Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies
Abstract
:1. Introduction
2. Materials and Methods
3. Results
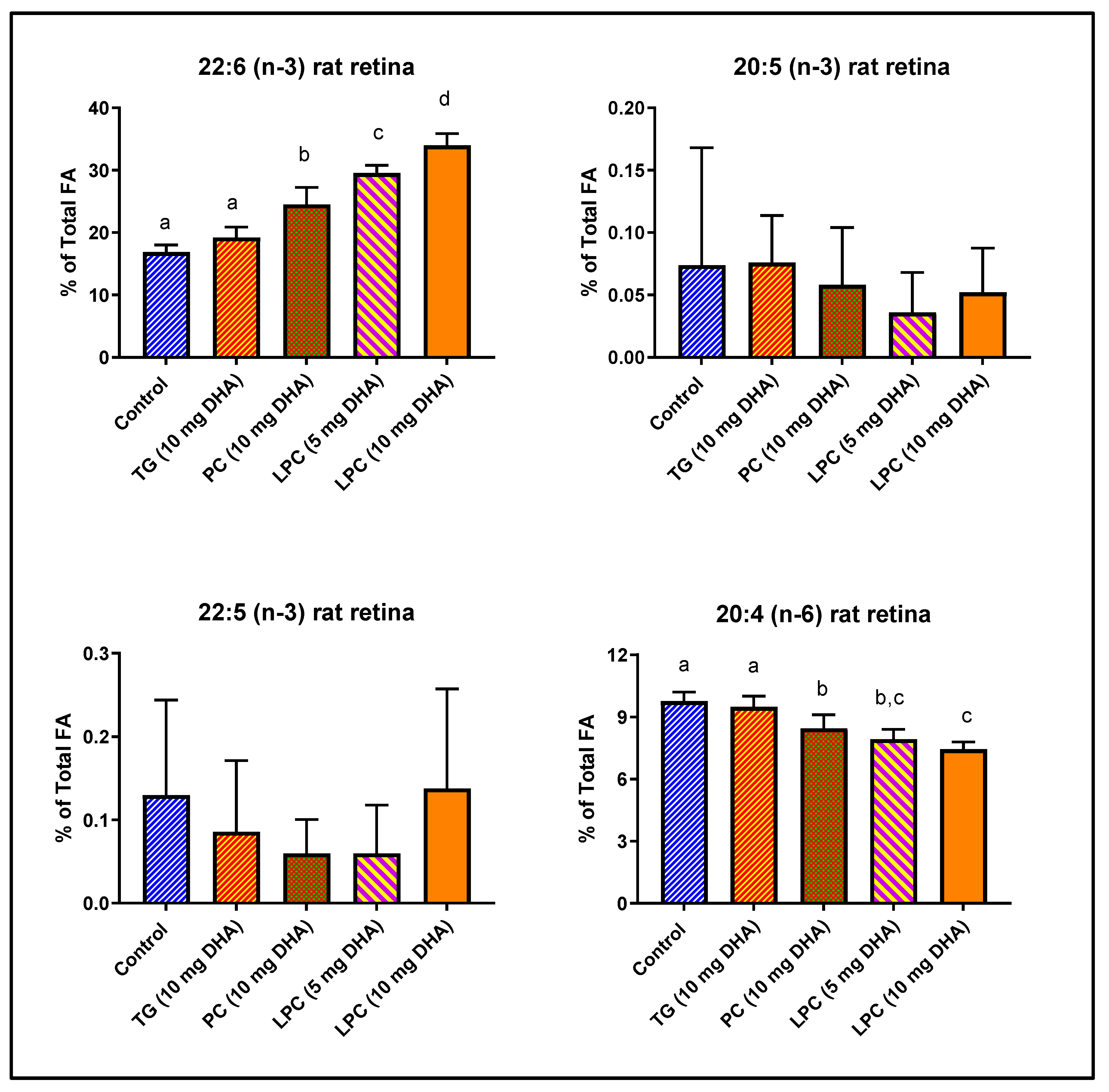
3.1. Comparative Effects of Dietary DHA in the Form of PC, TAG, and LPC in Rats
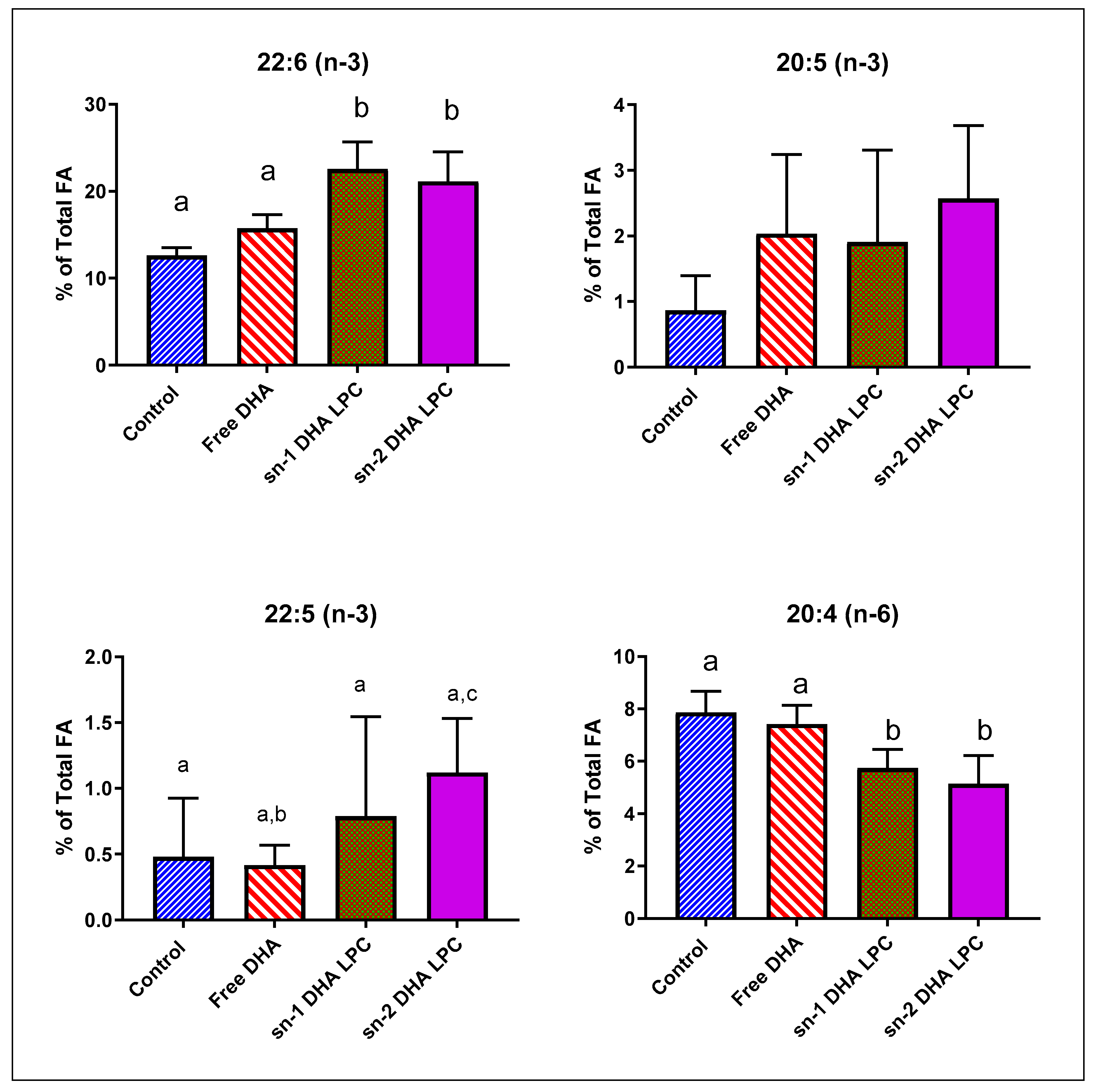
3.2. Comparative Effects of Free DHA and Isomers of LPC-DHA in Mice
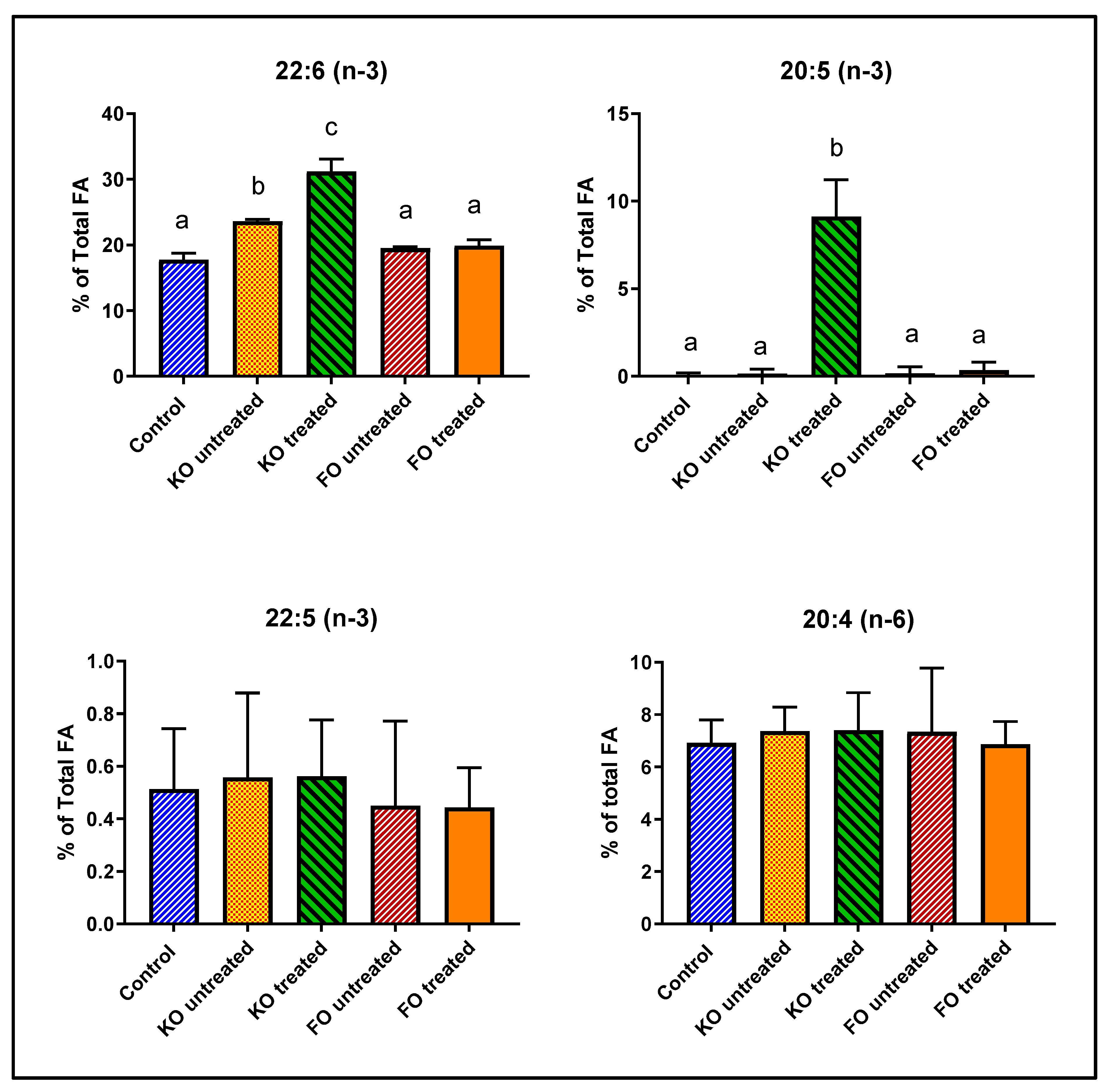
3.3. Effect of Fish Oil and Krill Oil on Mouse Retinal Omega-3 FA
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Stinson, A.M.; Wiegand, R.D.; Anderson, R.E. Recycling of docosahexaenoic acid in rat retinas during n-3 fatty acid deficiency. J. Lipid Res. 1991, 32, 2009–2017. [Google Scholar]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochr. Database Syst. Rev. 2017, 3, CD000376. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Progress Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Souied, E.H.; Aslam, T.; Garcia-Layana, A.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C. Omega-3 fatty acids and age-related macular degeneration. Ophthalmic Res. 2016, 55, 62–69. [Google Scholar] [CrossRef]
- Sapieha, P.; Chen, J.; Stahl, A.; Seaward, M.R.; Favazza, T.L.; Juan, A.M.; Hatton, C.J.; Joyal, J.S.; Krah, N.M.; Dennison, R.J.; et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabet. 2012, 2, e36. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.; Weymouth, A.E.; Fletcher, E.L.; Vingrys, A.J. A Role for Omega-3 Polyunsaturated Fatty Acid Supplements in Diabetic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1755–1764. [Google Scholar] [CrossRef] [Green Version]
- Hegde, K.R.; Varma, S.D. Electron impact mass spectroscopic studies on mouse retinal fatty acids: Effect of diabetes. Ophthalmic Res. 2009, 42, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Futterman, S.; Sturtevant, R.; Kupfer, C. Effect of alloxan diabetes on the fatty acid composition of the retina. Investig. Ophtalmol. Vis. Sci. 1969, 8, 542–544. [Google Scholar]
- Anderson, R.E.; Maude, M.B.; Bok, D. Low docosahexaenoic acid levels in rod outer segment membranes of mice with rds/peripherin and P216L peripherin mutations. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1715–1720. [Google Scholar]
- Gong, J.; Rosner, B.; Rees, D.G.; Berson, E.L.; Weigel-DiFranco, C.A.; Schaefer, E.J. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2596–2602. [Google Scholar]
- Martínez, M. Severe deficiency of docosahexaenoic acid in peroxisomal disorders: A defect of delta 4 desaturation? Neurology 1990, 40, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Hughbanks-Wheaton, D.K.; Pearson, N.S.; Fish, G.E.; Spencer, R.; Takacs, A.; Klein, M.; Locke, K.G.; Birch, D.G. Four-year placebo-controlled trial of docosahexaenoic acid in X-linked retinitis pigmentosa (DHAX trial): A randomized clinical trial. JAMA Ophthalmol. 2014, 132, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The nutritional AMD treatment 2 study. Ophthalmology 2013, 120, 1619–1631. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Bazan, N.G. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Asp. Med. 2018, 64, 18–33. [Google Scholar] [CrossRef]
- Tachikawa, M.; Akanuma, S.I.; Imai, T.; Okayasu, S.; Tomohiro, T.; Hatanaka, Y.; Hosoya, K.I. Multiple cellular transport and binding processes of unesterified docosahexaenoic acid in outer blood-retinal barrier retinal pigment epithelial cells. Biol. Pharm. Bull. 2018, 41, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.H.; Chan, J.P.; Cazenave-Gassiot, A.; Poh, R.W.; Foo, J.C.; Galam, D.L.; Ghosh, S.; Nguyen, L.N.; Barathi, V.A.; Yeo, S.W.; et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid (DHA) in eye and is important for photoreceptor cell development. J. Biol. Chem. 2016, 291, 10501–10514. [Google Scholar] [CrossRef] [Green Version]
- Yalagala, P.C.R.; Sugasini, D.; Zaldua, S.B.; Tai, L.M.; Subbaiah, P.V. Lipase treatment of dietary krill oil, but not fish oil, enables enrichment of brain eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Mol. Nutr. Food Res. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, P.T.; Milne, S.B.; Byrne, M.O.; Xiang, Y.; Brown, H.A. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Methods Enzymol. 2007, 432, 21–57. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, P.V.; Dammanahalli, K.J.; Yang, P.; Bi, J.; O’Donnell, J.M. Enhanced incorporation of dietary DHA into lymph phospholipids by altering its molecular carrier. Biochim. Biophys. Acta 2016, 1861, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachem, M.; Geloen, A.; Van, A.; Foumaux, B.; Fenart, L.; Gosselet, F.; Da Silva, P.; Breton, G.; Lagarde, M.; Picq, M.; et al. Efficient docosahexaenoic acid uptake by the brain from a structured phospholipid. Mol. Neurobiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Lyso-sn 1 Phosphatidylcholine Bound to Albumin: A Preferential Form for Rat Brain Uptake of Unsaturated Fatty Acids Compared to the Unesterified Form? In Proceedings of the 32nd International Conference on Biochemistry of Lipids, Granada, Spain, 18–21 September 1991; p. 3. [Google Scholar]
- Nishizawa, C.; Wang, J.-Y.; Sekine, S.; Saito, M. Effect of dietary DHA on DHA levels in retinal rod outer segments in young versus mature rats. Int. J. Vitam. Nutr. Res. 2003, 73, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.O.; Martins, S.V.; Lopes, P.A.; Miguueis, S.; Alfaia, C.M.; Pinto, R.M.A.; Rolo, E.A.; Bispo, P.; Batista, I.; Bandarra, N.M.; et al. Influence of feeding graded levels of canned sardines on the inflammatory markers and tissue fatty acid composition of Wistar rats. Br. J. Nutr. 2014, 112, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Begg, D.P.; Barr, D.; Garg, M.; Cameron-Smith, D.; Sinclair, A.J. Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br. J. Nutr. 2010, 103, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Tou, J.; Altman, S.; Gigliotti, J.; Benedito, V.; Cordonier, E. Different sources of omega-3 polyunsaturated fatty acids affects apparent digestibility, tissue deposition, and tissue oxidative stability in growing female rats. Lipids Health Dis. 2011, 10, 179. [Google Scholar] [CrossRef] [Green Version]
- Tikhonenko, M.; Lydic, T.A.; Opreanu, M.; Li Calzi, S.; Bozack, S.; McSorley, K.M.; Sochacki, A.L.; Faber, M.S.; Hazra, S.; Duclos, S.; et al. N-3 Polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS ONE 2013, 8, e55177. [Google Scholar] [CrossRef] [Green Version]
- Abcouwer, S.F.; Gardner, T.W. Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 2014, 1311, 174–190. [Google Scholar] [CrossRef] [Green Version]
- Rossino, M.G.; Casini, G. Nutraceuticals for the treatment of diabetic retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Glozman, S.; Weiner, L.; Yavin, E. Enhanced free radical scavenging and decreased lipid peroxidation in the rat fetal brain after treatment with ethyl docosahexaenoate. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2001, 1532, 203–212. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Robins, S.J.; Patton, G.M.; Sandberg, M.A.; Weigel-DiFranco, C.A.; Rosner, B.; Berson, E.L. Red blood cell membrane phosphatidylethanolamine fatty acid content in various forms of retinitis pigmentosa. J. Lipid Res. 1995, 36, 1427–1433. [Google Scholar] [PubMed]
- Kalogerou, M.; Kolovos, P.; Prokopiou, E.; Papagregoriou, G.; Deltas, C.; Malas, S.; Georgiou, T. Omega-3 fatty acids protect retinal neurons in the DBA/2J hereditary glaucoma mouse model. Exp. Eye Res. 2018, 167, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Morita, I.; Murota, S.I. Eicosapentaenoic acid attenuates vascular endothelial growth factor-induced proliferation via inhibiting Flk-1 receptor expression in bovine carotid artery endothelial cells. J. Cell Physiol. 1998, 176, 342–349. [Google Scholar] [CrossRef]
- McCusker, M.M.; Durrani, K.; Payette, M.J.; Suchecki, J. An eye on nutrition: The role of vitamins, essential fatty acids, and antioxidants in age-related macular degeneration, dry eye syndrome, and cataract. Clin. Dermatol. 2016, 34, 276–285. [Google Scholar] [CrossRef]
- Prokopiou, E.; Kolovos, P.; Georgiou, C.; Kalogerou, M.; Potamiti, L.; Sokratous, K.; Kyriacou, K.; Georgiou, T. Omega-3 fatty acids supplementation protects the retina from age-associated degeneration in aged C57BL/6J mice. BMJ Open Ophthalmol. 2019, 4, e000326. [Google Scholar] [CrossRef] [Green Version]
- Prokopiou, E.; Kolovos, P.; Kalogerou, M.; Neokleous, A.; Nicolaou, O.; Sokratous, K.; Kyriacou, K.; Georgiou, T. Omega-3 fatty acids supplementation: Therapeutic potential in a mouse model of stargardt disease. Investig. Ophtalmol. Vis. Sci. 2018, 59, 2757–2767. [Google Scholar] [CrossRef]
- Schnebelen, C.; Viau, S.; Grégoire, S.; Joffre, C.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L.; Acar, N. Nutrition for the eye: Different susceptibility of the retina and the lacrimal gland to dietary omega-6 and omega-3 polyunsaturated fatty acid incorporation. Ophthalmic Res. 2009, 41, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Brush, R.S.; Elliott, M.H.; Wicker, L.D.; Henry, K.R.; Anderson, R.E. High levels of retinal membrane docosahexaenoic acid increase susceptibility to stress-induced degeneration. J. Lipid Res. 2009, 50, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, M.; Sauvé, Y.; Merrells, K.J.; Kang, J.X.; Ma, D.W.L. Supranormal electroretinogram in F at-1 mice with retinas enriched in docosahexaenoic acid and n-3 very long chain fatty acids (C24–C36). Investig. Ophtalmol. Vis. Sci. 2009, 50, 4394–4401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of -ë-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Lobanova, E.S.; Schuhmann, K.; Finkelstein, S.; Lewis, T.R.; Cady, M.A.; Hao, Y.; Keuthan, C.; Ash, J.D.; Burns, M.E.; Shevchenko, A.; et al. Disrupted blood-retina lysophosphatidylcholine transport impairs photoreceptor health but not visual signal transduction. J. Neurosci. 2019, 39, 9689–9701. [Google Scholar] [CrossRef]
- Chen, C.T.; Bazinet, R.P. b-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot. Essent. Fatty Acids 2015, 92, 33–40. [Google Scholar] [CrossRef]
- Kaur, G.; Molero, J.C.; Weisinger, H.S.; Sinclair, A.J. Orally administered [14C] DPA and [14C] DHA are metabolised differently to [14C] EPA in rats. Br. J. Nutr. 2013, 109, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Yalagala, P.C.R.; Sugasini, D.; Dasarathi, S.; Pahan, K.; Subbaiah, P.V. Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: Potential treatment for depression. J. Lipid Res. 2019, 60, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Suh, M.; Clandinin, M.T. 20:5n-3 but not 22:6n-3 is a preferred substrate for synthesis of n-3 very-long-chain fatty acids (C24–C36) in retina. Curr. Eye Res. 2005, 30, 959–968. [Google Scholar] [CrossRef]
- Yu, M.; Benham, A.; Logan, S.; Brush, R.S.; Mandal, M.N.; Anderson, R.E.; Agbaga, M.P. ELOVL4 protein preferentially elongates 20:5n3 to very long chain PUFAs over 20:4n6 and 22:6n3. J. Lipid Res. 2012, 53, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, B.; Mukherjee, P.K.; Asatryan, A.; Kautzmann, M.A.; Heap, J.; Gordon, W.C.; Bhattacharjee, S.; Yang, R.; Petasis, N.A.; Bazan, N.G. Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity. Sci. Rep. 2017, 7, 5279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faiq, M.A.; Wollstein, G.; Schuman, J.S.; Chan, K.C. Cholinergic nervous system and glaucoma: From basic science to clinical applications. Prog. Retin. Eye Res. 2019, 72, 100767. [Google Scholar] [CrossRef] [PubMed]

| Control | TG-DHA | PC-DHA | LPC-DHA 5 mg | LPC-DHA 10 mg | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.A. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |||||||||
| 12:0 | 4.39 | ± | 1.19 | 4.71 | ± | 2.09 | 4.57 | ± | 1.49 | 3.10 | ± | 0.08 | 3.10 | ± | 0.60 | ||||
| 14:0 | 0.08 | ± | 0.04 | 0.17 | ± | 0.18 | 0.97 | ± | 1.97 | 0.52 | ± | 0.90 | 0.11 | ± | 0.13 | ||||
| 16:0 | 22.14 | ± | 0.65 | 21.52 | ± | 1.37 | 19.06 | ± | 0.76 | ** | 18.05 | ± | 0.84 | ** | 17.27 | ± | 0.94 | ** | |
| 16:1 (n-7) | 0.73 | ± | 0.06 | 0.74 | ± | 0.09 | 0.65 | ± | 0.04 | 0.66 | ± | 0.08 | 0.68 | ± | 0.05 | ||||
| 17:1 (n-7) | 0.02 | ± | 0.02 | 0.12 | ± | 0.03 | * | 0.03 | ± | 0.02 | 0.01 | ± | 0.00 | 0.02 | ± | 0.02 | |||
| 18:0 | 20.26 | ± | 0.92 | 19.09 | ± | 1.30 | 17.79 | ± | 0.97 | 16.84 | ± | 0.43 | ** | 15.62 | ± | 0.85 | ** | ||
| 18:1 (n-9) | 18.98 | ± | 0.90 | 18.21 | ± | 1.57 | 17.70 | ± | 1.26 | 17.07 | ± | 0.94 | 15.81 | ± | 1.46 | ||||
| 18:1(n-7) | 4.40 | ± | 0.28 | 4.09 | ± | 0.16 | 3.91 | ± | 0.25 | 3.79 | ± | 0.16 | 3.43 | ± | 0.28 | * | |||
| 18:2 (n-6) | 0.97 | ± | 0.05 | 1.05 | ± | 0.36 | 0.95 | ± | 0.22 | 0.93 | ± | 0.26 | 0.97 | ± | 0.26 | ||||
| 18:3 (n-6) | 0.39 | ± | 0.02 | 0.38 | ± | 0.11 | 0.49 | ± | 0.13 | 0.50 | ± | 0.11 | 0.37 | ± | 0.08 | ||||
| 18:3 (n-3) | 0.03 | ± | 0.02 | 0.09 | ± | 0.10 | 0.07 | ± | 0.07 | 0.08 | ± | 0.05 | 0.04 | ± | 0.04 | ||||
| 20:0 | 0.05 | ± | 0.04 | 0.08 | ± | 0.05 | 0.05 | ± | 0.03 | 0.04 | ± | 0.03 | 0.04 | ± | 0.03 | ||||
| 20:1 (n-9) | 0.05 | ± | 0.05 | 0.06 | ± | 0.05 | 0.07 | ± | 0.03 | 0.05 | ± | 0.03 | 0.03 | ± | 0.02 | ||||
| 20:2 (n-6) | 0.08 | ± | 0.04 | 0.04 | ± | 0.01 | 0.06 | ± | 0.05 | 0.05 | ± | 0.04 | 0.06 | ± | 0.04 | ||||
| 20:3 (n-6) | 0.08 | ± | 0.10 | 0.16 | ± | 0.18 | 0.06 | ± | 0.04 | 0.09 | ± | 0.13 | 0.13 | ± | 0.15 | ||||
| 20:4 (n-6) | 9.77 | ± | 0.44 | 9.49 | ± | 0.52 | 8.45 | ± | 0.67 | 7.94 | ± | 0.48 | * | 7.44 | ± | 0.37 | ** | ||
| 22:0 | 0.04 | ± | 0.02 | 0.05 | ± | 0.04 | 0.07 | ± | 0.07 | 0.03 | ± | 0.02 | 0.04 | ± | 0.02 | ||||
| 20:5 (n-3) | 0.07 | ± | 0.09 | 0.08 | ± | 0.04 | 0.06 | ± | 0.05 | 0.04 | ± | 0.03 | 0.05 | ± | 0.04 | ||||
| 22:2 (n-6) | 0.11 | ± | 0.08 | 0.13 | ± | 0.06 | 0.12 | ± | 0.05 | 0.12 | ± | 0.04 | 0.13 | ± | 0.03 | ||||
| 22:4 (n-6) | 0.05 | ± | 0.03 | 0.13 | ± | 0.17 | 0.07 | ± | 0.05 | 0.24 | ± | 0.45 | 0.32 | ± | 0.62 | ||||
| 22:5 (n-6) | 0.02 | ± | 0.01 | 0.03 | ± | 0.04 | 0.02 | ± | 0.01 | 0.02 | ± | 0.03 | 0.03 | ± | 0.02 | ||||
| 22:5 (n-3) | 0.13 | ± | 0.11 | 0.09 | ± | 0.09 | 0.06 | ± | 0.04 | 0.06 | ± | 0.06 | 0.14 | ± | 0.12 | ||||
| 22:6 (n-3) | 16.88 | ± | 1.15 | 19.20 | ± | 1.67 | 24.53 | ± | 2.73 | * | 29.56 | ± | 1.23 | ** | 33.98 | ± | 1.91 | ** | |
| 24:1 (n-9) | 0.06 | ± | 0.04 | 0.07 | ± | 0.04 | 0.05 | ± | 0.02 | 0.03 | ± | 0.02 | 0.03 | ± | 0.02 | ||||
| 16:0 DMA | 0.03 | ± | 0.03 | 0.09 | ± | 0.08 | 0.05 | ± | 0.08 | 0.12 | ± | 0.12 | 0.08 | ± | 0.10 | ||||
| 18:0 DMA | 0.16 | ± | 0.30 | 0.05 | ± | 0.03 | 0.03 | ± | 0.02 | 0.03 | ± | 0.02 | 0.02 | ± | 0.01 | ||||
| 18:1 DMA | 0.06 | ± | 0.05 | 0.08 | ± | 0.08 | 0.06 | ± | 0.04 | 0.05 | ± | 0.02 | 0.06 | ± | 0.06 | ||||
| Control | Free DHA | sn-1 DHA LPC | sn-2 DHA LPC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | ||
| 12:0 | 0.26 | ± | 0.14 | 0.60 | ± | 0.35 | 0.73 | ± | 0.47 | 0.98 | ± | 0.34 | ||
| 14:0 | 0.50 | ± | 0.25 | 0.20 | ± | 0.17 | 0.30 | ± | 0.17 | 0.33 | ± | 0.25 | ||
| 16:0 | 17.92 | ± | 1.41 | 16.11 | ± | 1.56 | 14.62 | ± | 1.22 | ** | 15.05 | ± | 1.56 | ** |
| 16:1 | 0.38 | ± | 0.17 | 0.55 | ± | 0.28 | 0.47 | ± | 0.34 | 0.45 | ± | 0.37 | ||
| 17:1 | 0.29 | ± | 0.13 | 0.43 | ± | 0.16 | 0.48 | ± | 0.38 | 0.61 | ± | 0.47 | ||
| 18:0 | 18.90 | ± | 1.05 | 17.32 | ± | 0.66 | 15.44 | ± | 1.50 | ** | 15.40 | ± | 0.58 | ** |
| 18:1 (n-9) | 18.23 | ± | 0.82 | 17.14 | ± | 0.78 | 15.56 | ± | 1.16 | ** | 15.69 | ± | 1.10 | ** |
| 18:1(n-7) | 4.64 | ± | 0.48 | 4.18 | ± | 0.34 | 3.82 | ± | 0.62 | 3.87 | ± | 0.61 | ||
| 18:2 (n-6) | 0.69 | ± | 0.45 | 0.99 | ± | 0.65 | 1.38 | ± | 0.49 | 1.05 | ± | 0.40 | ||
| 18:3 (n-6) | 0.36 | ± | 0.19 | 0.49 | ± | 0.36 | 0.60 | ± | 0.47 | 0.28 | ± | 0.13 | ||
| 18:3 (n-3) | 0.54 | ± | 0.43 | 0.49 | ± | 0.15 | 0.51 | ± | 0.42 | 0.49 | ± | 0.37 | ||
| 20:0 | 1.86 | ± | 0.69 | 1.90 | ± | 0.64 | 1.18 | ± | 0.72 | 2.13 | ± | 1.71 | ||
| 20:1 (n-9) | 3.71 | ± | 0.42 | 3.42 | ± | 0.59 | 2.87 | ± | 0.60 | 2.62 | ± | 0.48 | ||
| 20:2 (n-6) | 1.28 | ± | 0.84 | 0.71 | ± | 0.50 | 0.88 | ± | 0.95 | 1.06 | ± | 0.76 | ||
| 20:3 (n-6) | 0.69 | ± | 0.54 | 1.30 | ± | 0.66 | 1.44 | ± | 0.35 | 1.16 | ± | 0.77 | ||
| 20:4 (n-6) | 7.86 | ± | 0.81 | 7.42 | ± | 0.72 | 5.75 | ± | 0.71 | ** | 5.14 | ± | 1.08 | ** |
| 22:0 | 1.20 | ± | 0.41 | 1.08 | ± | 0.59 | 1.24 | ± | 0.95 | 1.27 | ± | 1.14 | ||
| 20:5 (n-3) | 0.86 | ± | 0.53 | 2.03 | ± | 1.21 | 1.91 | ± | 1.40 | 2.57 | ± | 1.11 | ||
| 22:2 | 0.80 | ± | 0.34 | 0.77 | ± | 0.51 | 1.00 | ± | 0.76 | 1.32 | ± | 0.80 | ||
| 22:4 (n-6) | 2.03 | ± | 0.91 | 2.51 | ± | 0.85 | 2.23 | ± | 0.87 | 2.10 | ± | 0.65 | ||
| 22:5 (n-3) | 0.48 | ± | 0.44 | 0.42 | ± | 0.15 | 0.79 | ± | 0.76 | 1.12 | ± | 0.41 | ||
| 22:6 (n-3) | 12.61 | ± | 0.91 | 15.74 | ± | 1.57 | 22.57 | ± | 3.12 | ** | 21.14 | ± | 3.39 | ** |
| 24:1 | 0.78 | ± | 0.48 | 0.51 | ± | 0.25 | 0.49 | ± | 0.17 | 0.54 | ± | 0.40 | ||
| 16:0 DMA | 1.16 | ± | 0.96 | 0.75 | ± | 0.56 | 1.02 | ± | 0.32 | 0.98 | ± | 0.47 | ||
| 18:0 DMA | 1.25 | ± | 1.04 | 2.10 | ± | 0.88 | 1.96 | ± | 0.30 | 1.78 | ± | 1.04 | ||
| 18:1 DMA | 0.73 | ± | 0.75 | 0.85 | ± | 0.56 | 0.78 | ± | 0.64 | 0.89 | ± | 0.51 | ||
| Control | KO Untreated | KO Treated | FO Untreated | FO Treated | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.A. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D | ||||||
| 12:0 | 0.02 | ± | 0.03 | 0.06 | ± | 0.06 | 0.07 | ± | 0.05 | 0.02 | ± | 0.01 | 0.06 | ± | 0.05 | |
| 14:0 | 0.17 | ± | 0.27 | 0.38 | ± | 0.44 | 0.09 | ± | 0.04 | 0.20 | ± | 0.33 | 0.03 | ± | 0.04 | |
| 16:0 | 22.07 | ± | 1.84 | 20.34 | ± | 1.94 | 12.51 | ± | 7.01 | 22.20 | ± | 1.90 | 22.67 | ± | 2.12 | |
| 16:1 (n-7) | 0.20 | ± | 0.31 | 0.19 | ± | 0.17 | 0.10 | ± | 0.13 | 0.06 | ± | 0.07 | 0.04 | ± | 0.04 | |
| 18:0 | 18.69 | ± | 0.42 | 17.20 | ± | 1.25 | 14.20 | ± | 1.62 | * | 17.84 | ± | 0.88 | 18.03 | ± | 0.13 |
| 18:1 (n-9) | 20.04 | ± | 0.64 | 14.99 | ± | 5.59 | 14.75 | ± | 1.50 | ** | 19.51 | ± | 0.76 | 19.32 | ± | 0.99 |
| 18:1(n-7) | 4.79 | ± | 0.21 | 4.36 | ± | 0.42 | 2.35 | ± | 2.08 | 4.46 | ± | 0.14 | 3.72 | ± | 2.09 | |
| 18:2 (n-6) | 0.88 | ± | 0.46 | 0.99 | ± | 0.51 | 1.10 | ± | 0.34 | 1.22 | ± | 0.19 | 0.87 | ± | 0.69 | |
| 18:3 (n-6) | 0.06 | ± | 0.04 | 0.06 | ± | 0.06 | 0.18 | ± | 0.16 | 0.06 | ± | 0.05 | 0.05 | ± | 0.01 | |
| 18:3 (n-3) | 0.81 | ± | 0.53 | 0.82 | ± | 0.74 | 0.29 | ± | 0.24 | 0.79 | ± | 0.68 | 0.41 | ± | 0.31 | |
| 20:0 | 0.05 | ± | 0.03 | 0.03 | ± | 0.03 | 0.04 | ± | 0.04 | 0.05 | ± | 0.03 | 0.02 | ± | 0.01 | |
| 20:1 (n-9) | 2.17 | ± | 1.17 | 2.00 | ± | 1.41 | 0.78 | ± | 0.44 | 2.13 | ± | 0.55 | 1.12 | ± | 0.92 | |
| 20:2 (n-6) | 0.22 | ± | 0.19 | 0.38 | ± | 0.25 | 0.19 | ± | 0.15 | 0.56 | ± | 0.39 | 0.21 | ± | 0.10 | |
| 20:3 (n-6) | 0.03 | ± | 0.02 | 0.11 | ± | 0.13 | 0.44 | ± | 0.41 | 0.05 | ± | 0.02 | 0.23 | ± | 0.41 | |
| 20:4 (n-6) | 6.92 | ± | 0.88 | 7.37 | ± | 0.92 | 7.41 | ± | 1.43 | 7.34 | ± | 2.44 | 6.87 | ± | 0.86 | |
| 22:0 | 0.10 | ± | 0.10 | 0.37 | ± | 0.32 | 0.09 | ± | 0.10 | 0.14 | ± | 0.25 | 0.06 | ± | 0.06 | |
| 20:5 (n-3) | 0.09 | ± | 0.10 | 0.15 | ± | 0.26 | 9.12 | ± | 2.09 | ** | 0.18 | ± | 0.36 | 0.36 | ± | 0.45 |
| 22:2 (n-6) | 0.09 | ± | 0.09 | 0.20 | ± | 0.18 | 0.15 | ± | 0.16 | 0.12 | ± | 0.13 | 0.14 | ± | 0.15 | |
| 22:4 (n-6) | 1.11 | ± | 1.40 | 2.56 | ± | 1.44 | 1.79 | ± | 1.55 | 0.55 | ± | 1.10 | 2.76 | ± | 0.53 | |
| 22:5 (n-6) | 0.06 | ± | 0.04 | 0.15 | ± | 0.09 | 0.24 | ± | 0.20 | 0.17 | ± | 0.19 | 0.06 | ± | 0.03 | |
| 22:5 (n-3) | 0.51 | ± | 0.23 | 0.56 | ± | 0.32 | 0.56 | ± | 0.22 | 0.45 | ± | 0.32 | 0.44 | ± | 0.15 | |
| 22:6 (n-3) | 17.76 | ± | 1.00 | 23.62 | ± | 0.26 | 31.18 | ± | 1.89 | ** | 19.52 | ± | 0.18 | 19.89 | ± | 0.91 |
| 24:1 (n-9) | 0.04 | ± | 0.03 | 0.11 | ± | 0.13 | 0.09 | ± | 0.06 | 0.04 | ± | 0.01 | 0.03 | ± | 0.03 | |
| 16:0 DMA | 0.05 | ± | 0.02 | 0.05 | ± | 0.03 | 0.06 | ± | 0.04 | 0.13 | ± | 0.17 | 0.03 | ± | 0.02 | |
| 18:0 DMA | 2.96 | ± | 0.467 | 2.642 | ± | 0.95 | 2.126 | ± | 0.56 | 1.97 | ± | 0.67 | 2.48 | ± | 0.47 | |
| 18:1 DMA | 0.02 | ± | 0.02 | 0.05 | ± | 0.05 | 0.04 | ± | 0.04 | 0.02 | ± | 0.02 | 0.03 | ± | 0.03 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 2020, 12, 3114. https://doi.org/10.3390/nu12103114
Sugasini D, Yalagala PCR, Subbaiah PV. Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients. 2020; 12(10):3114. https://doi.org/10.3390/nu12103114
Chicago/Turabian StyleSugasini, Dhavamani, Poorna C. R. Yalagala, and Papasani V. Subbaiah. 2020. "Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies" Nutrients 12, no. 10: 3114. https://doi.org/10.3390/nu12103114





