
| NNR | IOM | ||
|---|---|---|---|
| Age | MJ/d | Age | kcal/d |
| 11–18 | (0.0393 W + 1.04 H + 1.93)*PAL | 14–18 | 135.3 − (30.8 × age [y]) + PA × [(10.0 × weight [kg]) + (934 × height [m])] + 25 |
| 19–30 | (0.0546 W + 2.33)*PAL | >19 | 354 − (6.91 × age [y]) + PA × [(9.36 × weight [kg]) + (726 v height [m])] |
| 31–60 | (0.0433 W + 2.57 H − 1.180)*PAL |
| PAL | NNR |
| 1.1–1.2 | Bed-bound or chair-bound |
| 1.3–1.5 | Seated work with none or only little physical activity |
| 1.6–1.7 | Seated work with some movement or some physical activity |
| 1.8–1.9 | Work including standing and moving around or seated work with some movement and with frequent activity |
| 2.0–2.4 | Very strenuous work or daily competitive physical training |
| PA, age ≥19 (ages 14–18) | IOM |
| 1.0 (1.0) | Very low active level |
| 1.12 (1.16) | Low active level |
| 1.27 (1.31) | Active level |
| 1.45 (1.56) | Highly active level |
| Trimester | NNR | IOM |
|---|---|---|
| 1st trimester | 103 kcal | 0 kcal |
| 2nd trimester | 329 kcal | 340 kcal |
| 3rd trimester | 537 kcal | 452 kcal |
| Micronutrient | NNR | IOM |
|---|---|---|
| Folic acid, µg/day | 500 | 600 |
| 25-Hydroxyvitamin D, µg/day | 10 | 5 |
| Calcium, mg/day | 900 | 1000 |
| Iron, mg/day | 40 | 27 |
| Dietary Components | Recommendations |
|---|---|
| Energy | Excessive weight gain should be avoided and a calorie restriction of 30–33% is advisable in women with overweight or women who have already gained the recommended weight during pregnancy |
| Carbohydrates | Exact amount of carbohydrate should be individualized. A minimum of 175 g/d should be ensured. Patients should be guided to choose starchy foods such as vegetables, legumes, fruits, and whole grains.Carbohydrate intake should be distributed throughout the day. |
| Protein | Total amount of protein should be 10–35E% with a minimum of 71 g/d. Protein intake should primarily come from plants, lean meat, and fish. |
| Fat | Total amount of fat should be 20–40E% with a maximum of 10E% from saturated fat, a minimum of 10–20E% from MUFAs, and 5–10E% from PUFAs. An intake of a minimum 350g of fish/week may be advisable. |
| Folic acid | 500–600 µg/d is recommended. Daily supplement of 400 µg/d may be advisable for all women at childbearing age and during the first 12 week of gestation. |
| 25-Hydroxyvitamin D | 5–10 µg/d is recommended depending on how much sunlight the woman gets. |
| Calcium | 900–1000 mg/d is recommended. Supplement may be advisable in women with a lack of intake of dairy products. |
| Iron | 27–40 mg/d is recommended. |
| Probiotics | It remains unresolved whether probiotics have beneficial metabolic effects in women with GDM. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. https://doi.org/10.3390/nu12103050
Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients. 2020; 12(10):3050. https://doi.org/10.3390/nu12103050
Chicago/Turabian StyleRasmussen, Louise, Charlotte Wolff Poulsen, Ulla Kampmann, Stine Bech Smedegaard, Per Glud Ovesen, and Jens Fuglsang. 2020. "Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus" Nutrients 12, no. 10: 3050. https://doi.org/10.3390/nu12103050
APA StyleRasmussen, L., Poulsen, C. W., Kampmann, U., Smedegaard, S. B., Ovesen, P. G., & Fuglsang, J. (2020). Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients, 12(10), 3050. https://doi.org/10.3390/nu12103050





