Longitudinal Changes in Resting Metabolic Rates with Aging Are Accelerated by Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Chronic Diseases
2.3. Resting Metabolic Rate
2.4. Body Composition
2.5. Statistical Analyses
3. Results
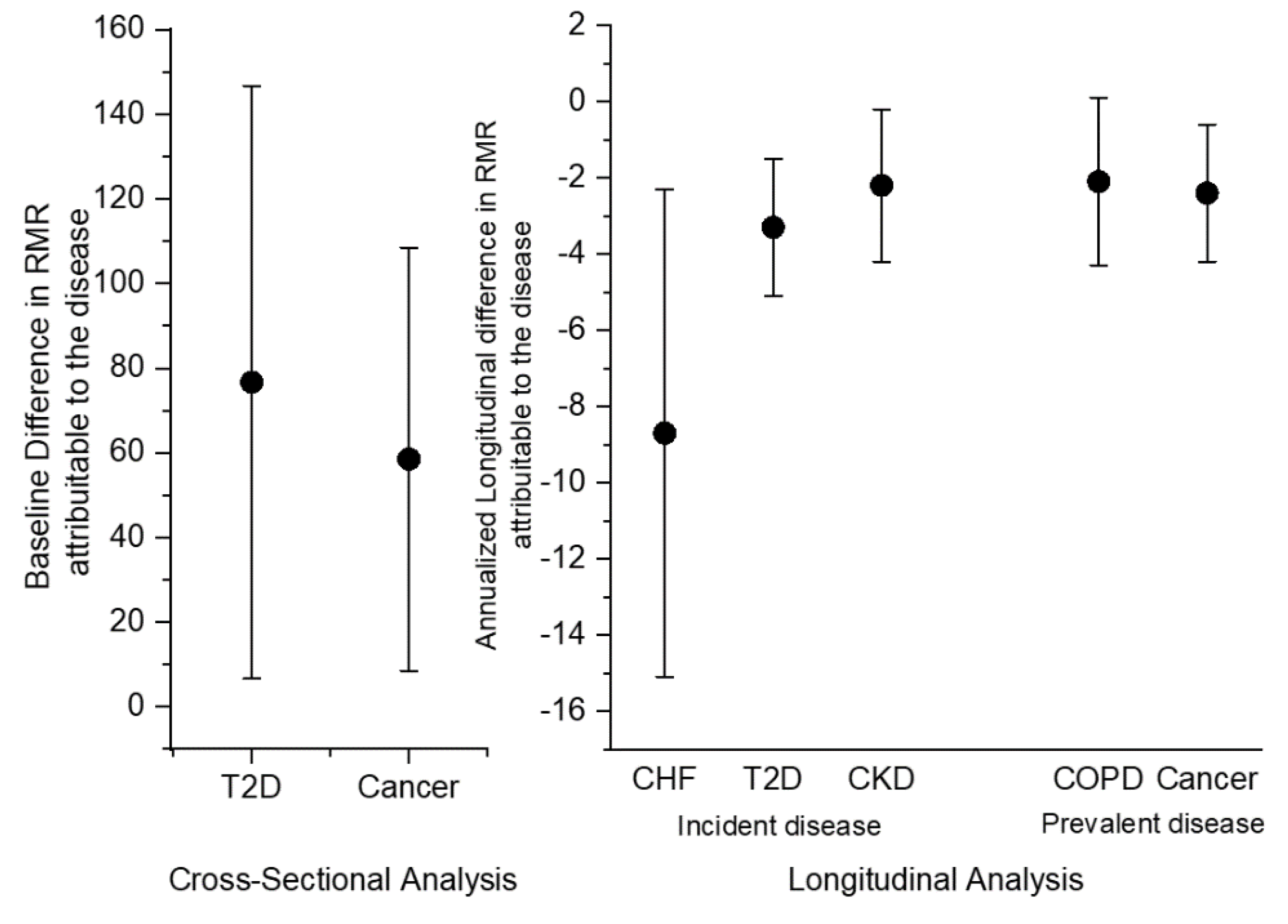
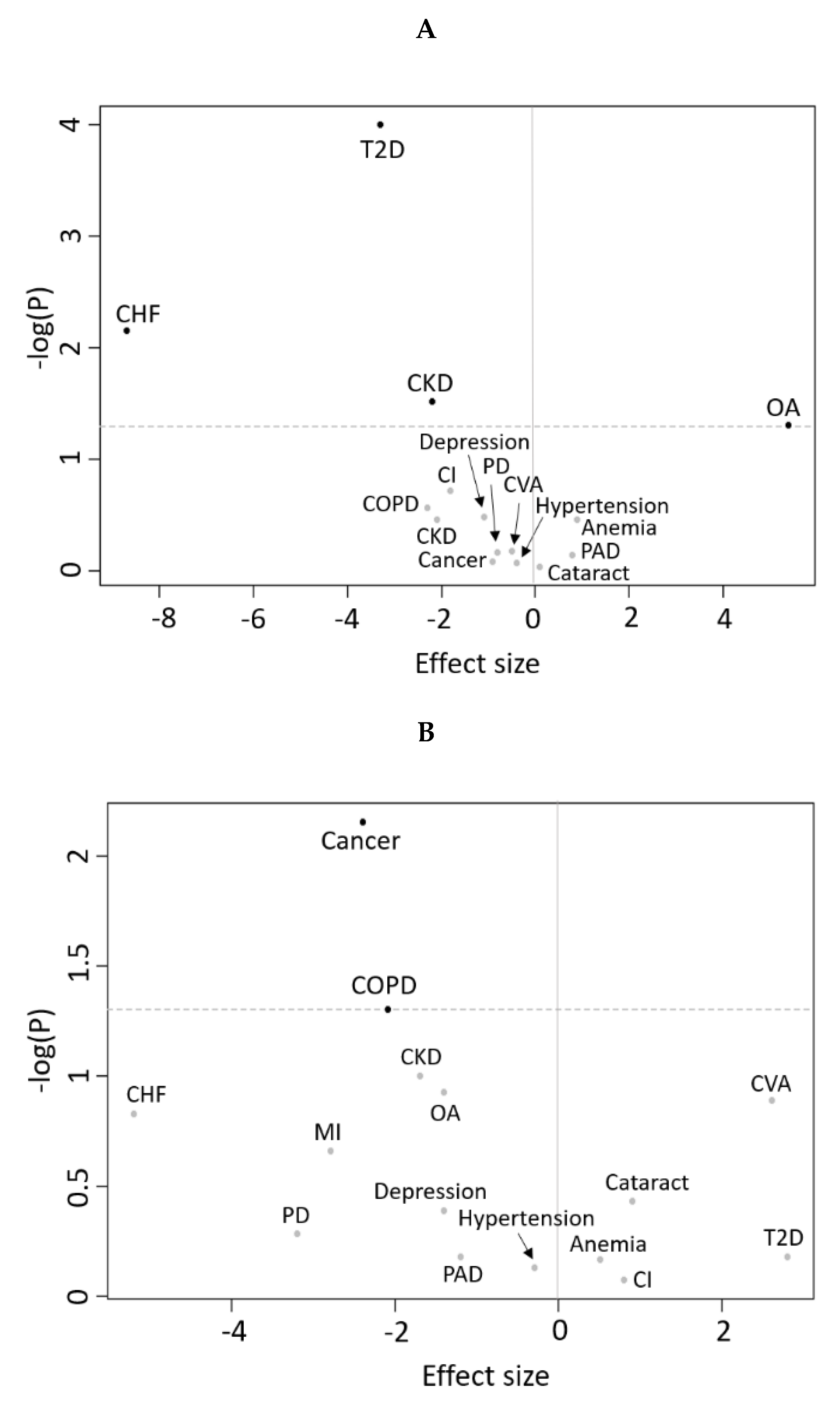
3.1. Cross-Sectional Analyses
3.2. Longitudinal Analyses
4. Discussion
4.1. Principal Findings
4.2. Cross-Sectional Associations Between Disease Status and RMR
4.3. Disease Status and Longitudinal Changes in RMR
4.4. Limitations and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ravussin, E.; Burnand, B.; Schutz, Y.; Jéquier, E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am. J. Clin. Nutr. 1982, 35, 566–573. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.G.; Soares, J.; Caspersen, C.J.; McCurdy, T. Examining variations of resting metabolic rate of adults. Med. Sci. Sports Exerc. 2014, 46, 1352–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, P.-L.; Schrack, J.A.; Shardell, M.D.; Levine, M.; Moore, A.Z.; An, Y.; Elango, P.; Karikkineth, A.; Tanaka, T.; De Cabo, R.; et al. A roadmap to build a phenotypic metric of ageing: Insights from the Baltimore Longitudinal Study of Aging. J. Intern. Med. 2020, 287, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J. Mechanisms of changes in basal metabolism during ageing. Eur. J. Clin. Nutr. 2000, 54, S77–S91. [Google Scholar] [CrossRef] [PubMed]
- Tzankoff, S.P.; Norris, A.H. Effect of muscle mass decrease on age-related BMR changes. J. Appl. Physiol. 1977, 43, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Hrmann, P.M.L.; Herbert, B.M.; User-Berthold, M.N. Effects of fat mass and body fat distribution on resting metabolic rate in the elderly. Metabolism 2001, 50, 972–975. [Google Scholar] [CrossRef]
- Schrack, J.A.; Knuth, N.D.; Simonsick, E.M.; Ferrucci, L. “IDEAL” aging is associated with lower resting metabolic rate: The Baltimore Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2014, 62, 667–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, E.; An, Y.; Schrack, J.A.; Gonzalez-Freire, M.; Zoli, M.; Simonsick, E.M.; Guralnik, J.M.; Boyd, C.M.; Studenski, S.A.; Ferrucci, L. Energy metabolism and the burden of multimorbidity in older adults: Results from the Baltimore Longitudinal Study of Aging. J. Gerontol. Ser. A: Boil. Sci. Med Sci. 2014, 70, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, A.; Jungert, A.; Spinneker, A.; Neuhäuser-Berthold, M. The impact of multimorbidity on resting metabolic rate in community-dwelling women over a ten-year period: A cross-sectional and longitudinal study. J. Nutr. Heal. Aging 2016, 21, 781–786. [Google Scholar] [CrossRef]
- Ruggiero, C.; Metter, E.J.; Melenovsky, V.; Cherubini, A.; Najjar, S.S.; Ble, A.; Senin, U.; Longo, D.L.; Ferrucci, L. High basal metabolic rate is a risk factor for mortality: The Baltimore Longitudinal Study of Aging. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2008, 63, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Jumpertz, R.; Hanson, R.; Sievers, M.L.; Bennett, P.H.; Nelson, R.G.; Krakoff, J. Higher energy expenditure in humans predicts natural mortality. J. Clin. Endocrinol. Metab. 2011, 96, E972–E976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrucci, L.; Schrack, J.A.; Knuth, N.D.; Simonsick, E.M. Aging and the energetic cost of life. J. Am. Geriatr. Soc. 2012, 60, 1768–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, B.J.L.; Norris, B.A.H. Activities and attitudes of participants in the Baltimore Longitudinal Study. J. Gerontol. 1966, 21, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Brach, J.S.; Simonsick, E.M.; Kritchevsky, S.; Yaffe, K.; Newman, A.B. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J. Am. Geriatr. Soc. 2004, 52, 502–509. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Fried, L.; Simonsick, E.; Ling, S.; Guralnik, J.M. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: The women’s health and aging study. Circulation 2000, 101, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function-measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [Green Version]
- Rumpler, W.V.; Seale, J.L.; Conway, J.M.; Moe, P.W. Repeatability of 24-h energy expenditure measurements in humans by indirect calorimetry. Am. J. Clin. Nutr. 1990, 51, 147–152. [Google Scholar] [CrossRef]
- Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. Comparison of the Cosmed K4b2 portable metabolic system in measuring steady-state walking energy expenditure. PLoS ONE 2010, 5, e9292. [Google Scholar] [CrossRef] [Green Version]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Exercise Physiology: Energy, Nutrition, and Human Performance; LWW; Lea & Febiger: Philadelphia, PA, USA, 1991. [Google Scholar]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef] [PubMed]
- Van Loan, M.D.; Mayclin, P.L. Body composition assessment: Dual-energy X-ray absorptiometry (DEXA) compared to reference methods. Eur. J. Clin. Nutr. 1992, 46, 125–130. [Google Scholar] [PubMed]
- Pritchard, J.E.; Nowson, C.A.; Strauss, B.J.; Carlson, J.S.; Kaymakci, B.; Wark, J.D. Evaluation of dual energy X-ray absorptiometry as a method of measurement of body fat. Eur. J. Clin. Nutr. 1993, 47, 216–228. [Google Scholar]
- Oberdier, M.T.; Morrell, C.H.; Lakatta, E.G.; Ferrucci, L.; Al Ghatrif, M. Subclinical longitudinal change in ankle-brachial index with aging in a community-dwelling population is associated with central arterial stiffening. J. Am. Hear. Assoc. 2019, 8, e011650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, M. Organ and tissue contribution to metabolic rate. Energy Metab. Tissue Determ. Cell. Corollaries 1992, 61–80. [Google Scholar]
- Armellini, F.; Zamboni, M.; Mino, A.; Bissoli, L.; Micciolo, R.; Bosello, O. Postabsorptive resting metabolic rate and thermic effect of food in relation to body composition and adipose tissue distribution. Metabolism 2000, 49, 6–10. [Google Scholar] [CrossRef]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef] [Green Version]
- Roubenoff, R. Inflammatory and hormonal mediators of cachexia. J. Nutr. 1997, 127, 1014S–1016S. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L. Session 2: Inflammation and cancer. Toxicol. Pathol. 2004, 32, 732. [Google Scholar] [CrossRef]
- Bogardus, C.; Taskinen, M.-R.; Zawadzki, J.; Lillioja, S.; Mott, D.; Howard, B.V. Increased resting metabolic rates in obese subjects with non-insulin-dependent diabetes mellitus and the effect of sulfonylurea therapy. Diabetes 1986, 35, 1–5. [Google Scholar] [CrossRef]
- Nair, K.; Webster, J.; Garrow, J.S. Effect of impaired glucose tolerance and type II diabetes on resting metabolic rate and thermic response to a glucose meal in obese women. Metabolism 1986, 35, 640–644. [Google Scholar] [CrossRef]
- Rigalleau, V.; Lasseur, C.; Pécheur, S.; Chauveau, P.; Combe, C.; Perlemoine, C.; Baillet, L.; Gin, H. Resting energy expenditure in uremic, diabetic, and uremic diabetic subjects. J. Diabetes Complicat. 2004, 18, 237–241. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [Green Version]
- Abbate, L.M.; Stevens, J.; Schwartz, T.A.; Renner, J.B.; Helmick, C.G.; Jordan, J.M. Anthropometric measures, body composition, body fat distribution, and knee osteoarthritis in women *. Obesity 2006, 14, 1274–1281. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; Van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Bar-Shai, A.; Sagiv, A.; Alon, R.; Krizhanovski, V. The role of Clara cell senescence in the pathogenesis of COPD. Eur. Respir. J. 2014, 44, 3245. [Google Scholar]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. Accelerated cellular senescence in degenerate intervertebral discs: A possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 2007, 9, R45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte senescence as a component of Alzheimer & rsquo;s disease. PLoS ONE 2012, 7, e45069. [Google Scholar] [CrossRef]
- Du, J.; Klein, J.D.; Hassounah, F.; Zhang, J.; Zhang, C.; Wang, X. Aging increases CCN1 expression leading to muscle senescence. Am. J. Physiol. Physiol. 2013, 306, C28–C36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampino, M.; Ferrucci, L.; Semba, R.D. Biomarkers in the path from cellular senescence to frailty. Exp. Gerontol. 2019, 129, 110750. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J.S. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef]
- Von Haehling, S.; Doehner, W.; Anker, S.D. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc. Res. 2007, 73, 298–309. [Google Scholar] [CrossRef]
- Berry, C. Catabolism in chronic heart failure. Eur. Hear. J. 2000, 21, 521–532. [Google Scholar] [CrossRef]
- Gea, J.; Agusti, A.; Roca, J. Pathophysiology of muscle dysfunction in COPD. J. Appl. Physiol. 2013, 114, 1222–1234. [Google Scholar] [CrossRef] [Green Version]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; De Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef] [Green Version]
- Kannus, R.; Jòzsa, L.; Renström, R.; Järvtoen, M.; Kvist, M.; Lento, M.; Oja, P.; Vuorl, I. The effects of training, immobilization and remobilization on musculoskeletal tissue: 1. Training and immobilization. Scand. J. Med. Sci. Sports 1992, 2, 100–118. [Google Scholar] [CrossRef]
- Adelnia, F.; Urbanek, J.; Osawa, Y.; Shardell, M.; Bs, N.A.B.; Fishbein, K.W.; Spencer, R.G.; Simonsick, E.M.; Schrack, J.A.; Ferrucci, L. Moderate-to-vigorous physical activity is associated with higher muscle oxidative capacity in older adults. J. Am. Geriatr. Soc. 2019, 67, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Zampino, M.; Semba, R.D.; Adelnia, F.; Spencer, R.G.; Fishbein, K.W.; Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. Greater skeletal muscle oxidative capacity is associated with higher resting metabolic rate: Results from the Baltimore Longitudinal Study of Aging. J. Gerontol. Ser. A: Boil. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Metter, E.J.; Griswold, M.E.; Harris, T.B.; Simonsick, E.M.; Longo, D.L.; Ferrucci, L. Changes in weight at the end of life: Characterizing weight loss by time to death in a cohort study of older men. Am. J. Epidemiology 2010, 172, 558–565. [Google Scholar] [CrossRef]
- Delgado, J.; Bowman, K.; Ble, A.; Masoli, J.A.H.; Han, Y.; Henley, W.; Welsh, S.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Blood pressure trajectories in the 20 years before death. JAMA Intern. Med. 2018, 178, 93–99. [Google Scholar] [CrossRef]
- Evans, W.J.; Hellerstein, M.; Orwoll, E.; Cummings, S.; Cawthon, P.M. D3-Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J. Cachex- Sarcopenia Muscle 2019, 10, 14–21. [Google Scholar] [CrossRef] [Green Version]

| Number of Participants | 997 (489 Males + 508 Females) |
|---|---|
| Age (years) | 65.9 ± 12.9 |
| Race, % White | 67.6 |
| % Black | 26.2 |
| % Other | 6.2 |
| Education, % Completed College | 84.2 |
| Current Smoking, % | 2.0 |
| Resting Metabolic Rate (kCal/day) | 1607 ± 429.6 in males, 1268.9 ± 331.9 in females |
| Physical Activity (min/week) | 93.9 ± 137.7 |
| Height (cm) | 168.9 ± 9.3 |
| Body Mass Index (kg/m2) | 27.0 ± 4.6 |
| Fat Body Mass (kg) | 27.0 ± 10.4 |
| Lean Body Mass (kg) | 47.5 ± 10.0 |
| Disease | Prevalence at Baseline (%) | β Value | p Value |
|---|---|---|---|
| Estimate (SD) | |||
| Chronic Heart Failure | 1 | 175.1 (110.8) | 0.11 |
| Sex | 26.2 (49.6) | 0.6 | |
| Age | −4.9 (1.0) | <0.001 | |
| Lean Mass | 22.2 (2.5) | <0.001 | |
| Fat Mass | 5.2 (1.3) | <0.001 | |
| Myocardial Infarction | 2.6 | 55.9 (68.1) | 0.41 |
| Sex | 47.4 (44.3) | 0.28 | |
| Age | −5.11 (0.9) | <0.001 | |
| Lean Mass | 20.2 (2.2) | <0.001 | |
| Fat Mass | 5.9 (1.1) | <0.001 | |
| Cerebrovascular Accident | 5.1 | −32.1 (50.7) | 0.52 |
| Sex | 44.7 (46.3) | 0.335 | |
| Age | −5.4 (1.0) | <0.001 | |
| Lean Mass | 20.6 (2.3) | <0.001 | |
| Fat Mass | 6.0 (1.2) | <0.001 | |
| Hypertension | 40.2 | 23.3 (2.9) | 0.9 |
| Sex | 48.2 (44.5) | 0.28 | |
| Age | −5.1 (0.9) | <0.001 | |
| Lean Mass | 20.2 (2.2) | <0.001 | |
| Fat Mass | 5.9 (1.2) | <0.001 | |
| Type 2 Diabetes Mellitus | 10.7 | 76.7 (36.0) | 0.04 |
| Sex | 32.7 (44.7) | 0.46 | |
| Age | −5.4 (0.9) | <0.001 | |
| Lean Mass | 20.7 (2.2) | <0.001 | |
| Fat Mass | 5.5 (1.2) | <0.001 | |
| Anemia | 12.1 | −12.8 (33.6) | 0.7 |
| Sex | 49.2 (44.3) | 0.27 | |
| Age | −5.0 (0.9) | <0.001 | |
| Lean Mass | 20.1 (2.2) | <0.001 | |
| Fat Mass | 6.0 (1.2) | <0.001 | |
| Peripheral Artery Disease | 1.8 | −31.9 (82.6) | 0.7 |
| Sex | 46.8 (44.9) | 0.3 | |
| Age | −5.0 (0.9) | <0.001 | |
| Lean Mass | 20.3 (2.3) | <0.001 | |
| Fat Mass | 5.9 (1.2) | <0.001 | |
| Cognitive Impairment | 0.6 | −47.2 (138.2) | 0.73 |
| Sex | 48.3 (46.4) | 0.3 | |
| Age | −5.3 (1.0) | <0.001 | |
| Lean Mass | 20.5 (2.3) | <0.001 | |
| Fat Mass | 6.0 (1.2) | <0.001 | |
| Depression | 4.4 | 26.2 (51.6) | 0.61 |
| Sex | 48.5 (44.3) | 0.27 | |
| Age | −5.0 (0.9) | <0.001 | |
| Lean Mass | 20.2 (2.2) | <0.001 | |
| Fat Mass | 6.0 (1.2) | <0.001 | |
| Parkinson’s Disease | 0.5 | 23.9 (150.6) | 0.87 |
| Sex | 48.2 (44.4) | 0.28 | |
| Age | −5.1 (0.9) | <0.001 | |
| Lean Mass | 20.2 (2.2) | <0.001 | |
| Fat Mass | 6.0 (1.2) | <0.001 | |
| Chronic Kidney Disease | 24.4 | 31.8 (28.6) | 0.71 |
| Sex | 58.2 (45.1) | 0.562 | |
| Age | −5.4 (1.0) | <0.001 | |
| Lean Mass | 20.2 (2.2) | <0.001 | |
| Fat mass | 6.0 (1.1) | <0.001 | |
| Cataract | 22.9 | −9.5 (27.6) | 0.73 |
| Sex | 49.5 (44.3) | 0.26 | |
| Age | −5.0 (1.0) | <0.001 | |
| Lean mass | 20.1 (2.2) | <0.001 | |
| Fat mass | 6.0 (1.2) | <0.001 | |
| Chronic obstructive pulmonary disease | 13.9 | 44.9 (31.4) | 0.15 |
| Sex | |||
| Age | 50.7 (44.3) | 0.25 | |
| Lean mass | −5.1 (0.9) | <0.001 | |
| Fat mass | 20.0 (2.2) | <0.001 | |
| 5.9 (1.2) | <0.001 | ||
| Cancer | 25.9 | 58.5 (25.7) | 0.02 |
| Sex | 44.4 (44.2) | 0.32 | |
| Age | −5.6 (0.9) | <0.001 | |
| Lean mass | 20.2 (2.2) | <0.001 | |
| Fat mass | 6.1 (1.2) | <0.001 | |
| Osteoarthritis | 22.1 | 36.7 (26.6) | 0.17 |
| Sex | 52.4 (44.3) | 0.24 | |
| Age | −5.3 (0.9) | <0.001 | |
| Lean mass | 20.0 (2.2) | <0.001 | |
| Fat mass | 5.8 (1.2) | <0.001 |
| Disease | β Value (SE) | p Value |
|---|---|---|
| Chronic Heart Failure | −8.7 (3.2) | 0.007 |
| Myocardial Infarction | −0.4 (2.1) | 0.85 |
| Cerebrovascular Accident | −0.8 (1.9) | 0.68 |
| Hypertension | −0.5 (1.1) | 0.67 |
| Diabetes Mellitus | −3.3 (0.9) | <0.001 |
| Anemia | 0.9 (1.0) | 0.35 |
| Peripheral Artery Disease | 0.8 (2.1) | 0.72 |
| Cognitive Impairment | −2.1 (2.2) | 0.35 |
| Depression | −1.8 (1.4) | 0.19 |
| Parkinson’s Disease | −0.9 (4.0) | 0.82 |
| Chronic Kidney Disease | −2.2 (1.0) | 0.03 |
| Cataract | 0.1 (0.9) | 0.92 |
| Chronic Obstructive Pulmonary Disease | −2.3 (2.1) | 0.27 |
| Cancer | −1.1 (1.1) | 0.33 |
| Osteoarthritis | 5.4 (2.7) | 0.05 |
| Disease | β Value (SE) | p Value |
|---|---|---|
| Chronic Heart Failure | −5.2 (3.7) | 0.15 |
| Myocardial Infarction | −2.8 (2.3) | 0.22 |
| Cerebrovascular Accident | 2.6 (1.7) | 0.13 |
| Hypertension | −0.3 (0.8) | 0.75 |
| Diabetes Mellitus | 2.8 (6.5) | 0.67 |
| Anemia | 0.5 (1.1) | 0.69 |
| Peripheral Artery Disease | −1.2 (2.8) | 0.67 |
| Cognitive Impairment | 0.8 (4.6) | 0.85 |
| Depression | −1.4 (1.7) | 0.41 |
| Parkinson’s Disease | −3.2 (5.1) | 0.52 |
| Chronic Kidney Disease | −1.7 (1.1) | 0.10 |
| Cataract | 0.9 (1.0) | 0.37 |
| Chronic Obstructive Pulmonary Disease | −2.1 (1.1) | 0.05 |
| Cancer | −2.4 (0.9) | 0.007 |
| Osteoarthritis | −1.4 (0.9) | 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampino, M.; AlGhatrif, M.; Kuo, P.-L.; Simonsick, E.M.; Ferrucci, L. Longitudinal Changes in Resting Metabolic Rates with Aging Are Accelerated by Diseases. Nutrients 2020, 12, 3061. https://doi.org/10.3390/nu12103061
Zampino M, AlGhatrif M, Kuo P-L, Simonsick EM, Ferrucci L. Longitudinal Changes in Resting Metabolic Rates with Aging Are Accelerated by Diseases. Nutrients. 2020; 12(10):3061. https://doi.org/10.3390/nu12103061
Chicago/Turabian StyleZampino, Marta, Majd AlGhatrif, Pei-Lun Kuo, Eleanor Marie Simonsick, and Luigi Ferrucci. 2020. "Longitudinal Changes in Resting Metabolic Rates with Aging Are Accelerated by Diseases" Nutrients 12, no. 10: 3061. https://doi.org/10.3390/nu12103061
APA StyleZampino, M., AlGhatrif, M., Kuo, P.-L., Simonsick, E. M., & Ferrucci, L. (2020). Longitudinal Changes in Resting Metabolic Rates with Aging Are Accelerated by Diseases. Nutrients, 12(10), 3061. https://doi.org/10.3390/nu12103061





