The Association between Nutritional Status and In-Hospital Mortality among Patients with Acute Coronary Syndrome—A Result of the Retrospective Nutritional Status Heart Study (NSHS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Nutritional Screening
2.4. Ethical Considerations
2.5. Statistical Analyses
3. Results
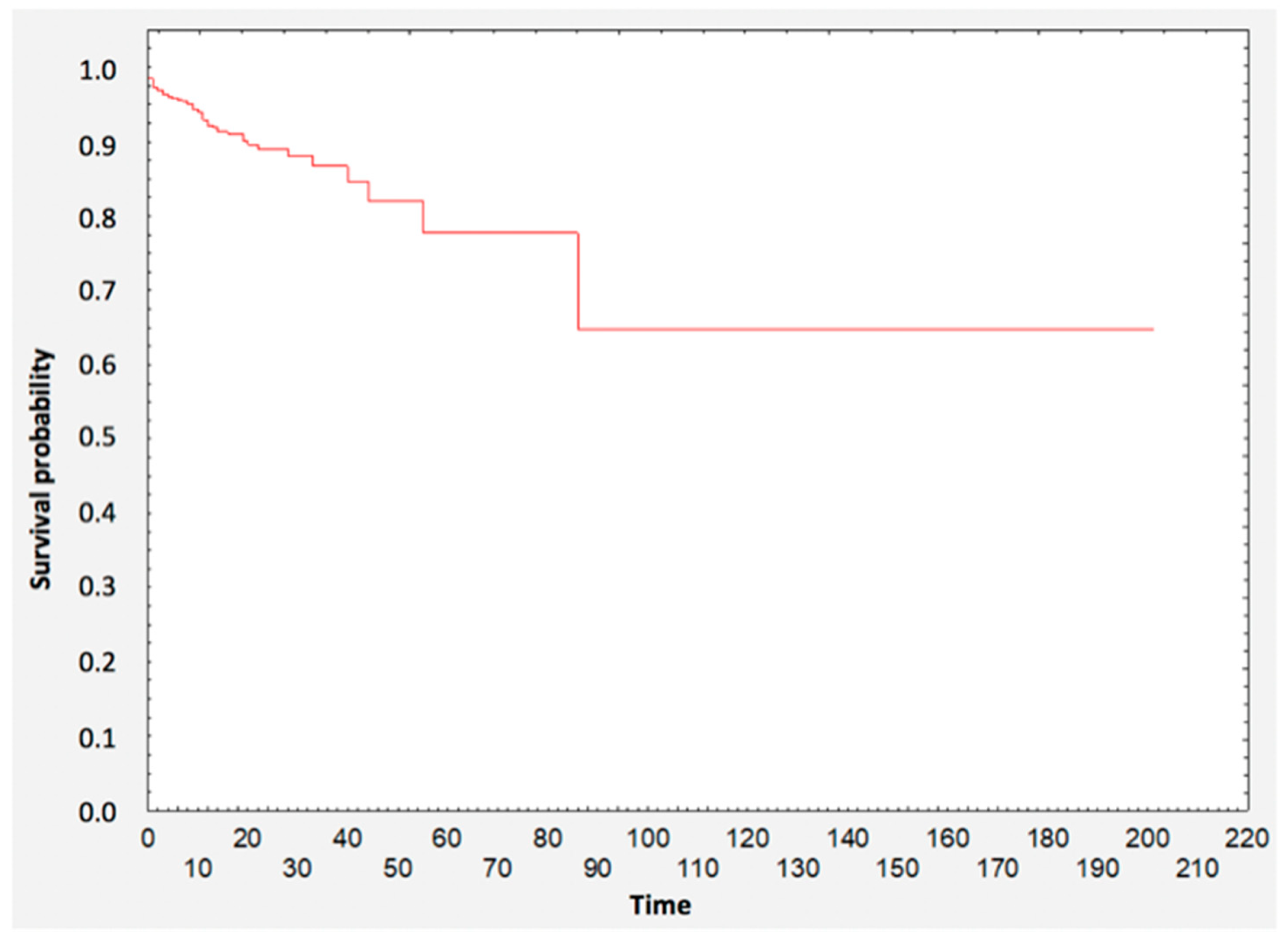
3.1. Survival Analysis
3.2. Survival Analysis—Group Comparisons
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Daviglus, M.L.; Loria, C.M.; Colangelo, L.A.; Spring, B.; Moller, A.C.; Lloyd-Jones, D.M. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: The Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation 2012, 125, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, T.; Jacobsen, R.K.; Toft, U.; Aadahl, M.; Glümer, C.; Pisinger, C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ 2014, 348, g3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, D.H.; Bopp, M.M.; Roberson, P.K. Protein-energy Undernutrition and Life-threatening Complications among the Hospitalized Elderly. J. Gen. Intern. Med. 2002, 17, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Brown, C.J.; Burgio, K.L.; Kilgore, M.L.; Ritchie, C.S.; Roth, D.L.; West, D.S.; Locher, J.L. Undernutrition at baseline and health services utilization and mortality over a 1-year period in older adults receiving Medicare home health services. J. Am. Med. Dir. Assoc. 2011, 12, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basta, G.; Chatzianagnostou, K.; Paradossi, U.; Botto, N.; Del Turco, S.; Taddei, A.; Berti, S.; Mazzone, A. The prognostic impact of objective nutritional indices in elderly patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Int. J. Cardiol. 2016, 221, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Söderström, L.; Rosenblad, A.; Thors Adolfsson, E.; Bergkvist, L. Malnutrition is associated with increased mortality in older adults regardless of the cause of death. Br. J. Nutr. 2017, 117, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.-C.; Gedeborg, R.; Nicholas, O.; James, S.; Jeppsson, A.; Wolfe, C.; Heuschmann, P.; Wallentin, L.; Deanfield, J.; Timmis, A.; et al. Acute myocardial infarction: A comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet 2014, 383, 1305–1312. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Ad Hoc ESPEN Working Group Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Boban, M.; Bulj, N.; Kolačević Zeljković, M.; Radeljić, V.; Krcmar, T.; Trbusic, M.; Delić-Brkljačić, D.; Alebic, T.; Vcev, A. Nutritional Considerations of Cardiovascular Diseases and Treatments. Nutr. Metab. Insights 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Miller, M.; Kaambwa, B.; Shahi, R.; Hakendorf, P.; Horwood, C.; Thompson, C. Factors influencing early and late readmissions in Australian hospitalised patients and investigating role of admission nutrition status as a predictor of hospital readmissions: A cohort study. BMJ Open 2018, 8, e022246. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.; Kondrup, J.; Prokopowicz, J.; Schiesser, M.; Krähenbühl, L.; Meier, R.; Liberda, M. EuroOOPS study group EuroOOPS: An international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin. Nutr. 2008, 27, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Kook, H.Y.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Jeong, M.H. Influence of undernutrition at admission on clinical outcomes in patients with acute myocardial infarction. J. Cardiol. 2017, 69, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Yoshihisa, A.; Kimishima, Y.; Kiko, T.; Shimizu, T.; Yamaki, T.; Kunii, H.; Nakazato, K.; Takeishi, Y. Prognostic impacts of nutritional status on long-term outcome in patients with acute myocardial infarction. Eur. J. Prev. Cardiol. 2019, 2047487319883723. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. NOURISH Study Group Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Rus, V.A.; Chitu, M.; Cernea, S.; Benedek, I.; Hodas, R.; Zavate, R.; Nyulas, T.; Hintea, M.; Benedek, T. Altered nutritional status, inflammation and systemic vulnerability in patients with acute myocardial infarction undergoing percutaneous coronary revascularisation: A prospective study in a level 3 cardiac critical care unit. Nutr. Diet. 2020, 77, 212–222. [Google Scholar] [CrossRef]
- Lu, Y.-W.; Lu, S.-F.; Chou, R.-H.; Wu, P.-S.; Ku, Y.-C.; Kuo, C.-S.; Chang, C.-C.; Tsai, Y.-L.; Wu, C.-H.; Huang, P.-H. Lipid paradox in patients with acute myocardial infarction: Potential impact of malnutrition. Clin. Nutr. 2019, 38, 2311–2318. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Cho, K.H.; Jeong, M.H.; Ahn, Y.; Kim, Y.J.; Chae, S.C.; Hong, T.J.; Seong, I.W.; Chae, J.K.; Kim, C.J.; Cho, M.C.; et al. Low-density lipoprotein cholesterol level in patients with acute myocardial infarction having percutaneous coronary intervention (the cholesterol paradox). Am. J. Cardiol. 2010, 106, 1061–1068. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Chu, C.-S.; Lin, T.-H.; Lee, K.-T.; Sheu, S.-H.; Lai, W.-T. Lipid paradox in acute myocardial infarction-the association with 30-day in-hospital mortality. Crit. Care Med. 2015, 43, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Charach, G.; George, J.; Roth, A.; Rogowski, O.; Wexler, D.; Sheps, D.; Grosskopf, I.; Weintraub, M.; Keren, G.; Rubinstein, A. Baseline low-density lipoprotein cholesterol levels and outcome in patients with heart failure. Am. J. Cardiol. 2010, 105, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Bui, Q.T.; Jacobs, J.R.; Begelman, S.M.; Miller, D.P.; French, W.J. Investigators of National Registry of Myocardial Infarction (NRMI) 4b–5 Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (the lipid paradox). Am. J. Cardiol. 2015, 115, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Li, Y.; Huang, F.; Chai, H.; Huang, B.; Li, Q.; Zhao, Z.; Liao, Y.; Zuo, Z.; Peng, Y.; et al. The triglyceride paradox in the mortality of coronary artery disease. Lipids Health Dis. 2019, 18, 21. [Google Scholar] [CrossRef] [Green Version]
- De Luca, G.; Verdoia, M.; Savonitto, S.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Morici, N.; Bossi, I.; Sganzerla, P.; Tortorella, G.; et al. Impact of body mass index on clinical outcome among elderly patients with acute coronary syndrome treated with percutaneous coronary intervention: Insights from the ELDERLY ACS 2 trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 730–737. [Google Scholar] [CrossRef]
- Song, C.; Fu, R.; Yang, J.; Xu, H.; Gao, X.; Feng, L.; Wang, Y.; Fan, X.; Ning, B.; Wan, S.; et al. The association between body mass index and in-hospital outcome among patients with acute myocardial infarction-Insights from China Acute Myocardial Infarction (CAMI) registry. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 808–814. [Google Scholar] [CrossRef]
- Holroyd, E.W.; Sirker, A.; Kwok, C.S.; Kontopantelis, E.; Ludman, P.F.; De Belder, M.A.; Butler, R.; Cotton, J.; Zaman, A.; Mamas, M.A.; et al. The Relationship of Body Mass Index to Percutaneous Coronary Intervention Outcomes: Does the Obesity Paradox Exist in Contemporary Percutaneous Coronary Intervention Cohorts? Insights from the British Cardiovascular Intervention Society Registry. JACC Cardiovasc. Interv. 2017, 10, 1283–1292. [Google Scholar] [CrossRef]
- Fukuoka, S.; Kurita, T.; Dohi, K.; Masuda, J.; Seko, T.; Tanigawa, T.; Saito, Y.; Kakimoto, H.; Makino, K.; Ito, M. Untangling the obesity paradox in patients with acute myocardial infarction after primary percutaneous coronary intervention (detail analysis by age). Int. J. Cardiol. 2019, 289, 12–18. [Google Scholar] [CrossRef]
- Niedziela, J.; Hudzik, B.; Niedziela, N.; Gąsior, M.; Gierlotka, M.; Wasilewski, J.; Myrda, K.; Lekston, A.; Poloński, L.; Rozentryt, P. The obesity paradox in acute coronary syndrome: A meta-analysis. Eur. J. Epidemiol. 2014, 29, 801–812. [Google Scholar] [CrossRef]
- Bucholz, E.M.; Beckman, A.L.; Krumholz, H.A.; Krumholz, H.M. Bucholz was affiliated with the Yale School of Medicine and Yale School of Public Health during the time that the work was conducted Excess weight and life expectancy after acute myocardial infarction: The obesity paradox reexamined. Am. Heart J. 2016, 172, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Dikaiou, P.; Björck, L.; Adiels, M.; Lundberg, C.E.; Mandalenakis, Z.; Manhem, K.; Rosengren, A. Obesity, overweight and risk for cardiovascular disease and mortality in young women. Eur. J. Prev. Cardiol. 2020, 2047487320908983. [Google Scholar] [CrossRef] [PubMed]
- Flicker, L.; McCaul, K.A.; Hankey, G.J.; Jamrozik, K.; Brown, W.J.; Byles, J.E.; Almeida, O.P. Body mass index and survival in men and women aged 70 to 75. J. Am. Geriatr. Soc. 2010, 58, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and all-cause mortality in older adults: A meta-analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cybulska, B.; Kłosiewicz-Latoszek, L. What does obesity paradox mean in coronary heart disease? Kardiol. Pol. 2013, 71, 963–968. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]



| Variables | Total | In-Hospital Mortality | p * | |||||
| Dead | Survivors | |||||||
| n | % | n | % | n | % | |||
| Sex (n = 1623) | M | 1063 | 65.6 | 59 | 56.2 | 1006 | 66.3 | 0.035 |
| BMI (n = 1007) | <18.5 | 20 | 2.0 | 1 | 2.6 | 19 | 2.0 | 0.024 |
| 18.5–24.9 | 287 | 28.5 | 19 | 50.0 | 268 | 27.6 | ||
| 25.0–29.9 | 384 | 38.1 | 11 | 28.9 | 373 | 38.5 | ||
| ≥30 | 317 | 31.4 | 7 | 18.4 | 309 | 32.0 | ||
| NRS (n = 1435) | <3 | 1331 | 92.8 | 66 | 79.5 | 1265 | 93.6 | <0.001 |
| ≥3 | 104 | 7.2 | 17 | 20.5 | 87 | 6.4 | ||
| HF (n = 1623) | Yes | 442 | 27.2 | 56 | 53.3 | 386 | 25.4 | <0.001 |
| CS (n = 1623) | Yes | 153 | 9.4 | 23 | 21.9 | 130 | 8.6 | <0.001 |
| MI (n = 1623) | STEMI | 494 | 30.4 | 36 | 34.3 | 458 | 30.2 | 0.005 |
| NSTEMI | 1045 | 64.4 | 57 | 54.3 | 988 | 65.1 | ||
| No info. | 84 | 5.2 | 12 | 11.4 | 72 | 4.7 | ||
| HT (n = 1623) | Yes | 356 | 21.9 | 69 | 65.7 | 287 | 18.9 | <0.001 |
| DM (n = 1623) | Yes | 327 | 20.1 | 43 | 41.0 | 284 | 18.7 | <0.001 |
| LDL (n = 1493) | ≥70 | 1184 | 79.3 | 37 | 59.7 | 1147 | 80.2 | <0.001 |
| Variables | x̅ | SD | x̅ | SD | x̅ | SD | p ** | |
| Age (years) (n = 1623) | 69 | 12 | 75.4 | 11.2 | 68.8 | 12.1 | <0.001 | |
| Body weight (kg) (n = 989) | 79.0 | 17.2 | 69.2 | 14.5 | 79.4 | 17.2 | <0.001 | |
| Height (cm) (n = 986) | 168.5 | 8.9 | 165.7 | 9.8 | 168.6 | 8.8 | 0.049 | |
| BMI (kg/m2) (n = 1007) | 27.9 | 5.3 | 25.5 | 4.7 | 28.0 | 5.3 | 0.004 | |
| TC (mg/dL) (n = 1522) | 178.1 | 53.3 | 149.8 | 55.7 | 179.3 | 52.9 | <0.001 | |
| TG (mg/dL) (n = 1511) | 141.3 | 90.4 | 126.9 | 69.2 | 143.1 | 91.2 | 0.16 | |
| HDL (mg/dL) (n = 1511) | 42.7 | 11.9 | 36.4 | 13.7 | 43.0 | 11.7 | <0.001 | |
| LDL (mg/dL) (n = 1493) | 107.3 | 43.7 | 87.8 | 44.3 | 108.2 | 43.4 | <0.001 | |
| Variables | Total | BMI | p * | |||||||||
| <18.5 n = 20 | 18.5–24.9 n = 287 | 25.0–29.9 n = 384 | ≥30 n = 317 | |||||||||
| n | % | n | % | n | % | n | % | n | % | |||
| Sex | M | 1065 | 65.6 | 10 | 50.0 | 187 | 65.2 | 266 | 69.3 | 202 | 63.7 | 0.18 |
| HF | Yes | 442 | 27.2 | 7 | 35.0 | 74 | 25.8 | 90 | 23.4 | 97 | 30.6 | 0.15 |
| CS | Yes | 153 | 9.4 | 0 | 0.0 | 34 | 11.8 | 32 | 8.3 | 21 | 6.6 | 0.06 |
| MI | STEMI | 494 | 30.4 | 2 | 10.0 | 87 | 30.3 | 128 | 33.6 | 79 | 25.2 | 0.041 |
| NSTEMI | 1045 | 64.4 | 17 | 85.0 | 182 | 63.4 | 243 | 63.3 | 223 | 70.4 | ||
| No info. | 84 | 5.2 | 1 | 5.0 | 18 | 6.3 | 12 | 3.1 | 14 | 4.4 | ||
| HT | Yes | 356 | 21.9 | 3 | 15.0 | 62 | 22.0 | 86 | 22.4 | 61 | 19.1 | 0.68 |
| DM | Yes | 327 | 20.1 | 3 | 15.0 | 38 | 13.2 | 62 | 16.2 | 95 | 30.1 | <0.001 |
| LDL | ≥70 | 1184 | 79.3 | 12 | 63.2 | 214 | 78.7 | 313 | 85.5 | 235 | 78.6 | 0.013 |
| Variables | x̅ | SD | x̅ | SD | x̅ | SD | x̅ | SD | x̅ | SD | p ** | |
| Age (years) | 69.2 | 12.1 | 72.4 | 10.5 | 70.1 | 12.7 | 68.9 | 12.2 | 67.6 | 11.6 | 0.058 | |
| Body weight (kg) | 79.0 | 17.2 | 44.2 | 7.6 | 64.6 | 9.0 | 78.7 | 9.3 | 95.9 | 14.6 | - | |
| Height (cm) | 168.5 | 8.9 | 166.7 | 9.9 | 168.0 | 8.7 | 169.3 | 8.6 | 168.1 | 9.2 | 0.13 | |
| BMI (kg/m2) | 28.0 | 5.3 | 16.0 | 2.3 | 22.8 | 1.6 | 27.4 | 1.4 | 34.1 | 3.7 | - | |
| TC (mg/dL) | 178.1 | 53.3 | 157.5 | 56.3 | 174.7 | 52.1 | 186.4 | 51.3 | 179.5 | 54.0 | 0.011 | |
| TG (mg/dL) | 142.4 | 90.4 | 114.9 | 42.3 | 127.6 | 112.8 | 136.4 | 74.6 | 164.7 | 96.4 | <0.001 | |
| HDL (mg/dL) | 42.7 | 11.9 | 41.1 | 17.6 | 45.1 | 13.1 | 44.4 | 11.0 | 39.8 | 10.1 | <0.001 | |
| LDL (mg/dL) | 107.3 | 43.7 | 93.8 | 41.1 | 104.7 | 41.2 | 114.8 | 43.0 | 106.9 | 44.6 | 0.006 | |
| Variables | Total n = 1623 | NRS < 3 n = 1331 | NRS ≥ 3 n = 104 | p * | ||||
| n | % | n | % | n | % | |||
| Sex | M | 1065 | 65.6 | 879 | 66.0 | 59 | 56.7 | 0.06 |
| BMI | <18.5 | 20 | 2.0 | 12 | 1.4 | 5 | 8.2 | <0.001 |
| 18.5–24.9 | 287 | 28.5 | 244 | 27.6 | 23 | 37.7 | ||
| 25.0–29.9 | 384 | 38.1 | 349 | 39.5 | 18 | 29.5 | ||
| ≥30 | 317 | 31.4 | 279 | 31.6 | 15 | 24.6 | ||
| HF | Yes | 442 | 27.2 | 366 | 27.5 | 36 | 34.6 | 0.12 |
| CS | Yes | 153 | 9.4 | 126 | 9.5 | 14 | 13.5 | 0.19 |
| MI | STEMI | 494 | 30.4 | 418 | 31.4 | 22 | 21.2 | 0.06 |
| NSTEMI | 1045 | 64.4 | 857 | 64.4 | 75 | 72.1 | ||
| No info. | 84 | 5.2 | 56 | 4.2 | 7 | 6.7 | ||
| HT | Yes | 356 | 21.9 | 294 | 22.1 | 20 | 19.2 | 0.50 |
| DM | Yes | 327 | 20.1 | 256 | 19.9 | 23 | 22.1 | 0.56 |
| LDL | ≥70 | 1184 | 79.3 | 999 | 80.0 | 55 | 63.2 | <0.001 |
| Variables | x̅ | SD | x̅ | SD | x̅ | SD | p ** | |
| Age (years) | 69.2 | 12.1 | 68.9 | 12.0 | 76.8 | 11.7 | <0.001 | |
| Body weight (kg) | 79.0 | 17.2 | 79.6 | 16.8 | 71.3 | 20.7 | <0.001 | |
| Height (cm) | 168.5 | 8.9 | 168.8 | 8.8 | 164.5 | 8.3 | <0.001 | |
| BMI (kg/m2) | 28.0 | 5.3 | 28.1 | 5.2 | 26.2 | 6.1 | 0.006 | |
| TC (mg/dL) | 178.1 | 53.3 | 179.6 | 53.2 | 156.3 | 52.9 | <0.001 | |
| TG (mg/dL) | 142.4 | 90.4 | 141.8 | 78.6 | 131.5 | 88.0 | 0.23 | |
| HDL (mg/dL) | 42.7 | 11.9 | 42.6 | 11.6 | 43.2 | 14.4 | 0.61 | |
| Age (years) | 107.3 | 43.7 | 108.9 | 43.9 | 85.4 | 36.5 | <0.001 | |
| Variables | Descriptive Statistics | |||||
|---|---|---|---|---|---|---|
| Me | x̅ | SD | n—Deaths | n—Survivors | ||
| BMI | <18.5 | 7.0 | 9.0 | 6.3 | 1 | 19 |
| 18.5–24.9 | 7.0 | 10.7 | 10.5 | 19 | 268 | |
| 25.0–29.9 | 7.0 | 10.2 | 10.0 | 11 | 373 | |
| ≥30 | 7.0 | 10.5 | 14.2 | 7 | 310 | |
| NRS | ≤3 | 7.0 | 10.7 | 11.8 | 66 | 1265 |
| >3 | 11.0 | 13.9 | 12.6 | 17 | 87 | |
| LDL | <70 | 9.0 | 12.2 | 14.9 | 25 | 284 |
| ≥70 | 7.0 | 10.6 | 10.5 | 37 | 1147 | |
| Single-Factor Model | Multi-Factor Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | p | HR | 95%CI HR (Lower) | 95%CI HR (Upper) | p | HR | 95%CI HR (Lower) | 95%CI HR (Upper) | |
| Sex | M | 0.051 | 0.68 | 0.46 | 1.01 | - | - | - | - |
| HF | Yes | <0.001 | 2.15 | 1.46 | 3.17 | 0.002 | 4.22 | 1.70 | 10.50 |
| BMI | <18.5 | 0.643 | 1.50 | 0.12 | 18.99 | Not included in the model | |||
| 25.0–29.9 | 0.389 | 0.44 | 0.21 | 0.93 | |||||
| ≥30 | 0.155 | 0.35 | 0.15 | 0.82 | |||||
| NRS | >3 | <0.001 | 2.77 | 1.62 | 4.75 | 0.005 | 4.66 | 1.60 | 16.61 |
| CS | Yes | 0.001 | 2.17 | 1.36 | 3.47 | - | - | - | - |
| MI | NSTEMI | 0.019 | 0.60 | 0.39 | 0.92 | - | - | - | - |
| HT | Yes | <0.001 | 7.38 | 4.93 | 11.05 | <0.001 | 37.82 | 12.70 | 112.60 |
| DM | Yes | <0.001 | 2.59 | 1.75 | 3.83 | - | - | - | - |
| LDL | ≥70 | 0.002 | 0.44 | 0.26 | 0.73 | - | - | - | - |
| Age (years) | <0.001 | 1.04 | 1.03 | 1.06 | <0.001 | 1.11 | 1.06 | 1.16 | |
| Body weight (kg) | 0.001 | 0.97 | 0.95 | 0.99 | Not included in the model | ||||
| Height (cm) | 0.087 | 0.97 | 0.93 | 1.00 | Not included in the model | ||||
| BMI (kg/m2) | 0.005 | 0.91 | 0.85 | 0.97 | 0.001 | 0.85 | 0.77 | 0.94 | |
| TC (mg/dL) | 0.001 | 0.99 | 0.98 | 0.995 | 0.017 | 0.99 | 0.98 | 0.99 | |
| TG (mg/dL) | 0.22 | 0.99 | 0.99 | 1.00 | 0.007 | 1.02 | 1.01 | 1.02 | |
| HDL (mg/dL) | <0.001 | 0.96 | 0.94 | 0.98 | - | - | - | - | |
| LDL (mg/dL) | 0.005 | 0.99 | 0.98 | 0.997 | Not included in the model | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czapla, M.; Karniej, P.; Juárez-Vela, R.; Łokieć, K. The Association between Nutritional Status and In-Hospital Mortality among Patients with Acute Coronary Syndrome—A Result of the Retrospective Nutritional Status Heart Study (NSHS). Nutrients 2020, 12, 3091. https://doi.org/10.3390/nu12103091
Czapla M, Karniej P, Juárez-Vela R, Łokieć K. The Association between Nutritional Status and In-Hospital Mortality among Patients with Acute Coronary Syndrome—A Result of the Retrospective Nutritional Status Heart Study (NSHS). Nutrients. 2020; 12(10):3091. https://doi.org/10.3390/nu12103091
Chicago/Turabian StyleCzapla, Michał, Piotr Karniej, Raúl Juárez-Vela, and Katarzyna Łokieć. 2020. "The Association between Nutritional Status and In-Hospital Mortality among Patients with Acute Coronary Syndrome—A Result of the Retrospective Nutritional Status Heart Study (NSHS)" Nutrients 12, no. 10: 3091. https://doi.org/10.3390/nu12103091








