Protective Effect of Vitis labrusca Leaves Extract on Cardiovascular Dysfunction through HMGB1-TLR4-NFκB Signaling in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Vitis labrusca Leaf (HP1) Extract
2.2. High-Performance Liquid Chromatography (HPLC) Analysis Method2
2.3. Experimental Animals and Blood Pressure
2.4. Electrocardiography Recording and Analysis (ECG)
2.5. Echocardiographic Analysis
2.6. Histological Analysis
2.7. Changes in Relaxation Response of Thoracic Aorta to Acetylcholine
2.8. Western Blot Analysis and Antibodies
2.9. Assessment of Cardiac Injury Biomarkers in Plasma
2.10. Statistical Analyses
3. Results
3.1. HPLC Chromatograms of Quercetin-3-O-Glucuronide from HP1 Extract
3.2. Effect of HP1 on Blood Pressure after Continuous Administration
3.3. Effects of HP1 on the ECG Changes in SHRs
3.4. Effect of HP1 on LV Remodeling and LV Function
3.5. Effect of HP1 on Cardiac Hypertrophy and Fibrosis in SHRs
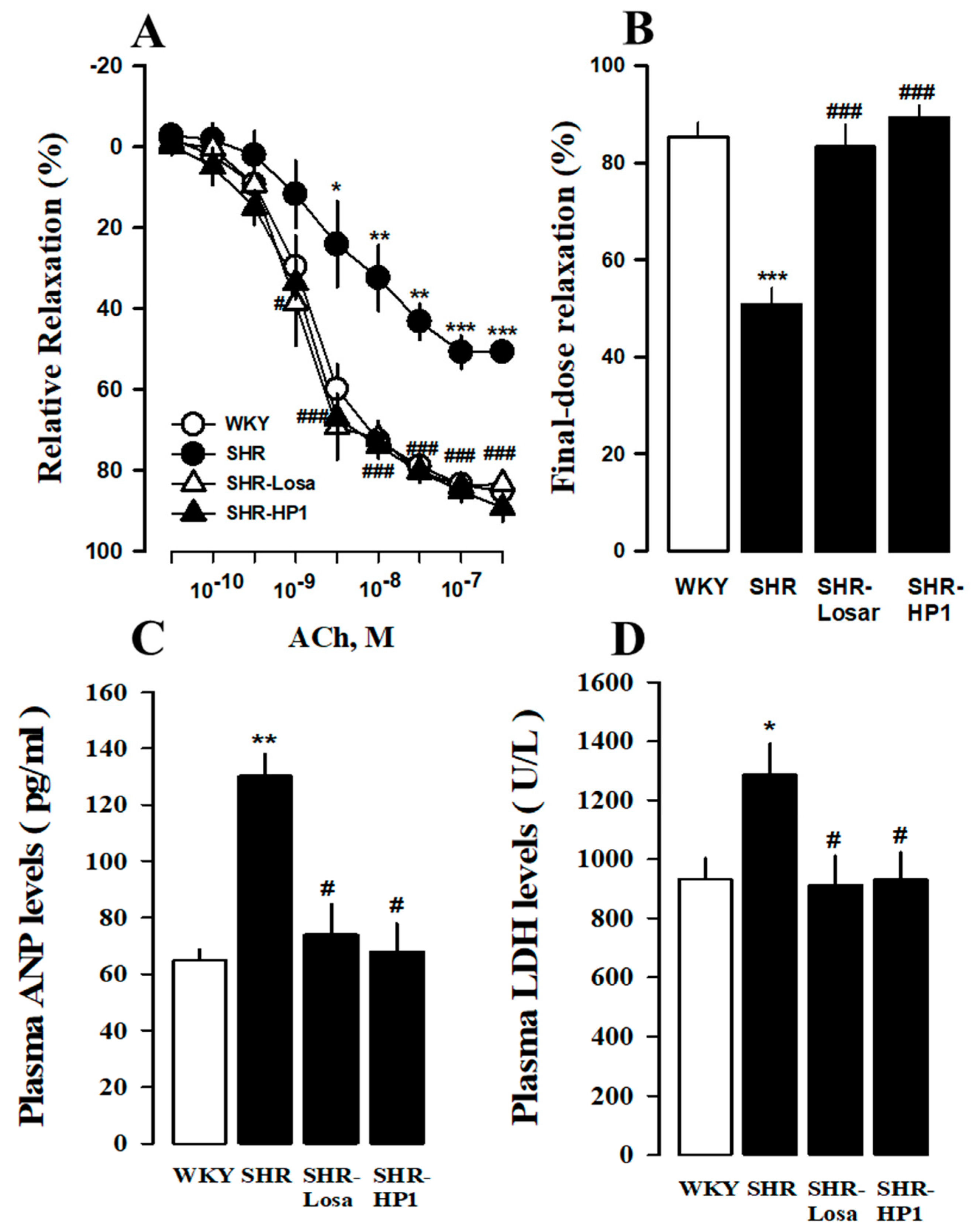
3.6. Effects of HP1 on Vascular Remodeling in SHRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katholi, R.E.; Couri, D.M. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. Int. J. Hypertens. 2011, 2011, 495349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otaki, Y.; Watanabe, T.; Takahashi, H.; Narumi, T.; Kadowaki, S.; Honda, Y.; Arimoto, T.; Shishido, T.; Miyamoto, T.; Konta, T.; et al. Association of renal tubular damage with cardio-renal anemia syndrome in patients with heart failure. Int. J. Cardiol. 2014, 173, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Banks, W.A.; Shah, G.N.; Gu, Z.; Sowers, J.R. Cardiorenal metabolic syndrome and diabetic cognopathy. Cardiorenal Med. 2013, 3, 265–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.; Kim, M.; Ko, Y.S.; Lee, H.Y.; Song, M.; Kim, H.K.; Cho, W.Y.; Jo, S.K. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol. Dial. Transplant. 2013, 28, 2766–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenvinkel, P. Inflammation in end-stage renal disease: The hidden enemy. Nephrology 2006, 11, 36–41. [Google Scholar] [CrossRef]
- Cardinale, J.P.; Sriramula, S.; Pariaut, R.; Guggilam, A.; Mariappan, N.; Elks, C.; Francis, J. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension 2010, 56, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Elks, C.M.; Mariappan, N.; Haque, M.; Guggilam, A.; Majid, D.S.; Francis, J. Chronic NF- {kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am. J. Physiol. Renal Physiol. 2009, 296, F298–F305. [Google Scholar] [CrossRef] [Green Version]
- Moubarak, M.; Jabbour, H.; Smayra, V.; Chouery, E.; Saliba, Y.; Jebara, V.; Fares, N. Cardiorenal syndrome in hypertensive rats: Microalbuminuria, inflammation and ventricular hypertrophy. Physiol. Res. 2012, 61, 13–24. [Google Scholar] [CrossRef]
- Eriguchi, M.; Tsuruya, K.; Haruyama, N.; Yamada, S.; Tanaka, S.; Suehiro, T.; Noguchi, H.; Masutani, K.; Torisu, K.; Kitazono, T. Renal denervation has blood pressure-independent protective effects on kidney and heart in a rat model of chronic kidney disease. Kidney Int. 2014, 87, 16–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.; Qu, H.; Wu, J.; Li, L.; Tang, Y. Endothelial microparticles activate endothelial cells to facilitate the inflammatory response. Mol. Med. Rep. 2017, 15, 1291–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.S. Wine Science: Principles and Applications; Academic Press: Boston, MA, USA, 1994. [Google Scholar]
- Dani, C.; Oliboni, L.S.; Vanderlinde, R.; Bonatto, D.; Salvador, M.; Henriques, J.A. Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. In Vitro 2010, 24, 148–153. [Google Scholar] [CrossRef]
- Oliboni, L.S.; Dani, C.; Funchal, C.; Henriques, J.A.; Salvador, M. Hepatoprotective, cardioprotective, and renal-protective effects of organic and conventional grapevine leaf extracts (Vitis labrusca var. Bordo) on Wistar rat tissues. An. Acad. Bras. Ciênc. 2011, 83, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Scola, G.; Fernandes, C.C.; Menin, E.; Salvador, M. Suppression of oncoprotein Her-2 and DNA damage after treatment with flavan-3-ol Vitis labrusca extract. Anticancer Agents. Med. Chem. 2013, 13, 1088–1095. [Google Scholar] [CrossRef]
- Kwon, S.U.; Lee, H.Y.; Xin, M.; Ji, S.J.; Cho, H.K.; Kim, D.S.; Kim, D.K.; Lee, Y.M. Antithrombotic activity of Vitis labrusca extract on rat platelet aggregation. Blood Coagul. Fibrinolysis 2016, 27, 141–146. [Google Scholar] [CrossRef]
- Kim, H.Y.; Oh, H.C.; Li, X.; Cho, K.W.; Kang, D.G.; Lee, H.S. Ethanol extract of seeds of Oenotheraodorata induces vasorelaxation via endothelium-dependent NO-cGMP signaling through activation of Akt-eNOS-sGC pathway. J. Ethnopharmacol. 2011, 133, 315–323. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic acid increases plasma ANP levels via an accentuation of cardiac ANP synthesis and secretion in rats. Eur. J. Pharmacol. 2013, 710, 73–79. [Google Scholar] [CrossRef]
- Stephan, L.S.; Almeida, E.D.; Markoski, M.M.; Garavaglia, J.; Marcadenti, A. Red Wine, Resveratrol and Atrial Fibrillation. Nutrients 2017, 9, 1190. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Ghiselli, A.; Buchetti, B.; Carnevale, R.; Natella, F.; Germanò, G.; Fimognari, F.; Di Santo, S.; Lenti, L.; Violi, F. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. Atherosclerosis 2006, 188, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of Wine, Alcohol and Polyphenols on Cardiovascular Disease Risk Factors: Evidences from Human Studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moura, R.S.; Viana, F.S.C.; Souza, M.A.V.; Kovary, K.; Guedes, D.C.; Oliveira, E.P.B.; Rubenich, L.M.S.; Carvalho, L.C.R.M.; Oliveira, R.M.; Tano, T.; et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J. Pharm. Pharmacol. 2002, 54, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.P.; Kaye, D.M.; Lambert, E.; Sommerville, M.; Socratous, F.; Esler, M.D. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 2003, 108, 560–565. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Paul, M.; Ganten, D. Lessons from rat models of hypertension: From Goldblatt to genetic engineering. Cardiovasc. Res. 1998, 39, 77–88. [Google Scholar] [CrossRef]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef]
- Conrad, C.H.; Brooks, W.W.; Hayes, J.A.; Sen, S.; Robinson, K.G.; Bing, O.H. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 1995, 91, 161–170. [Google Scholar] [CrossRef]
- Bing, O.H.; Brooks, W.W.; Robinson, K.G.; Slawsky, M.T.; Hayes, J.A.; Litwin, S.E.; Sen, S.; Conrad, C.H. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J. Mol. Cell. Cardiol. 1995, 27, 383–396. [Google Scholar] [CrossRef]
- Friberg, P.; Sundelin, B.; Bohman, S.O.; Bobik, A.; Nilsson, H.; Wickman, A.; Gustafsson, H.; Petersen, J.; Adams, M.A. Renin-angiotensin system in neonatal rats: Induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int. 1994, 45, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Tufro-McReddie, A.; Romano, L.M.; Harris, J.M.; Ferder, L.; Gomez, R.A. Angiotensin II regulates nephrogenesis and renal vascular development. Am. J. Physiol. 1995, 269, F110–F115. [Google Scholar] [CrossRef]
- Lee, R.M.; Berecek, K.H.; Tsoporis, J.; McKenzie, R.; Triggle, C.R. Prevention of hypertension and vascular changes by captopril treatment. Hypertension 1991, 17, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.N.; Berecek, K.H. Prevention of genetic hypertension by early treatment of spontaneously hypertensive rats with the angiotensin converting enzyme inhibitor captopril. Hypertension 1993, 22, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, J.J.; Beattie, E.C.; MacPherson, F. Macpherson Angiotensin II receptor antagonist losartan has persistent effects on blood pressure in the young spontaneously hypertensive rat: Lack of relation to vascular structure. J. Vasc. Res. 1992, 29, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Regan, C.P.; Bishop, S.P.; Berecek, K.H. Early, short-term treatment with captopril permanently attenuates cardiovascular changes in spontaneously hypertensive rats. Clin. Exp. Hypertens. 1997, 19, 1161–1177. [Google Scholar] [CrossRef]
- Allessie, M.A.; Boyden, P.A.; Camm, A.J.; Kleber, A.G.; Lab, M.J.; Legato, M.J.; Rosen, M.R.; Schwartz, P.J.; Spooner, P.M.; Wagoner, D.R.; et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001, 103, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation: The Framingham Heart Study. Circulation 1994, 89, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, S.M.; Larson, M.G.; Lauer, M.S.; Benjamin, E.J.; Levy, D. Influence of blood pressure on left atrial size: The Framingham Heart Study. Hypertension 1995, 25, 1155–1160. [Google Scholar] [CrossRef]
- Hennersdorf, M.G.; Strauer, B.E. Arterial hypertension and cardiac arrhythmias. J. Hypertens. 2001, 19, 167–177. [Google Scholar] [CrossRef]
- Frohlich, E.D. An updated concept of left ventricular hypertrophy risk in hypertension. Ochsner. J. 2009, 9, 181–190. [Google Scholar]
- Raman, S.V. The hypertensive heart. J. Am. Coll. Cardiol. 2010, 55, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Devereux, R.B.; Pickering, T.G.; Alderman, M.H.; Chien, S.; Borer, J.S.; Laragh, J.H. Left ventricular hypertrophy in hypertension. Prevalence and relationship to pathophysiologic variables. Hypertension 1987, 9, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanchetti, A. Cardiac hypertrophy as a target of antihypertensive therapy. Nat. Rev. Cardiol. 2010, 7, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, A.M.; Aalkjaer, C.; Bund, S.J.; Korsgaard, N.; Mulvany, M.J. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension 1993, 21, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J. Appl. Physiol. 2012, 113, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; Parise, G.; Bellamy, L.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS ONE 2014, 9, e89431. [Google Scholar] [CrossRef]
- Chou, T.C.; Yen, M.H.; Li, C.Y.; Ding, Y.A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension 1998, 31, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Song, X.A.; Jia, L.L.; Cui, W.; Zhang, M.; Chen, W.; Yuan, Z.Y.; Guo, J.; Li, H.H.; Zhu, G.Q.; Liu, H.; et al. Inhibition of TNF-α in hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by inhibiting neurohumoral excitation in spontaneously hypertensive rats. Toxicol. Appl. Pharmacol. 2014, 281, 101–108. [Google Scholar] [CrossRef]
- Li, Y.B.; Xu, P.; Xu, K.; Cai, Y.S.; Sun, M.Y.; Yang, L.; Sun, J.; Lu, S.M. Methotrexate affects HMGB1 expression in rheumatoid arthritis, and the downregulation of HMGB1 prevents rheumatoid arthritis progression. Mol. Cell. Biochem. 2016, 420, 161–170. [Google Scholar] [CrossRef]
- Nomura, S.; Fujita, S.; Ozasa, R.; Nakanishi, T.; Miyaji, M.; Mori, S.; Ito, T.; Ishii, K. The correlation between platelet activation markers and HMGB1 in patients with disseminated intravascular coagulation and hematologic malignancy. Platelets 2011, 22, 396–397. [Google Scholar] [CrossRef]
- Wang, W.J.; Yin, S.J.; Rong, R.Q. PKR and HMGB1 expression and function in rheumatoid arthritis. Genet. Mol. Res. 2015, 14, 17864–17870. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, H.; Ding, A.; Golenbock, D.T.; Latz, E.; Czura, C.J.; Fenton, M.J.; Tracey, K.J.; Yang, H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006, 26, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Chen, C.H.; Zhang, L.L.; Cao, X.J.; Ma, Q.L.; Deng, P.; Zhu, G.; Gao, C.Y.; Li, B.H.; Pi, Y.; et al. IRAK1 mediates TLR4-induced ABCA1 downregulation and lipid accumulation in VSMCs. Cell Death Dis. 2015, 6, e1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| SBP | DBP | MAP | HR | Flow | Volume | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WKY | 106.31 | ± | 3.82 | 94.68 | ± | 5.00 | 100.03 | ± | 4.61 | 353.30 | ± | 8.24 | 2.23 | ± | 0.39 | 3.26 | ± | 1.29 |
| SHR | 192.79 | ± | 4.06 *** | 167.18 | ± | 5.53 *** | 170.51 | ± | 5.37 *** | 477.03 | ± | 8.49 ** | 5.08 | ± | 1.08 * | 10.01 | ± | 1.90 ** |
| SHR-Losa | 147.93 | ± | 3.09 ## | 135.03 | ± | 4.11 ## | 129.12 | ± | 3.60 ## | 377.31 | ± | 9.82 # | 4.79 | ± | 1.72 | 5.44 | ± | 1.80 # |
| SHR-HP1 | 151.23 | ± | 3.50 ## | 120.86 | ± | 7.35 ## | 152.98 | ± | 6.82 ## | 365.61 | ± | 9.30 # | 3.87 | ± | 0.81 | 7.72 | ± | 1.77 # |
| LA | RA | LV | RV | Sep | WH | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WKY | 15 | ± | 0.001 | 19 | ± | 0.002 | 388 | ± | 0.021 | 128 | ± | 0.009 | 146 | ± | 0.012 | 699 | ± | 0.015 |
| SHR | 17 | ± | 0.001 * | 23 | ± | 0.001 * | 515 | ± | 0.01 ** | 153 | ± | 0.006 ** | 177 | ± | 0.01 ** | 882 | ± | 0.005 ** |
| SHR-Losa | 14 | ± | 0.001 | 18 | ± | 0.001 | 415 | ± | 0.012 # | 146 | ± | 0.004 | 153 | ± | 0.001 # | 746 | ± | 0.016 # |
| SHR-HP1 | 15 | ± | 0.001 | 20 | ± | 0.001 | 429 | ± | 0.031 # | 149 | ± | 0.010 | 170 | ± | 0.01 # | 782 | ± | 0.032 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.Y.; Hong, M.H.; Yoon, J.J.; Kim, D.S.; Na, S.W.; Jang, Y.J.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Protective Effect of Vitis labrusca Leaves Extract on Cardiovascular Dysfunction through HMGB1-TLR4-NFκB Signaling in Spontaneously Hypertensive Rats. Nutrients 2020, 12, 3096. https://doi.org/10.3390/nu12103096
Kim HY, Hong MH, Yoon JJ, Kim DS, Na SW, Jang YJ, Lee YJ, Kang DG, Lee HS. Protective Effect of Vitis labrusca Leaves Extract on Cardiovascular Dysfunction through HMGB1-TLR4-NFκB Signaling in Spontaneously Hypertensive Rats. Nutrients. 2020; 12(10):3096. https://doi.org/10.3390/nu12103096
Chicago/Turabian StyleKim, Hye Yoom, Mi Hyeon Hong, Jung Joo Yoon, Dae Sung Kim, Se Won Na, Youn Jae Jang, Yun Jung Lee, Dae Gill Kang, and Ho Sub Lee. 2020. "Protective Effect of Vitis labrusca Leaves Extract on Cardiovascular Dysfunction through HMGB1-TLR4-NFκB Signaling in Spontaneously Hypertensive Rats" Nutrients 12, no. 10: 3096. https://doi.org/10.3390/nu12103096
APA StyleKim, H. Y., Hong, M. H., Yoon, J. J., Kim, D. S., Na, S. W., Jang, Y. J., Lee, Y. J., Kang, D. G., & Lee, H. S. (2020). Protective Effect of Vitis labrusca Leaves Extract on Cardiovascular Dysfunction through HMGB1-TLR4-NFκB Signaling in Spontaneously Hypertensive Rats. Nutrients, 12(10), 3096. https://doi.org/10.3390/nu12103096





