Supplementation with Resveratrol, Piperine and Alpha-Tocopherol Decreases Chronic Inflammation in a Cluster of Older Adults with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and PMN Isolation
2.2. Neutrophils Viability
2.3. PMNs Count and Viability
2.4. PMN Oxygen Consumption
2.5. PMN Chemiluminescence
2.6. Statistics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Diaz, A.; Espeche, W.; March, C.; Flores, R.; Parodi, R.; Genesio, M.; Sabio, R.; Poppe, S. Prevalencia del síndrome metabólico en Argentina en los últimos 25 años: Revisión sistemática de estudios observacionales poblacionales. Hipertensión y Riesgo Vascular 2018, 35, 64–69. [Google Scholar] [CrossRef]
- Marquez-Sandoval, F.; Macedo-Ojeda, G.; Hörner, D.V.; Ballart, J.F.; Salas-Salvadó, J.; Vizmanos, B. The prevalence of metabolic syndrome in Latin America: A systematic review. Public Health Nutr. 2011, 14, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, W.; Lun, Z.; Zhang, H.; Sun, Z.; Kanu, J.S.; Qiu, S.; Cheng, Y.; Liu, Y. Prevalence of metabolic syndrome in mainland china: A meta-analysis of published studies. BMC Public Health 2016, 16, 296. [Google Scholar] [CrossRef] [Green Version]
- Vidigal, F.d.C.; Bressan, J.; Babio, N.; Salas-Salvadó, J. Prevalence of metabolic syndrome in Brazilian adults: A systematic review. BMC Public Health 2013, 13, 1198. [Google Scholar]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the Metabolic Syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef]
- Ansarimoghaddam, A.; Adineh, H.A.; Zareban, I.; Iranpour, S.; Hosseinzadeh, A.; Kh, F. Prevalence of metabolic syndrome in Middle-East countries: Meta-analysis of cross-sectional studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 195–201. [Google Scholar] [CrossRef]
- Raposo, L.; Severo, M.; Barros, H.; Santos, A.C. The prevalence of the metabolic syndrome in Portugal: The PORMETS study. BMC Public Health 2017, 17, 555. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Taverne, Y.J.H.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life. BioEssays 2018, 40, 1700158. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Chielle, E.O.; Gens, F.; Rossi, E.M. Oxidative, inflammatory and cardiometabolic biomarkers of clinical relevance in patients with metabolic syndrome. J. Bras. Patol. Med. Lab. 2018, 54, 213–219. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Zhang, S.M.; Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Buring, J.E.; Manson, J.E. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: A randomized trial. J. Am. Med. Assoc. 2008, 300, 2012–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the Prevention of Cardiovascular Disease in Men. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smithjr, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhns, D.B.; Priel, D.A.L.; Chu, J.; Zarember, K.A. Isolation and Functional Analysis of Human Neutrophils. Curr. Protoc. Immunol. 2015, 111, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Valdez, L.B.; Boveris, A. Nitric Oxide and Superoxide Radical Production by Human Mononuclear Leukocytes. Antioxid. Redox Signal. 2001, 3, 505–513. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Repetto, M. Clinical use of chemiluminescence assays for the determination of systemic oxidative stress. In Handbook of Chemiluminescent Methods in Oxidative Stress Assessment; Popov, I., Lewin, G., Eds.; Transword Research Network: Kerala, India, 2008; Volume 9, pp. 163–194. ISBN 978-81-7895-334-2. [Google Scholar]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. Lipid Peroxidation 2012, 1, 3–30. [Google Scholar] [CrossRef] [Green Version]
- Bomfim, G.F.; Rodrigues, F.; Carneiro, F.S. Are the innate and adaptive immune systems setting hypertension on fire? Pharmacol. Res. 2017, 117, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Caillon, A.; Paradis, P.; Schiffrin, E.L. Role of immune cells in hypertension. Br. J. Pharmacol. 2018, 176, 1818–1828. [Google Scholar] [CrossRef]
- Mikolajczyk, T.P.; Guzik, T.J. Adaptive Immunity in Hypertension. Curr. Hypertens. Rep. 2019, 21, 68. [Google Scholar] [CrossRef] [Green Version]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Haase, J.; Weyer, U.; Immig, K.; Klöting, N.; Blüher, M.; Eilers, J.; Bechmann, I.; Gericke, M. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia 2013, 57, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Raciti, G.A.; Spinelli, R.; Desiderio, A.; Longo, M.; Parrillo, L.; Nigro, C.; D’Esposito, V.; Mirra, P.; Fiory, F.; Pilone, V.; et al. Specific CpG hyper-methylation leads to Ankrd26 gene down-regulation in white adipose tissue of a mouse model of diet-induced obesity. Sci. Rep. 2017, 7, 43526. [Google Scholar] [CrossRef] [PubMed]
- Jukema, R.A.; Ahmed, T.A.N.; Tardif, J.-C. Does low-density lipoprotein cholesterol induce inflammation? If so, does it matter? Current insights and future perspectives for novel therapies. BMC Med. 2019, 17, 197. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Franco, O.H.; Hu, F.B.; Cai, L.; Yu, Z.; Li, H.; Ye, X.; Qi, Q.; Ye, X.; Pan, A.; et al. Ferritin Concentrations, Metabolic Syndrome, and Type 2 Diabetes in Middle-Aged and Elderly Chinese. J. Clin. Endocrinol. Metab. 2008, 93, 4690–4696. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, Y.; Zhang, F.; Zhang, S.; Zhou, X.; Ji, L. Association of serum ferritin levels with metabolic syndrome and insulin resistance in a Chinese population. J. Diabetes Complicat. 2017, 31, 364–368. [Google Scholar] [CrossRef]
- Suárez-Ortegón, M.F.; Ensaldo-Carrasco, E.; Shi, T.; McLachlan, S.; Fernández-Real, J.M.; Wild, S.H. Ferritin, metabolic syndrome and its components: A systematic review and meta-analysis. Atherosclerosis 2018, 275, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Singh, U.; Jialal, I. The Evolving Role of C-Reactive Protein in Atherothrombosis. Clin. Chem. 2009, 55, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Singh, U.; Jialal, I. Human C-reactive protein and the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Shityakov, S.; Bigdelian, E.; Hussein, A.A.; Hussain, M.B.; Tripathi, Y.C.; Khan, M.U.; Shariati, M.A. Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019, 176, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Chuchawankul, S.; Khorana, N.; Poovorawan, Y. Piperine inhibits cytokine production by human peripheral blood mononuclear cells. Genet. Mol. Res. 2012, 11, 617–627. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.; Jialal, I. Anti-inflammatory Effects of α-Tocopherol. Ann. N. Y. Acad. Sci. 2004, 1031, 195–203. [Google Scholar] [CrossRef]
- Skalicky, J.; Muzakova, V.; Kandar, R.; Meloun, M.; Roušar, T.; Palicka, V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin. Chem. Lab. Med. 2008, 46, 499–505. [Google Scholar] [CrossRef]



| Patients (Male–Female) | 22 (M 13–F 9) |
| Age (years) | 68 ± 4.7 |
| Weight (kg) | 82 ± 17.5 |
| Body Mass Index (kg/m2) | 29.25 ± 3.4 |
| Systolic blood pressure (mmHg) | 135 ± 25.85 |
| Diastolic blood pressure (mmHg) | 86 ± 18.32 |
| Waist circumference (cm) | 109 ± 23.30 |
| Blood glucose (mg/dL) | 103 ± 21 |
| HDL Cholesterol (mg/dL) | 57.95 ± 12.32 |
| Triglycerides (mg/dL) | 126 ± 26.86 |
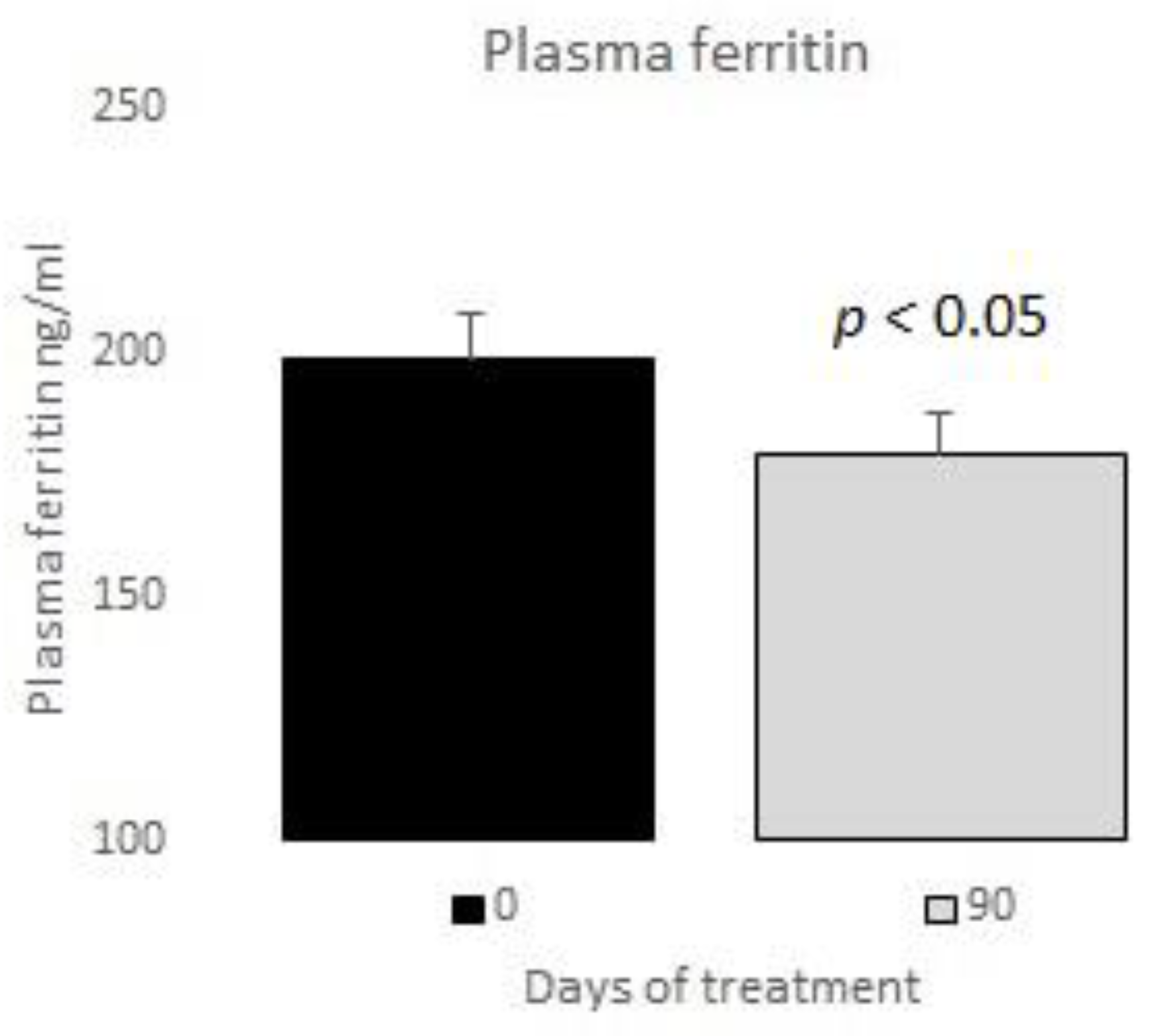
| Plasma ferritin (ng/mL) | 198.45 ± 38.11 |
| Ultrasensitive C reactive protein (mg/L) | 4.10 ± 0.87 |
| Oxygen consumption (nmol O2/min/ 106 cells) | 13 ± 2 |
| Chemiluminescence (cps/mL cells) | 134 ± 47 |
| Arterial Hypertension | Waist Circumf | Triglycerides | HDL Cholesterol | High Glucose or Diabetes | Patients/CVD Risk Factors in MetS | |
|---|---|---|---|---|---|---|
| Patients | 20/22 91% | 19/22 86% | 18/22 82% | 18/22 82% | 16/22 73% | 10/5 5/4 7/3 |
| Patient ID | BP | WC | TG | HDL Col. | BG |
|---|---|---|---|---|---|
| HB | 180/100 | 114 | 71 | 48 | 97 |
| CF | 120/80 | 88 | 105 | 77 | 99 |
| MG | 148/86 | 93 | 64 | 51 | 91 |
| PH | 105/78 | 107 | 90 | 81 | 106 |
| HL | 116/60 | 98.5 | 169 | 45 | 90 |
| RS | 130/90 | 110 | 90 | 61 | 90 |
| ES | 130/96 | 101 | 133 | 40 | 98 |
| Patient ID | BP | WC | TG | HDL Chol. | BG |
|---|---|---|---|---|---|
| BH | 150/90 | 114 | 79 | 39 | 105 |
| AD | 160/100 | 99 | 57 | 77 | 101 |
| LV | 120/80 | 104 | 107 | 76 | 90 |
| EV | 130/80 | 123 | 282 | 39 | 89 |
| MG | 100/86 | 93 | 64 | 51 | 91 |
| Patient ID | BP | WC | TG | HDL Chol. | BG |
|---|---|---|---|---|---|
| JB | 140/90 | 112 | 65 | 60 | 105 |
| RB | 140/92 | 140 | 131 | 37 | 136 |
| SN | 120/70 | 99 | 50 | 66 | 99 |
| CL | 160/84 | 128 | 155 | 79 | 116 |
| JP | 138/86 | 128 | 238 | 64 | 104 |
| AS | 120/80 | 114.5 | 354 | 30 | 111 |
| MS | 130/90 | 119 | 100 | 68 | 119 |
| ET | 130/90 | 110 | 202 | 43 | 110 |
| LA | 140/98 | 95 | 104 | 89 | 110 |
| HA | 170/100 | 103 | 58 | 54 | 102 |
| Biomarkers/ Basal vs. Final | Basal | Final | p | Δ % |
|---|---|---|---|---|
| Plasma ferritin (ng/mL) | 198.45 ± 38.11 | 178.75 ± 21.90 | <0.05 | 10 |
| Oxygen consumption (nmol O2/min/ 106 cells) | 13 ± 2 | 6 ± 1 | <0.0001 | 55 |
| Ultrasensitive C reactive protein (mg/L) | 4.10 ± 0.87 | 2.74 ± 0.59 | <0.0001 | 33 |
| Chemiluminescence (cps/mL cells) | 134 ± 47 | 100 ± 22 | <0.005 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, R.F.; Repetto, M.G.; Lairion, F.; Lazarowski, A.; Merelli, A.; Manfredi Carabetti, Z.; Pastor, I.; Pastor, E.; Iermoli, L.V.; Bavasso, C.A.; et al. Supplementation with Resveratrol, Piperine and Alpha-Tocopherol Decreases Chronic Inflammation in a Cluster of Older Adults with Metabolic Syndrome. Nutrients 2020, 12, 3149. https://doi.org/10.3390/nu12103149
Pastor RF, Repetto MG, Lairion F, Lazarowski A, Merelli A, Manfredi Carabetti Z, Pastor I, Pastor E, Iermoli LV, Bavasso CA, et al. Supplementation with Resveratrol, Piperine and Alpha-Tocopherol Decreases Chronic Inflammation in a Cluster of Older Adults with Metabolic Syndrome. Nutrients. 2020; 12(10):3149. https://doi.org/10.3390/nu12103149
Chicago/Turabian StylePastor, Raúl Francisco, Marisa Gabriela Repetto, Fabiana Lairion, Alberto Lazarowski, Amalia Merelli, Zulma Manfredi Carabetti, Isabel Pastor, Elena Pastor, Laura Valeria Iermoli, Carlos Amadeo Bavasso, and et al. 2020. "Supplementation with Resveratrol, Piperine and Alpha-Tocopherol Decreases Chronic Inflammation in a Cluster of Older Adults with Metabolic Syndrome" Nutrients 12, no. 10: 3149. https://doi.org/10.3390/nu12103149
APA StylePastor, R. F., Repetto, M. G., Lairion, F., Lazarowski, A., Merelli, A., Manfredi Carabetti, Z., Pastor, I., Pastor, E., Iermoli, L. V., Bavasso, C. A., & Iermoli, R. H. (2020). Supplementation with Resveratrol, Piperine and Alpha-Tocopherol Decreases Chronic Inflammation in a Cluster of Older Adults with Metabolic Syndrome. Nutrients, 12(10), 3149. https://doi.org/10.3390/nu12103149





