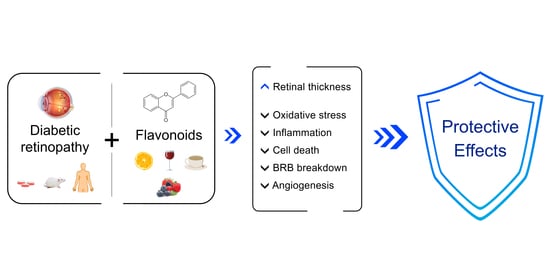
The Benefits of Flavonoids in Diabetic Retinopathy
Abstract
1. Diabetes and Diabetic Retinopathy
2. Pathogenesis of Diabetic Retinopathy
3. The Importance of Nutraceuticals
4. Diabetic Retinopathy and the Benefits of Flavonoids
4.1. Anthocyanins
4.2. Flavanols
4.3. Flavanones
4.4. Flavones
4.5. Flavonols
4.6. Isoflavones
5. Clinical Studies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Thomas, R.L.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019, 157, 107840. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in diabetes. Diabetes Care 2004, 27, s84–s87. [Google Scholar] [CrossRef] [PubMed]
- Ligda, G.; Ploubidis, D.; Foteli, S.; Kontou, P.I.; Nikolaou, C.; Tentolouris, N. Quality of life in subjects with type 2 diabetes mellitus with diabetic retinopathy: A case–control study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Shaya, F.T.; Aljawadi, M. Diabetic retinopathy. Clin. Ophthalmol. 2007, 1, 259–265. [Google Scholar]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L.; Hodgson, L.A.; Lim, L.L.; Al-Qureshi, S. Diabetic retinopathy in pregnancy: A review. Clin. Exp. Ophthalmol. 2016, 44, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Jeng, C.J.; Hsieh, Y.T.; Yang, C.M.; Yang, C.H.; Lin, C.L.; Wang, I.J. Development of diabetic retinopathy after cataract surgery. PLoS ONE 2018, 13, e0202347. [Google Scholar] [CrossRef]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrósio, A.F.; Santiago, A.R. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediat. Inflamm. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ames, A.; Li, Y.Y.; Heher, E.C.; Kimble, C.R. Energy metabolism of rabbit retina as related to function: High cost of Na+ transport. J. Neurosci. 1992, 12, 840–853. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. Studies on the permeability of the blood-retinal barrier 3. Breakdown of the blood-retinal barrier by circulatory disturbances a topographical study of the vascular tree. Br. J. Ophthalmol. 1966, 50, 505–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardner, T.W.; Antonetti, D.A.; Barber, A.J.; Lieth, E.; Tarbell, J.A. The molecular structure and function of the inner blood-retinal barrier. Doc. Ophthalmol. 1999, 97, 25–33. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. Pathophysiology of diabetic retinopathy. Br. J. Ophthalmol. 1978, 62, 351–355. [Google Scholar] [CrossRef]
- Adamis, A.P.; Miller, J.W.; Bernal, M.T.; D’Amico, D.J.; Folkman, J.; Yeo, T.-K.; Yeo, K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef]
- Miller, J.W.; Adamis, A.P.; Shima, D.T.; D’Amore, P.A.; Moulton, R.S.; O’Reilly, M.S.; Folkman, J.; Dvorak, H.F.; Brown, L.F.; Berse, B.; et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994, 145, 574–584. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E.K. The incidence of vision loss in a diabetic population. Ophthalmology 1988, 95, 1340–1348. [Google Scholar] [CrossRef]
- Park, H.Y.L.; Kim, J.H.; Park, C.K. Neuronal cell death in the inner retina and the influence of vascular endothelial growth factor inhibition in a diabetic rat model. Am. J. Pathol. 2014, 184, 1752–1762. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A. Retinal neurodegeneration: Early pathology in diabetes. Clin. Exp. Ophthalmol. 2000, 28, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.B.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; Devries, J.H.; Schlingemann, R.O.; Abràmoff, M.D. Early neurodegeneration in the retina of type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715–2719. [Google Scholar] [CrossRef]
- Santiago, A.R.; Madeira, M.H.; Boia, R.; Aires, I.D.; Rodrigues-Neves, A.C.; Santos, P.F.; Ambrósio, A.F. Keep an eye on adenosine: Its role in retinal inflammation. Pharmacol. Ther. 2020, 210, 107513. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of inflammation in diabetic retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Wu, H.; Hwang, D.K.; Song, X.; Tao, Y. Association between Aqueous cytokines and diabetic retinopathy stage. J. Ophthalmol. 2017, 2017, 9402198. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Xi, A.; Tang, J.; Kern, T.S. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes 2002, 51, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.A.; Mohr, S. Inhibition of caspase-1/interleukin-1β signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes 2007, 56, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Odenbach, S. Role of interleukin-1β in the pathogenesis of diabetic retinopathy. Br. J. Ophthalmol. 2004, 88, 1343–1347. [Google Scholar] [CrossRef]
- Joussen, A.M.; Doehmen, S.; Le, M.L.; Koizumi, K.; Radetzky, S.; Krohne, T.U.; Poulaki, V.; Semkova, I.; Kociok, N. TNF-α mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol. Vis. 2009, 15, 1418–1428. [Google Scholar] [PubMed]
- Costa, G.N.; Vindeirinho, J.; Cavadas, C.; Ambrósio, A.F.; Santos, P.F. Contribution of TNF receptor 1 to retinal neural cell death induced by elevated glucose. Mol. Cell. Neurosci. 2012, 50, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.; Manz, R.; Ziegenhorn, A.; Weih, M.; Angstwurm, K.; Döcke, W.D.; Meisel, A.; Schumann, R.R.; Schönfelder, G.; Dirnagl, U.; et al. Cerebral endothelial cells release TNF-α after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J. Immunol. 1999, 163, 4308–4314. [Google Scholar] [PubMed]
- Gregersen, R.; Lambertsen, K.; Finsen, B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J. Cereb. Blood Flow Metab. 2000, 20, 53–65. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Targan, S.R. Tumor necrosis factor: Biology and therapeutic inhibitors. Gastroenterology 2000, 119, 1148–1157. [Google Scholar] [CrossRef]
- Omri, S.; Behar-Cohen, F.; De Kozak, Y.; Sennlaub, F.; Verissimo, L.M.; Jonet, L.; Savoldelli, M.; Omri, B.; Crisanti, P. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: Role of PKCζ in the Goto Kakizaki rat model. Am. J. Pathol. 2011, 179, 942–953. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress: Annual review of biochemistry. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol. Res. 2015, 99, 137–148. [Google Scholar] [CrossRef]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative stress and microvascular alterations in diabetic retinopathy: Future therapies. Oxidative Med. Cell. Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef]
- Geraldes, P.; Hiraoka-Yamamoto, J.; Matsumoto, M.; Clermont, A.; Leitges, M.; Marette, A.; Aiello, L.P.; Kern, T.S.; King, G.L. Activation of PKC-and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009, 15, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Amin, H.; Prajapati, P.K. Plant kingdom Nutraceuticals for diabetes. J. Ayurvedic Herb. Med. 2016, 2, 224–228. [Google Scholar]
- Rossino, M.G.; Casini, G. Nutraceuticals for the treatment of diabetic retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The possible role of flavonoids in the prevention of diabetic complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Ola, M.S.; Al-Dosari, D.; Alhomida, A.S. Role of oxidative stress in diabetic retinopathy and the beneficial effects of flavonoids. Curr. Pharm. Des. 2018, 24, 2180–2187. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Putta, S.; Yarla, N.S.; Kumar, E.K.; Lakkappa, D.B.; Kamal, M.A.; Scotti, L.; Scotti, M.T.; Ashraf, G.M.; Rao, B.S.B.; Sarala Kumari, D.; et al. Preventive and therapeutic potentials of anthocyanins in diabetes and associated complications. Curr. Med. Chem. 2017, 25, 5347–5371. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxidative Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes—Induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef]
- Bonetti, F.; Brombo, G.; Zuliani, G. Nootropics, functional foods, and dietary patterns for prevention of cognitive decline. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 211–232. [Google Scholar]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V.; Santos-Buelga, C.; Feliciano, A.S. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Mechanisms of neuronal cell death in AGE-exposed retinas—Research and literature review. Curr. Diabetes Rev. 2017, 13, 280–288. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Yamamoto, S. Neuronal cell death and regeneration in diseases associated with advanced glycation end-products accumulation. Neural Regen. Res. 2014, 9, 701–702. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Moon, M.K.; Kim, J.S. Epicatechin breaks preformed glycated serum albumin and reverses the retinal accumulation of advanced glycation end products. Eur. J. Pharmacol. 2015, 748, 108–114. [Google Scholar] [CrossRef]
- Skopinski, P.; Szaflik, J.; Duda-Król, B.; Nartowska, J.; Sommer, E.; Chorostowska-Wynimko, J.; Demkow, U.; Skopinska-Rózewska, E. Suppression of angiogenic activity of sera from diabetic patients with non-proliferative retinopathy by compounds of herbal origin and sulindac sulfone. Int. J. Mol. Med. 2004, 14, 707–711. [Google Scholar] [CrossRef]
- Kodama, D.H.; Gonçalves, A.E.D.S.S.; Lajolo, F.M.; Genovese, M.I. Flavonoides, fenólicos totais e capacidade antioxidante: Comparação entre bebidas comerciais à base de chá verde. Cienc. Tecnol. Aliment. 2010, 30, 1077–1082. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.B.; Hamassaki-Britto, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.O.; De Faria, J.B.L.; De Faria, J.M.L. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Mustata, G.T.; Rosca, M.; Biemel, K.M.; Reihl, O.; Smith, M.A.; Viswanathan, A.; Strauch, C.; Du, Y.; Tang, J.; Kern, T.S.; et al. Paradoxical effects of green tea (Camellia sinensis) and antioxidant vitamins in diabetic rats: Improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes 2005, 54, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Jin, W.; Xing, Y.; Yang, A. Catechin weakens diabetic retinopathy by inhibiting the expression of NF-κB signaling pathway-mediated inflammatory factors. Ann. Clin. Lab. Sci. 2018, 48, 594–600. [Google Scholar]
- Tundis, R.; Acquaviva, R.; Bonesi, M.; Malfa, G.A.; Tomasello, B.; Loizzo, M.R. Citrus flavanones. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2020; pp. 1–30. [Google Scholar]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid naringenin attenuates oxidative stress, apoptosis and improves neurotrophic effects in the diabetic rat retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef]
- Mancino, R.; Pierro, D.; Varesi, C.; Cerulli, A.; Feraco, A.; Cedrone, C.; Pinazo-Duran, M.D.; Coletta, M.; Nucci, C. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol. Vis. 2011, 17, 1298–1304. [Google Scholar]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. J. Cell. Biochem. 2019, 120, 5644–5651. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.P.; Nag, T.C.; Srivastava, S.; Saxena, R. Hesperetin ameliorates hyperglycemia induced retinal vasculopathy via anti-angiogenic effects in experimental diabetic rats. Vasc. Pharmacol. 2012, 57, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.P.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 2013, 87, 65–74. [Google Scholar] [CrossRef]
- Cai, Y.; Cheng, T.; Yao, Y.; Li, X.; Ma, Y.; Li, L.; Zhao, H.; Bao, J.; Zhang, M.; Qiu, Z.; et al. In vivo genome editing rescues photoreceptor degeneration via a Cas9/RecA-mediated homology-directed repair pathway. Sci. Adv. 2019, 5, eaav3335. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Molecules 2012, 17, 12868–12881. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Liou, S.S.; Hong, T.Y.; Liu, I.M. Protective effects of hesperidin (Citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients 2017, 9, 1312. [Google Scholar] [CrossRef]
- Liu, W.Y.; Liou, S.S.; Hong, T.Y.; Liu, I.M. Hesperidin prevents high glucose-induced damage of retinal pigment epithelial cells. Planta Med. 2018, 84, 1030–1037. [Google Scholar] [CrossRef]
- Duodu, K.G.; Awika, J.M. Phytochemical-related health-promoting attributes of sorghum and millets. In Sorghum and Millets, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 225–258. [Google Scholar]
- Yang, L.-P.; Sun, H.; Wu, L.-M.; Guo, X.-J.; Dou, H.-L.; Tso, M.O.M.; Zhao, L.; Li, S. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Ahmad, S.; Megyerdi, S.; Mussell, R.; Choksi, K.; Maddipati, K.R.; Elmarakby, A.; Rizk, N.; Al-Shabrawey, M. 12/15-lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: Contribution of NADPH oxidase. PLoS ONE 2013, 8, e57254. [Google Scholar] [CrossRef]
- Xiao, J.R.; Do, C.W.; To, C.H. Potential therapeutic effects of baicalein, baicalin, and wogonin in ocular disorders. J. Ocul. Pharmacol. Ther. 2014, 30, 605–614. [Google Scholar] [CrossRef]
- Dai, C.; Jiang, S.; Chu, C.; Xin, M.; Song, X.; Zhao, B. Baicalin protects human retinal pigment epithelial cell lines against high glucose-induced cell injury by up-regulation of microRNA-145. Exp. Mol. Pathol. 2019, 106, 123–130. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. MiRNA biogenesis and regulation of diseases: An overview. Methods Mol. Biol. 2017, 1509, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yin, Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-κB signaling. Life Sci. 2018, 207, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Xie, J.; Liu, Y.; Li, Y.; Su, G. Differentially expressed MicroRNAs in the Development of early diabetic retinopathy. J. Diabetes Res. 2017, 2017, 4727942. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Teramachi, M.; Yasuda, H.; Kamiya, T.; Hara, H. Contribution of p38 MAPK, NF-κB and glucocorticoid signaling pathways to ER stress-induced increase in retinal endothelial permeability. Arch. Biochem. Biophys. 2012, 520, 30–35. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, T.; Ouyang, H.; Lu, B.; Wang, Z.; Ji, L. Scutellarin alleviates blood-retina-barrier oxidative stress injury initiated by activated microglia cells during the development of diabetic retinopathy. Biochem. Pharmacol. 2019, 159, 82–95. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Gu, J.; Yang, H.; Liu, N.; Lin, Y.; Li, X.; Shao, C. Scutellarin inhibits high glucose-induced and hypoxia-mimetic agent-induced angiogenic effects in human retinal endothelial cells through reactive oxygen species/hypoxia-inducible factor-1a/vascular endothelial growth factor pathway. J. Cardiovasc. Pharmacol. 2014, 64, 218–227. [Google Scholar] [CrossRef]
- Chen, X.; Han, R.; Hao, P.; Wang, L.; Liu, M.; Jin, M.; Kong, D.; Li, X. Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells. Biomed. Pharmacother. 2018, 101, 87–93. [Google Scholar] [CrossRef]
- Zhang, H.T.; Shi, K.; Baskota, A.; Zhou, F.L.; Chen, Y.X.; Tian, H.M. Silybin reduces obliterated retinal capillaries in experimental diabetic retinopathy in rats. Eur. J. Pharmacol. 2014, 740, 233–239. [Google Scholar] [CrossRef]
- Kang, M.K.; Lee, E.J.; Kim, Y.H.; Kim, D.Y.; Oh, H.; Kim, S.I.; Kang, Y.H. Chrysin ameliorates malfunction of retinoid visual cycle through blocking activation of age-rage-er stress in glucose-stimulated retinal pigment epithelial cells and diabetic eyes. Nutrients 2018, 10, 1046. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020. [Google Scholar] [CrossRef] [PubMed]
- –Kocka, A.B.; Woźniak, M.; Feldo, M.; Kocki, J.; Szewczyk, K. Diosmin—Isolation techniques, determination in plant material and pharmaceutical formulations, and clinical use. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Tong, N.; Zhang, Z.; Zhang, W.; Qiu, Y.; Gong, Y.; Yin, L.; Qiu, Q.; Wu, X. Diosmin alleviates retinal edema by protecting the blood-retinal barrier and reducing retinal vascular permeability during ischemia/reperfusion injury. PLoS ONE 2013, 8, e61794. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Zhang, Z.; Gong, Y.; Yin, L.; Wu, X. Diosmin protects rat retina from ischemia/reperfusion injury. J. Ocul. Pharmacol. Ther. 2012, 28, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Liou, S.-S.; Hong, T.-Y.; Liu, I.-M. The benefits of the citrus flavonoid diosmin on human retinal pigment epithelial cells under high-glucose conditions. Molecules 2017, 22, 2251. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhao, C.; Peng, Q.; Xie, P.; Liu, Q.H. Kaempferol inhibited VEGF and PGF expression and in vitro angiogenesis of hrecs under diabetic-like environment. Braz. J. Med Biol. Res. 2017, 50, e5396. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Wu, H.Y.; Chu, G.X.; Xie, Z.W.; Bao, G.H. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef]
- Chen, B.; He, T.; Xing, Y.; Cao, T. Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp. Ther. Med. 2017, 14, 6022–6026. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wang, H.; Shi, J. Quercetin attenuates high glucose induced injury in human retinal pigment epithelial cell line ARPE-19 by up-regulation of miR-29b. J. Biochem. 2020, 167, 495–502. [Google Scholar] [CrossRef]
- Tang, W.; Guo, J.; Gu, R.; Lei, B.; Ding, X.; Ma, J.; Xu, G. MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells. Mol. Vis. 2020, 26, 64–75. [Google Scholar] [PubMed]
- Ola, M.S.; Ahmed, M.M.; Ahmad, R.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J. Mol. Neurosci. 2015, 56, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sharma, H.P.; Das, U.; Velpandian, T.; Saklani, R. Effect of rutin on retinal VEGF, TNF-α, aldose reductase, and total antioxidant capacity in diabetic rats: Molecular mechanism and ocular pharmacokinetics. Int. Ophthalmol. 2020, 40, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mei, X.; Ouyang, H.; Lu, B.; Yu, Z.; Wang, Z.; Ji, L. Natural flavonoid galangin alleviates microglia-trigged blood–retinal barrier dysfunction during the development of diabetic retinopathy. J. Nutr. Biochem. 2019, 65, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.; Kim, K.M.; Jung, D.H.; Choi, S.; Kim, C.S.; Kim, J.S. Myricetin inhibits advanced glycation end product (AGE)-induced migration of retinal pericytes through phosphorylation of ERK1/2, FAK-1, and paxillin in vitro and in vivo. Biochem. Pharmacol. 2015, 93, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Palanisamy, U.; Chua, K.H.; Kuppusamy, U.R. Protective effect of myricetin derivatives from Syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Mol. Vis. 2019, 25, 47–59. [Google Scholar] [PubMed]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Amelioration of hyperglycemia-induced oxidative damage in ARPE-19 cells by myricetin derivatives isolated from Syzygium malaccense. J. Funct. Foods 2020, 67, 103844. [Google Scholar] [CrossRef]
- Xin, H.; Zhou, F.; Liu, T.; Li, G.Y.; Liu, J.; Gao, Z.Z.; Bai, G.Y.; Lu, H.; Xin, Z.C. Icariin ameliorates streptozotocin-induced diabetic retinopathy in Vitro and in Vivo. Int. J. Mol. Sci. 2012, 13, 866–878. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Mehrabadi, M.E.; Salemi, Z.; Babaie, S.; Panahi, M. Effect of biochanin a on retina levels of vascular endothelial growth factor, tumor necrosis factor-alpha and interleukin-1beta in rats with streptozotocin-induced diabetes. Can. J. Diabetes 2018, 42, 639–644. [Google Scholar] [CrossRef]
- Jia, W.C.; Liu, G.; Zhang, C.D.; Zhang, S.P. Formononetin attenuates hydrogen peroxide (H2O 2)-induced apoptosis and NF-kB activation in RGC-5 cells. Eur. Rev. Med Pharmacol. Sci. 2014, 18, 2191–2197. [Google Scholar] [PubMed]
- Teng, Y.; Cui, H.; Yang, M.; Song, H.; Zhang, Q.; Su, Y.; Zheng, J. Protective effect of puerarin on diabetic retinopathy in rats. Mol. Biol. Rep. 2009, 36, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, K.Y.; Xu, S.; Lü, W.H.; Chen, H.; Zhang, H.Q. Experiment effect of puerarin on retina in diabetic rats induced by streptozotocin and its mechanisms. Chin. Pharmacol. Bull. 2011, 27, 1279–1284. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, X.; Xu, X.; Yu, Y. Effects of puerarin on the retina and STAT3 expression in diabetic rats. Exp. Ther. Med. 2017, 14, 5480–5484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, F.; Zhang, H.Q.; Zhu, J.; Liu, K.Y.; Cheng, H.; Li, G.L.; Xu, S.; Lv, W.H.; Xie, Z.G. Puerarin enhances superoxide dismutase activity and inhibits RAGE and VEGF expression in retinas of STZ-induced early diabetic rats. Asian Pac. J. Trop. Med. 2012, 5, 891–896. [Google Scholar] [CrossRef]
- Chen, F.; Liu, K.Y.; Xu, S.; Li, W.H.; Li, G.L.; Zhang, H.Q. Protective effect of puerarin on retinopathy and its inhibitory effect on NF-κB activity in diabetic mellitus rats. Chin. J. Pharmacol. Toxicol. 2011, 25, 296–300. [Google Scholar] [CrossRef]
- Hao, L.N.; Zhang, Y.Q.; Shen, Y.H.; Wang, Z.Y.; Wang, Y.H.; Zhang, H.F.; He, S.Z. Effect of puerarin on retinal pigment epithelial cells apoptosis induced partly by peroxynitrite via fas/fasl pathway. Int. J. Ophthalmol. 2010, 3, 283–287. [Google Scholar] [CrossRef]
- Hao, L.N.; Wang, M.; Ma, J.L.; Yang, T. Puerarin decreases apoptosis of retinal pigment epithelial cells in diabetic rats by reducing peroxynitrite level and iNOS expression. Sheng li xue bao: [Acta physiologica Sinica] 2012, 64, 199–206. [Google Scholar]
- Kim, J.; Kim, K.M.; Kim, C.S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free Radic. Biol. Med. 2012, 53, 357–365. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, M.; Wang, K.; Zhang, K.; Gao, Y.; Zhu, L.; Zhou, F. The effect of puerarin against IL-1β-mediated leukostasis and apoptosis in retinal capillary endothelial cells (TR-iBRB2). Mol. Vis. 2014, 20, 1815. [Google Scholar]
- Lv, B.; Huo, F.; Dang, X.; Xu, Z.; Chen, T.; Zhang, T.; Yang, X. Puerarin attenuates N-methyl-d-aspartic acid-induced apoptosis and retinal ganglion cell damage through the JNK/p38 MAPK pathway. J. Glaucoma 2016, 25, e792–e801. [Google Scholar] [CrossRef]
- Kumar, M.P.; Sankeshi, V.; Naik, R.R.; Thirupathi, P.; Das, B.; Raju, T.N. The inhibitory effect of Isoflavones isolated from Caesalpinia pulcherrima on aldose reductase in STZ induced diabetic rats. Chem. Biol. Interact. 2015, 237, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-X.; Xu, Y.-L.; Li, S.-H.; Hui, R.; Wu, Y.-J.; Huang, X.-H. Effects of green tea catechins with or without caffeine on glycemic control in adults: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Cennamo, G.; Bonavolontà, P.; Pascotto, A.; de Crecchio, G.; Cennamo, G. Long-term follow-up of oral administration of flavonoids, Centella asiatica and Melilotus, for diabetic cystoid macular edema without macular thickening. J. Ocul. Pharmacol. Ther. 2013, 29, 733–737. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Qiu, Y.; Liu, Y.; Wang, L.; Yang, J.; Wang, C.; Ma, J. Anthocyanins increase serum adiponectin in newly diagnosed diabetes but not in prediabetes: A randomized controlled trial. Nutr. Metab. 2020, 17, 78. [Google Scholar] [CrossRef]
- Mahoney, S.E.; Loprinzi, P.D. Influence of flavonoid-rich fruit and vegetable intake on diabetic retinopathy and diabetes-related biomarkers. J. Diabetes Complicat. 2014, 28, 767–771. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, D.; Sun, H.-P.; Yan, N.; Xu, Y.; Pan, C.-W. Regular Chinese green tea consumption is protective for diabetic retinopathy: A clinic-based case-control study. J. Diabetes Res. 2015, 2015, 231570. [Google Scholar] [CrossRef]
- Schönlau, F.; Rohdewald, P. Pycnogenol® for diabetic retinopathy. Int. Ophthalmol. 2001, 24, 161–171. [Google Scholar] [CrossRef]
- Steigerwalt, R.; Belcaro, G.; Cesarone, M.R.; Di Renzo, A.; Grossi, M.G.; Ricci, A.; Dugall, M.; Cacchio, M.; Schönlau, F. Pycnogenol improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy. J. Ocul. Pharmacol. Ther. 2009, 25, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Domanico, D.; Fragiotta, S.; Cutini, A.; Carnevale, C.; Zompatori, L.; Vingolo, E.M. Circulating levels of reactive oxygen species in patients with nonproliferative diabetic retinopathy and the influence of antioxidant supplementation: 6-month follow-up. Indian J. Ophthalmol. 2015, 63, 9–14. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; del-Rio-Vellosillo, M. Update on the effects of antioxidants on diabetic retinopathy: In vitro experiments, animal studies and clinical trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]

| Name of Substances/Compound | Dose | Model | Observations | Reference |
|---|---|---|---|---|
| Blueberry anthocyanins | 20, 40 or 80 mg/kg orally administered for 12 weeks | STZ-induced T1DM rats | ↑ antioxidant capacity of the retina | [56] |
| ↑ GSH and GPx activity | ||||
| ↑ Nrf2 and HO-1 mRNA and protein levels | ||||
| ↓ weight loss and blood glucose levels | ||||
| ↓ VEGF level and ROS levels | ||||
| ↓ MDA and IL-1β levels | ||||
| 10 μg/mL of BAE, Mv, Mv-3-glc or Mv-3-gal for 24 h | High glucose-exposed HRCECs | ↑ CAT and SOD, ICAM-1 and NF-κB pathway | [55] | |
| ↑ cell viability | ||||
| ↓ VEGF levels and Akt pathway | ||||
| ↓ ROS, eNOS and NO levels | ||||
| ↓ Nox4 expression | ||||
| Vaccinium myrtillus extract | 100 mg/kg orally administered for 6 weeks | STZ-induced T1DM rats | ↑ zonula occludens-1, occludin and claudin-5 levels | [57] |
| ↓ fluorescein leakage | ||||
| ↓ VEGF levels |
| Name of Substances/Compound | Dose | Model | Observations | Reference |
|---|---|---|---|---|
| (−)—Epicatechin | 50 and 100 mg/kg for 2 weeks | Unmodified rat serum albumin and AGE-modified rat serum albumin intravenous injected (normoglycemic) rats | ↑ AGE breaking activity | [62] |
| ↓ AGE burden and vascular apoptosis | ||||
| 10, 25, 50, 100, 250, 500 and 1000 μg/mL | Collagen–glycated bovine serum albumin complexes and glycated human serum albumin | ↑ AGE breaking activity | ||
| Epigallocatechin | 2, 20 and 200 µg | Balb/c mice intradermally injected with NPDR patients serum | ↓ angiogenic effects | [63] |
| Green tea | 5.7 g/kg for 12 weeks | STZ-induced T1DM rats | ↓ occludin, NMDAr1 subunit and GLAST levels | [65] |
| ↓ BRB breakdown | ||||
| ↓ impaired electroretinography recordings | ||||
| ↓ GFAP, oxidative retinal markers and glutamine synthetase levels | ||||
| 1, 10 or 100 µg/mL | High glucose-exposed PRRMC, rMC-1 and ARPE-19 cells | ↑ GSH, GLAST and NMDAR1 protein levels | ||
| ↓ ROS and glutamine synthetase | ||||
| ↓ claudin-1 levels | ||||
| 100 mL for 12 months | STZ-induced T1DM rats | ↓ superoxide production, acellular capillaries and pericyte ghosts | [66] | |
| Epigallocatechin gallate | 2, 20 and 200 µg/mL | Balb/c mice intradermally injected with NPDR patients serum | ↓ angiogenic effects | [63] |
| 200 mg/kg orally administered for 4 days | VEGF intradermally injected Balb/c mice and Sprague-Dawley rats | ↓ vascular leakage and permeability by BRB breakdown | [67] | |
| 1, 10, 25 and 50 μM for 1.5, 24 or 72 h | TPA and TNF-α-exposed HRPECs and ARPE-19 cells | ↓ MMP-9 levels | ||
| ↓ cell death and MMP-9, VEGF and VEGF Receptor-2 mRNA expression | ||||
| H2O2-exposed ARPE-19 cells | ||||
| ↓ ROS levels | ||||
| VEGF-exposed HRMECs | ↓ cell proliferation, vascular permeability and tube formation | |||
| Catechin | 50, 100 and 200 mg/kg by intravitreal injection for 8 weeks | STZ-induced diabetic rats | ↑ HSP27 levels | [68] |
| ↓ IL-1β, IL-6 and TNF-α levels | ||||
| ↓ NF-κB pathway |
| Name of Substances/Compound | Dose | Model | Observations | Reference |
|---|---|---|---|---|
| Naringenin | 50 mg/kg orally administered for 5 weeks | STZ-induced T1DM rats | ↑ GSH levels | [70] |
| ↑ BDNF, TrkB and synaptophysin levels | ||||
| ↑ Bcl-2 levels | ||||
| ↓ TBARs levels | ||||
| ↓ Bax and caspase-3 levels | ||||
| Eriodictyol | 0.1, 1 and 10 mg/kg orally administered for 10 days | STZ-induced T1DM rats | ↓ TNF-α, ICAM-1, VEGF and eNOS levels | [72] |
| ↓ plasma lipid peroxidation | ||||
| ↓ BRB breakdown | ||||
| 5, 10 and 20 μM for 24 h | High glucose-exposed RGC-5 cells | ↑ SOD, GPx and CAT activity | [73] | |
| ↑ cell viability and heme-oxygenase-1 expression | ||||
| ↑ Nrf2 nuclear translocation | ||||
| ↓ ROS levels | ||||
| ↓ TNF-α and IL-8 levels | ||||
| ↓ cell apoptosis | ||||
| Hesperetin | 200 mg/kg orally administered for 24 weeks | STZ-induced T1DM rats | ↓ VEGF and PKC-β expression | [74] |
| ↓ vascular permeability and leakage | ||||
| ↓ dilated vessels | ||||
| ↓ basement membrane thickness | ||||
| 100 mg/kg orally administered for 24 weeks | STZ-induced T1DM rats | ↑ GSH levels | [75] | |
| ↓ TNF-α and IL-1β levels | ||||
| ↓ caspase-3, GFAP and aquaporin-4 expression | ||||
| ↓ photoreceptors cell death | ||||
| ↓ edematous Müller cells’ feet, degenerated photoreceptor layer and basement membrane thickness | ||||
| Hesperidin | 100 and 200 mg/kg intragastrically administered for 12 weeks | STZ-induced T1DM rats | ↑ retina thickness | [77] |
| ↑ SOD activity | ||||
| ↓ blood glucose, aldose reductase activity, ICAM-1, IL-1β, TNF-α, VEGF and AGEs levels | ||||
| ↓ BRB breakdown | ||||
| ↓ MDA levels | ||||
| 12.5, 25 and 50 µmol/L for 48 h | High glucose-exposed RGC-5 cells | ↑ SOD, CAT, glutathione peroxidase activities and GSH levels | [78] | |
| ↓ cell loss and cytochrome c release | ||||
| Reverted ∆Ψm loss | ||||
| ↓ ROS, MDA and protein carbonyl levels | ||||
| ↓ caspase-9, caspase-3 and Bax/Bcl-2 levels | ||||
| ↓ c-Jun N-terminal kinase phosphorylation and p38 MAPK | ||||
| 10, 20 or 40 µM for 48 h | High glucose-exposed ARPE-19 cells | ↑ cell viability | [79] | |
| ↑ glutathione peroxidase, SOD and CAT activities and GSH levels | ||||
| ↓ ROS production | ||||
| ↓ caspase-9/3, cytochrome c release and Bax/Bcl-2 ratio |
| Name of Substances/Compound | Dose | Model | Observations | Reference |
|---|---|---|---|---|
| Baicalein | 75, 150 and 300 mg/kg orally administered for 24 weeks | STZ-induced T1DM rats | ↓ microglial activation and IL-18, TNF-α and IL-1β expression | [81] |
| ↓ GFAP and VEGF expression in Müller cells | ||||
| ↓ vascular abnormality and ganglion cell loss | ||||
| 75 mg/kg in drinking water | Ins2Akita mice | ↑ pSHP1 levels | [83] | |
| ↓ HETE, ICAM-1, VCAM-1 and IL-6 levels | ||||
| ↓ ROS generation, and NOX2 expression | ||||
| ↓ pVEGF-R2 levels | ||||
| Baicalin | 2.5, 5, 10, 50 and 100 μM for 12 h | High glucose-exposed ARPE-19 and HRMECs | ↑ miR-145 levels | [84] |
| ↑ cell proliferation | ||||
| ↓ apoptosis | ||||
| ↓ IL-1β, IL-6 and IL-8 release and ROS levels | ||||
| ↓ NF-κB and p38 MAPK pathways | ||||
| Scutellarin | 0.1, 1, 10 or 100 nM; 0,1 or 1 μM for 48 h | High glucose and hypoxia-mimetic agent-exposed HRECs | ↓ cell proliferation, migration, and tube formation | [90] |
| ↓ VEGF levels | ||||
| ↓ HIF-1α protein and mRNA levels | ||||
| ↓ NADPH oxidase activity | ||||
| 20 and 50 μM for 6 h | High glucose-exposed BV-2 cells | ↓ NF-κB and TNF-α expression | [89] | |
| ↓ microglia cell activation | ||||
| ↓ BRB damage | ||||
| ↓ ERK1/2 phosphorylation | ||||
| 20 and 50 μM for 6 h | TNF-α-exposed HRECs and ARPE-19 cells | ↑ claudin-1 and claudin-19 expression | ||
| ↑ Nrf2 nuclear accumulation | ||||
| ↓ ROS formation and BRB damage | ||||
| 5 and 10 mg/kg intragastric administration for 1 month | STZ-induced T1DM rats | ↑ claudin-1 and claudin-19 expression | ||
| ↓ microglia cell activation | ||||
| ↓ BRB breakdown | ||||
| ↓ ERK1/2 phosphorylation | ||||
| Nepetin | 2.5, 5 and 10 µM for 24 h | IL-1β-exposed ARPE-19 cells | ↓ IL-6, IL-8 and MCP-1 levels | [91] |
| ↓ nuclear translocation of NF-κB p65 | ||||
| ↓ phosphorylation of inhibitor of nuclear factor kappa B and IκB kinase | ||||
| ↓ phosphorylation of ERK1/2, JNK and p38 MAPK | ||||
| Silybin | 15 and 30 mg/kg orally administered for 22 weeks | STZ and high-fat diet-induced T2DM rats | ↓ obliterated retinal capillaries | [92] |
| ↓ leukostasis and ICAM-1 levels | ||||
| Chrysin | 1, 10 and 20 µM for 3 days | Glucose-exposed RPE cells | ↑ PEDF levels | [93] |
| ↓ VEGF and IGF-1 levels | ||||
| ↓ AGE secretion and RAGE induction | ||||
| ↓ ER stress | ||||
| 1, 10 and 20 µM for 3 days | AGE-BSA-exposed RPE cells | ↑ PEDF, RPE65, LRAT and RDH5 levels | ||
| ↓ ER stress | ||||
| 10 mg/kg orally administered for 10 weeks | db/db mice | ↑ ONL thickness | ||
| ↑ RPE65, LRAT, RDH5, CRBP, CRALBP, IRBP, STRA6 and rhodopsin levels | ||||
| ↓ AGE secretion and RAGE induction | ||||
| ↓ ER stress | ||||
| Diosmin | 100 mg/kg intragastric administration | Retinal ischemia/reperfusion in rats | ↑ SOD, GPx and CAT activities | [97] |
| ↑ retinal a- and b-wave amplitudes | ||||
| ↑ INL, IPL, ONL and total retinal thicknesses | ||||
| ↑ ganglion cells number | ||||
| ↓ edema | ||||
| ↓ MDA | ||||
| ↑ retinal a- and b-wave amplitudes | [96] | |||
| ↑ ZO-1 and occludin levels | ||||
| ↓ VEGF levels | ||||
| ↓ Evans blue leakage | ||||
| 0.1, 1 and 10 µg/mL | High glucose-exposed ARPE-19 cells | ↑ cell viability | [98] | |
| ↑ Bcl-2/Bax | ||||
| ↓ capase-3 activity and cytochrome c release | ||||
| ↓ ROS levels | ||||
| ↓ JNK and p38 phosphorylation |
| Name of Substances/Compound | Dose | Model | Observations | Reference |
|---|---|---|---|---|
| Kaempferol | 5 and 25 μM for 24 h | High glucose-stimulated HRECs | ↓ VEGF and PGF mRNA levels | [100] |
| ↓ cell proliferation, migration, migration distance and sprouting of HRECs | ||||
| ↓ PI3K expression and ERK1/2, Src, and Akt1 activation | ||||
| 10, 15, 20, 25, 30 and 35 µM | N/A | ↓ α-glucosidase and α-amylase activities | [101] | |
| Quercetin | 25 and 50 mg/kg orally administered for 24 weeks | STZ-induced T1DM rats | ↑ GSH levels | [103] |
| ↑ SOD and CAT activities | ||||
| ↑ ganglion cells number and retinal thickness | ||||
| ↓ TNF-α and IL-1β levels | ||||
| ↓ NF-kB and caspase-3 levels | ||||
| ↓ GFAP and aquaporin-4 levels | ||||
| 150 mg/kg by intragastric injection for 20 weeks | STZ-induced T1DM rats | ↓ MMP-9 and VEGF serum levels | [102] | |
| ↓ MMP-9 and VEGF RNA and protein levels | ||||
| 10, 20, 30, 40 and 50 μM for 24 h | High glucose-exposed ARPE-19 cells | ↑ CyclinD1, CDK4 and Bcl-2 levels | [104] | |
| ↑ MiR-29b expression | ||||
| ↑ PTEN/AKT pathway | ||||
| ↓ NF-κB pathway via a miR-29b-dependent way | ||||
| ↓ viability loss, apoptosis, MCP-1 and IL-6 production and ROS generation | ||||
| ↓ p53, Bax and cleaved-caspase-3 expression | ||||
| Rutin | 100 mg/kg orally administered for 5 weeks | STZ-induced T1DM rats | ↑ blood insulin levels | [106] |
| ↑ Bcl-2 levels | ||||
| ↑ BDNF, NGF and GSH | ||||
| ↓ blood glucose levels | ||||
| ↓ TBARs | ||||
| ↓ caspase-3 levels | ||||
| 50 mg/kg orally administered for 24 weeks | STZ-induced T1DM rats | ↑ total antioxidant capacity of the retinas | [107] | |
| ↓ VEGF, TNF-α and aldose reductase protein levels | ||||
| ↓ vascular leakage of fluorescein | ||||
| Galangin | 20 and 50 μM for 6 h | D-glucose-stimulated microglial BV2 cells | ↓ BRB damage | [108] |
| ↓ ROS formation | ||||
| ↓ microglia cells activation | ||||
| ↓ ERK1/2 phosphorylation | ||||
| ↓ NF-κB and Egr1 protein levels | ||||
| ↓ TNF-α levels | ||||
| 20 and 50 μM for 6 h | TNF-α-exposed HRECs and ARPE-19 cells | ↑ claudin-1 and occludin levels | ||
| ↑ Nrf2 activation | ||||
| ↓ BRB damage | ||||
| ↓ ROS formation | ||||
| 1 and 10 mg/kg injection for 1 month | STZ-induced T1DM mice | ↓ BRB damage | ||
| ↓ ROS formation | ||||
| ↓ microglia cells activation | ||||
| ↓ ERK1/2 phosphorylation, NF-κB Egr1 protein | ||||
| ↓ TNF-α levels | ||||
| Myricetin | N/A | AGE-BSA-exposed bovine retinal pericytes | ↓ pericytes migration | [109] |
| ↓ ERK1/2-FAK-1-paxillin phosphorylation | ||||
| 5 or 10 mM intravitreally injection | AGE-BSA-intravitreally injected rats | ↓ pericytes migration | ||
| ↓ ERK1/2-FAK-1-paxillin phosphorylation | ||||
| 2.5, 5, 10, 20 and 40 μg/mL for 4 h | Glucose oxidase-exposed ARPE-19 cells | ↑ Nrf2 and SOD2 levels | [110] | |
| ↓ production of H2O2 and intracellular ROS | ||||
| ↓ nitric oxide producer transcription | ||||
| 0.02, 0.2, 2, 20 and 40 μg/mL for 48 h | High glucose-exposed ARPE-19 cells | ↑ antioxidant proteins and other protective factors | [111] | |
| ↑ Nrf2 pathway | ||||
| ↓ intracellular ROS levels and | ||||
| AGE formation | ||||
| ↓ NFkB1 expression | ||||
| and RAGE | ||||
| Icariin | 5 mg/kg orally administered for 12 weeks | STZ-induced T1DM rats | ↑ RECA, Thy-1, Brn3a, and Collagen IV and CA-II levels | [112] |
| ↑ VEGF levels | ||||
| 0, 10, 100 and 1000 nmol/mL for 3 days | RGC cells from control and diabetic rats | ↑ neurite growth |
| Name of Substances/Compound | Dose | Model | Observations | Reference |
|---|---|---|---|---|
| Biochanin A | 10 and 15 mg/kg orally administered for 6 weeks | STZ-induced T1DM rats | ↓ blood glucose levels | [114] |
| ↓ VEGF, TNF-α and IL-1β levels | ||||
| Formononetin | 0.1, 0.5, 1, 5 and 10 µmol/L for 24 h | H2O2-exposed RGC-5 cells | ↑ RGC-5 cell viability | [115] |
| ↑ MnSOD activity | ||||
| ↓ superoxide anions, MDA and 8-OHdG levels | ||||
| ↓ apoptosis and NF-κB pathway activation | ||||
| Puerarin | 6 intraperitoneal injections80 mg/kg | STZ-induced T1DM rats | ↓ morphological changes of INL and ONL | [116] |
| ↓ VEGF and HIF-1α mRNA | ||||
| 140 mg/kg | RPE cells from STZ-induced T1DM rats | ↓ apoptosis | [121] | |
| ↓ NT, C3, iNOS mRNA expression | ||||
| ↓ Fas/FasL | ||||
| Low and high doses for 4 weeks | STZ-induced diabetic rats | ↑ ONL thickness (high-dose puerarin) | [117] | |
| ↓ VEGF levels | ||||
| ↓ AGEs accumulation (high-dose puerarin) | ||||
| ↓ RAGE levels (high-dose puerarin) | ||||
| 125, 250 and 500 mg/kg intragastric administered for 4 weeks | STZ-induced T1DM rats | ↑ retinal b-wave amplitude | [120] | |
| ↓ retinal cell apoptosis | ||||
| ↓ NF-κB p65 activity | ||||
| 500 mg/kg intragastric administration for 4 weeks | STZ-induced T1DM rats | ↑ SOD activity | [119] | |
| ↓ MDA content | ||||
| ↓ RAGE and VEGF levels | ||||
| 1, 5 and 10 µM for 1 h | AGE-BSA-exposed bovine retinal pericytes | ↓ apoptosis | [123] | |
| ↓ ROS generation and NADPH oxidase activity | ||||
| ↓ p47phox and Rac1 phosphorylation | ||||
| ↓ NF-kB activation | ||||
| 10 µM for 1 h | Intravitreal injection of AGE-modified rat serum albumin rats | ↓ apoptosis of the retinal pericyte of rats | ||
| 140 mg/kg intraperitoneal injection for 60 (56) days | RPE cells from STZ-induced T1DM rats | ↓ apoptosis | [122] | |
| ↓ NT and iNOS mRNA levels | ||||
| ↓ Fas/FasL protein expression | ||||
| 10, 25 and 50 µM for 24 h | IL-1β-exposed TR-iBRB2 cells | ↓ leukostasis and apoptosis | [124] | |
| ↓ VCAM-1 and ICAM-1 expression | ||||
| ↓ mitochondrial dysfunction | ||||
| 10−7,10−6,10−5 and 10−4 mol/L for 36 h | NMDA-exposed RGCs cells | ↑ SOD and NO production | [125] | |
| ↑ Bcl-2 expression | ||||
| ↓ RGCs injury | ||||
| ↓ ROS and MDA levels | ||||
| ↓ nNOS and iNOS expression | ||||
| ↓ Bax expression and caspase-3 activity | ||||
| ↓ JNK and p38 phosphorylation | ||||
| 10−7,10−6,10−5 and 10−4 mol/L for 36 h | NMDA-intravitreally injected rats | ↓ RGCs loss | ||
| 250 and 500 mg/kg intragastric administration for 4 weeks | STZ-induced T1DM rats | ↑ insulin levels | [118] | |
| ↑ retinal b-wave amplitude | ||||
| ↑ SOD activity | ||||
| ↓ blood glucose levels | ||||
| ↓ MDA | ||||
| ↓ STAT3 mRNA and protein levels | ||||
| 3,6,7,40,50-pentamethoxy-5,30-dihydroxyflavone | 160 mg/kg for 8 weeks | STZ (35 mg/kg)-induced DM rats | ↓ aldose reductase, SOD, CAT, and GPx and GSH levels | [126] |
| ↓ TBARs and protein carbonyl levels | ||||
| ↓ sorbitol accumulation |
| Study | Participants | Dose | Duration | Results | Reference |
|---|---|---|---|---|---|
| Contribution of flavonoid intake for decreasing the risk of development chronic diseases | n = 10,054, of which 546 were addressed to measure the risk of developing diabetes | N/A | 1 year | ↓ risk of developing T2DM associated with higher quercetin and myricetin intake | [127] |
| Green tea catechins impact, with or without caffeine, on glycemic control markers | n = 1584 out of twenty-two eligible trials | N/A | N/A | ↓ FBG levels | [128] |
| No significant difference in FBI, HbA1c and HOMA-IR | |||||
| Orally administered combination of flavonoids with Centella asiatica and Melilotus effect for the treatment of CME without macular thickening | n = 70 with T2DM and CME without macular thickening | Oral combination of 300 mg diosmin, 15 mg C. asiatica and 160 mg Melilotus per day | 36 months | ↑ retina sensitivity | [129] |
| No differences in visual acuity, mean central retinal thickness, stability fixation, HbA1c percentage, microalbuminuria and blood pressure | |||||
| Purified anthocyanins effect to increase serum adiponectin | n = 160 prediabetic or newly diagnosed diabetic | 2 × 320 mg per day | 12 weeks | Anthocyanins supplementation increased serum adiponectin and decreased fasting glucose in newly diagnosed diabetics but not in prediabetic patients | [130] |
| Chinese green tea consumption and the risk of diabetic retinopathy | n = 100 with DR | Drink Chinese green tea for at least once a week | 1 year | ↓ 50% risk of developing DR in people who regularly drink green tea than those who do not | [132] |
| n = 100 diabetic without DR | |||||
| Pycnogenol® effect on the progression of visual acuity | n = 1169 with T1DM and T2DM that presented DR | 60–120 mg per day | 6 months | ↓ progression of visual loss | [133,134] |
| No significant improvements in patients’ sight | |||||
| Effect of Pycnogenol® in early stages of DR | n = 46 diagnosed with moderate degree of diabetic macular edema | 150 mg daily | 2 months | Visual and baseline improvement | [134] |
| Flavonoid-rich diet impact on DR and diabetes-related biomarkers | n = 381 diabetic patient from NHANES 2003–2006 | N/A | N/A | ↓ risk of developing DR by 30% | [131] |
| ↓ C-reactive protein levels, HbA1C and glucose | |||||
| Effects of antioxidant supplementation in ROS circulating levels and in changes in CMT | n = 68 with NPDR | One tablet containing 50 mg of pycnogenol, 30 mg of Vitamin E and 20 mg of CoQ per day | 6 months | ↓ ROS levels and CMT | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, A.L.; Bruno, D.F.; Ambrósio, A.F.; Santos, P.F. The Benefits of Flavonoids in Diabetic Retinopathy. Nutrients 2020, 12, 3169. https://doi.org/10.3390/nu12103169
Matos AL, Bruno DF, Ambrósio AF, Santos PF. The Benefits of Flavonoids in Diabetic Retinopathy. Nutrients. 2020; 12(10):3169. https://doi.org/10.3390/nu12103169
Chicago/Turabian StyleMatos, Ana L., Diogo F. Bruno, António F. Ambrósio, and Paulo F. Santos. 2020. "The Benefits of Flavonoids in Diabetic Retinopathy" Nutrients 12, no. 10: 3169. https://doi.org/10.3390/nu12103169
APA StyleMatos, A. L., Bruno, D. F., Ambrósio, A. F., & Santos, P. F. (2020). The Benefits of Flavonoids in Diabetic Retinopathy. Nutrients, 12(10), 3169. https://doi.org/10.3390/nu12103169