Temporal Leptin to Determine Cardiovascular and Metabolic Fate throughout the Life
Abstract
1. Introduction
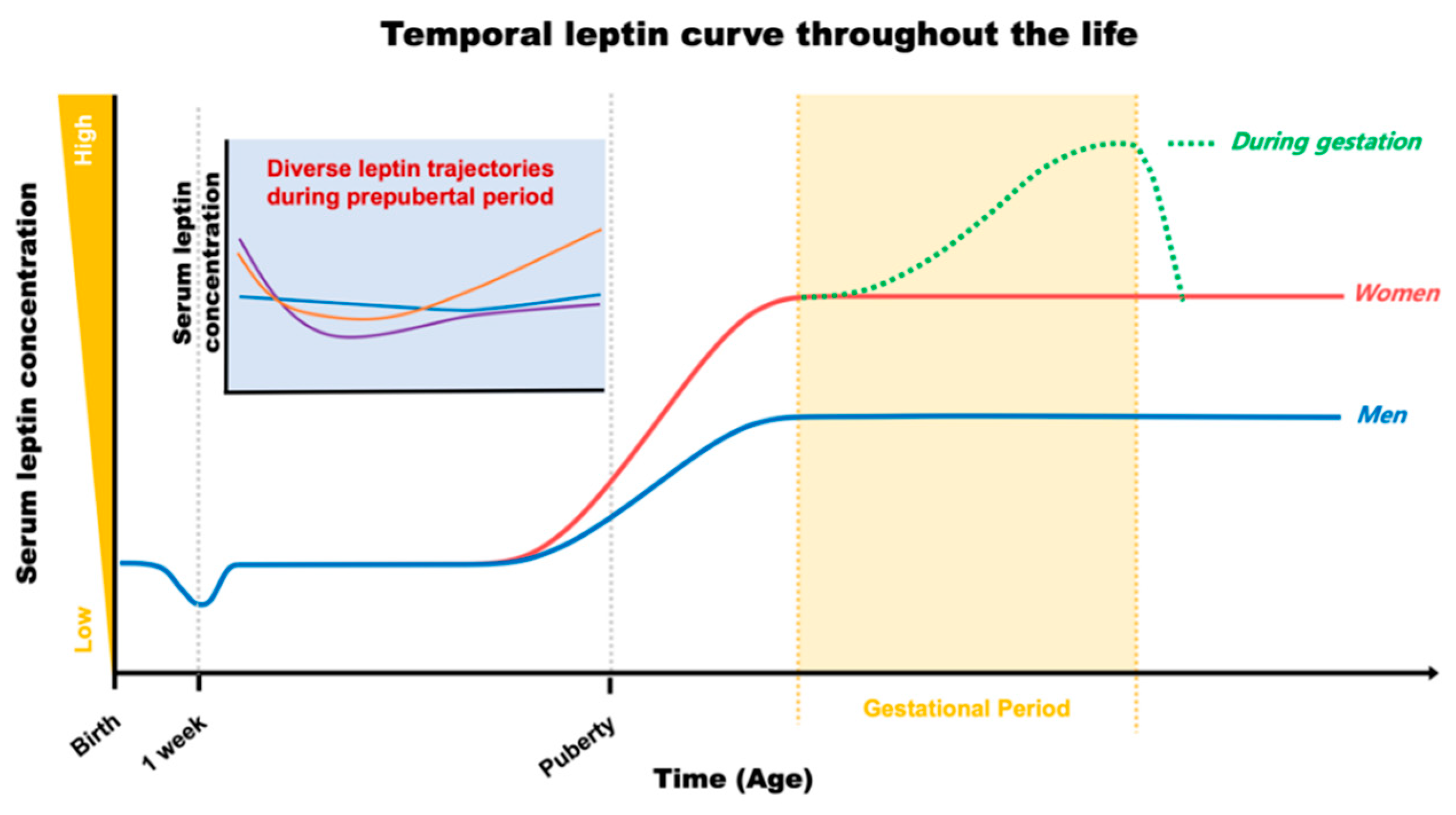
2. Early Leptin for Future Cardiometabolic Output
2.1. Maternal and Cord Leptin during Gestation
2.2. Postnatal Leptin
2.3. Merge of Leptin with Early Environmental Factors
3. Leptin as a Hallmark of Cardiometabolic Fate in Adulthood
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Spiezio, A.; Sandin, E.S.; Dore, R.; Muller-Fielitz, H.; Storck, S.E.; Bernau, M.; Mier, W.; Oster, H.; Johren, O.; Pietrzik, C.U.; et al. The LepR-mediated leptin transport across brain barriers controls food reward. Mol. Metab. 2018, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Suyama, S.; Koch, M.; Jin, S.; Argente-Arizon, P.; Argente, J.; Liu, Z.W.; Zimmer, M.R.; Jeong, J.K.; Szigeti-Buck, K.; et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 2014, 17, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.K.; Kim, J.G.; Lee, B.J. Participation of the central melanocortin system in metabolic regulation and energy homeostasis. Cell Mol. Life Sci. 2014, 71, 3799–3809. [Google Scholar] [CrossRef] [PubMed]
- Simonds, S.E.; Pryor, J.T.; Cowley, M.A. Does leptin cause an increase in blood pressure in animals and humans? Curr. Opin. Nephrol. Hypertens. 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.; Morgan, D.A.; Butler, S.D.; Mark, A.L.; Davisson, R.L. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension 2013, 61, 737–744. [Google Scholar] [CrossRef]
- Young, C.N.; Morgan, D.A.; Butler, S.D.; Rahmouni, K.; Gurley, S.B.; Coffman, T.M.; Mark, A.L.; Davisson, R.L. Angiotensin type 1a receptors in the forebrain subfornical organ facilitate leptin-induced weight loss through brown adipose tissue thermogenesis. Mol. Metab. 2015, 4, 337–343. [Google Scholar] [CrossRef]
- Palou, M.; Pico, C.; Palou, A. Leptin as a breast milk component for the prevention of obesity. Nutr. Rev. 2018, 76, 875–892. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef]
- Ramos-Lobo, A.M.; Teixeira, P.D.; Furigo, I.C.; Melo, H.M.; Silva, N.D.M.L.E.; De Felice, F.G.; Donato, J., Jr. Long-term consequences of the absence of leptin signaling in early life. Elife 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Funcke, J.B.; Lennerz, B.; Kuhnle-Krahl, U.; Lahr, G.; Debatin, K.M.; Vatter, P.; Gierschik, P.; Moepps, B.; Fischer-Posovszky, P. Biologically inactive leptin and early-onset extreme obesity. N. Engl. J. Med. 2015, 372, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, Y.; Liang, D.; Li, L.; Liang, Y.; Chen, L.; Tong, L.; Zhou, J.; Li, H.; Zhang, H. Association of polymorphisms in LEPR with type 2 diabetes and related metabolic traits in a Chinese population. Lipids Health Dis. 2018, 17, 2. [Google Scholar] [CrossRef]
- Nesrine, Z.; Haithem, H.; Imen, B.; Fadoua, N.; Asma, O.; Fadhel, N.M.; Ali, B. Leptin and Leptin receptor polymorphisms, plasma Leptin levels and obesity in Tunisian volunteers. Int. J. Exp. Pathol. 2018, 99, 121–130. [Google Scholar] [CrossRef]
- De Luis, D.A.; Aller, R.; Izaola, O.; Sagrado, M.G.; Conde, R.; Aller, R.; Primo, D. Effect of Lys656Asn Polymorphism of Leptin Receptor Gene on Cardiovascular Risk Factors and Serum Adipokine Levels after a High Polyunsaturated Fat Diet in Obese Patients. J. Clin. Lab. Anal. 2014, 29, 432–436. [Google Scholar] [CrossRef]
- Santana, M.G.; De Velasco, P.C.; De Oliveira, O.R.C.; Santo, R.E.; Spreafico, F.; De Almeida, L.B.; Sardinha, F.L.D.C.; Carmo, M.D.G.T.D. Adiponectin, insulin and leptin levels in the cord plasma of the neonates from adolescent and adult mothers and their relationship with anthropometric parameters and fetal sex-gender. J. Perinatol. 2018, 38, 489–495. [Google Scholar] [CrossRef]
- Walsh, J.M.; Byrne, J.; Mahony, R.; Foley, M.E.; McAuliffe, F.M. Leptin, fetal growth and insulin resistance in non-diabetic pregnancies. Early Hum. Dev. 2014, 90, 271–274. [Google Scholar] [CrossRef]
- Galletti, F.; Barbato, A.; Versiero, M.; Iacone, R.; Russo, O.; Barba, G.; Siani, A.; Cappuccio, F.P.; Farinaro, E.; Della Valle, E.; et al. Circulating leptin levels predict the development of metabolic syndrome in middle-aged men: An 8-year follow-up study. J. Hypertens. 2007, 25, 1671–1677. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Lowe, G.; Rumley, A.; Cherry, L.; Whincup, P.H.; Sattar, N. Adipokines and risk of type 2 diabetes in older men. Diabetes Care 2007, 30, 1200–1205. [Google Scholar] [CrossRef]
- Neville, C.E.; Patterson, C.C.; Linden, G.; Love, K.; McKinley, M.C.; Kee, F.; Blankenberg, S.; Evans, A.; Yarnell, J.W.G.; Woodside, J.V. The relationship between adipokines and the onset of type 2 diabetes in middle-aged men: The PRIME study. Diabetes Res. Clin. Pract. 2016, 120, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Fava, C.; Targher, G.; Franchini, M.; Danese, E.; Bonafini, S.; De Cata, A.; Salvagno, G.L.; Ruzzenente, O.; Guidi, G.C.; et al. Plasma Leptin in Patients at Intermediate to High Cardiovascular Risk With and Without Type 2 Diabetes Mellitus. J. Clin. Lab. Anal. 2016, 31, e22031. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, M.; Dmoch-Gajzlerska, E.; Mazurkiewicz, B.; Gajzlerska-Majewska, W. Maternal serum and cord blood leptin concentrations at delivery. PLoS ONE 2019, 14, e0224863. [Google Scholar] [CrossRef]
- Hardie, L.; Trayhurn, P.; Abramovich, D.; Fowler, P. Circulating leptin in women: A longitudinal study in the menstrual cycle and during pregnancy. Clin. Endocrinol. 1997, 47, 101–106. [Google Scholar] [CrossRef]
- Herrera, E.; Lasunción, M.A.; Huerta, L.; Martin, A. Plasma leptin levels in rat mother and offspring during pregnancy and lactation. Biol. Neonate 2000, 78, 315–320. [Google Scholar] [CrossRef]
- Briffa, J.F.; McAinch, A.J.; Romano, T.; Wlodek, M.E.; Hryciw, D.H. Leptin in pregnancy and development: A contributor to adulthood disease? Am. J. Physiol. Endocrinol. Metab. 2015, 308, E335–E350. [Google Scholar] [CrossRef]
- Lin, R.; Ju, H.; Yuan, Z.; Zhang, C.; Zeng, L.; Sun, Y.; Su, Z.; Jin, L. Effects of maternal and fetal LEP common variants on maternal glycemic traits in pregnancy. Sci. Rep. 2017, 7, 17710. [Google Scholar] [CrossRef] [PubMed]
- Ladyman, S.; Grattan, D.R. Central Effects of Leptin on Glucose Homeostasis are Modified during Pregnancy in the Rat. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Ladyman, S.; Tups, A.; Augustine, R.A.; Swahn-Azavedo, A.; Kokay, I.C.; Grattan, D.R. Loss of hypothalamic response to leptin during pregnancy associated with development of melanocortin resistance. J. Neuroendocr. 2009, 21, 449–456. [Google Scholar] [CrossRef]
- Ramos-Lobo, A.M.; Furigo, I.C.; Teixeira, P.D.S.; Zampieri, T.T.; Wasinski, F.; Buonfiglio, D.C.; Donato, J. Maternal metabolic adaptations are necessary for normal offspring growth and brain development. Physiol. Rep. 2018, 6, e13643. [Google Scholar] [CrossRef]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Godínez-Martínez, E.; Sánchez-Jiménez, B.; Montiel-Ojeda, D.; Tolentino, M. Serum Concentration of Leptin in Pregnant Adolescents Correlated with Gestational Weight Gain, Postpartum Weight Retention and Newborn Weight/Length. Nutrients 2017, 9, 1067. [Google Scholar] [CrossRef]
- Erichart-Perera, O.; Muñoz-Manrique, C.; Reyes-López, A.; Tolentino-Dolores, M.; Sosa, S.E.Y.; Ramírez-González, M.C. Metabolic markers during pregnancy and their association with maternal and newborn weight status. PLoS ONE 2017, 12, e0180874. [Google Scholar]
- Okdemir, D.; Hatipoglu, N.; Kurtoglu, S.; Siraz, U.G.; Akar, H.H.; Muhtaroglu, S.; Kutuk, M.S. The Role of Irisin, Insulin and Leptin in Maternal and Fetal Interaction. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 307–315. [Google Scholar] [CrossRef]
- Elzein, A.O.; Ali, A.A.; Hamdan, H.Z.; Elhassan, E.M.; Shrif, N.E.; Adam, I. Materno-foetal leptin and insulin-like growth factor in low birth weight neonates. J. Obs. Gynaecol. 2016, 36, 31–33. [Google Scholar] [CrossRef]
- Gijón-Conde, T.; Graciani, A.; Guallar-Castillón, P.; Aguilera, M.T.; Rodríguez-Artalejo, F.; Banegas, J.R. Leptin Reference Values and Cutoffs for Identifying Cardiometabolic Abnormalities in the Spanish Population. Rev. Esp. Cardiol. 2015, 68, 672–679. [Google Scholar] [CrossRef]
- Shang, M.; Dong, X.; Hou, L. Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns. J. Obs. Gynaecol. Res. 2018, 44, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.M.; Dias-Rocha, C.P.; Reis-Gomes, C.F.; Wang, H.; Atella, G.C.; Cordeiro, A.; Pazos-Moura, C.C.; Joss-Moore, L.A.; Trevenzoli, I.H. Maternal high-fat diet impairs leptin signaling and up-regulates type-1 cannabinoid receptor with sex-specific epigenetic changes in the hypothalamus of newborn rats. Psychoneuroendocrinology 2019, 103, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Purcell, R.H.; Terrillion, C.E.; Yan, J.; Moran, T.H.; Tamashiro, K.L. Maternal High-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 2012, 61, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Nilsson, C.; Secher, A.; Kildegaard, J.; Skovgaard, T.; Nielsen, M.O.; Grove, K.; Raun, K. Differential hypothalamic leptin sensitivity in obese rat offspring exposed to maternal and postnatal intake of chocolate and soft drink. Nutr. Diabetes 2017, 7, e242. [Google Scholar] [CrossRef] [PubMed]
- Yau-Qiu, Z.X.; Pico, C.; Rodriguez, A.M.; Palou, A. Leptin Distribution in Rat Foetal and Extraembryonic Tissues in Late Gestation: A Physiological View of Amniotic Fluid Leptin. Nutrients 2020, 12, 2542. [Google Scholar] [CrossRef]
- Kadakia, R.; Scholtens, D.M.; Rouleau, G.; Talbot, O.; Ilkayeva, O.; George, T.; Josefson, J.L. Cord Blood Metabolites Associated with Newborn Adiposity and Hyperinsulinemia. J. Pediatr. 2018, 203, 144–149 e141. [Google Scholar] [CrossRef]
- Kadakia, R.; Zheng, Y.; Zhang, Z.; Zhang, W.; Josefson, J.L.; Hou, L. Association of cord blood methylation with neonatal leptin: An epigenome wide association study. PLoS ONE 2019, 14, e0226555. [Google Scholar] [CrossRef]
- Telschow, A.; Ferrari, N.; Deibert, C.; Flöck, A.; Merz, W.M.; Gembruch, U.; Ehrhardt, C.; Dötsch, J.; Graf, C. High Maternal and Low Cord Blood Leptin Are Associated with BMI-SDS Gain in the First Year of Life. Obes. Facts 2019, 12, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Karakosta, P.; Roumeliotaki, T.; Chalkiadaki, G.; Sarri, K.; Vassilaki, M.; Venihaki, M.; Malliaraki, N.; Kampa, M.; Castanas, E.; Kogevinas, M.; et al. Cord blood leptin levels in relation to child growth trajectories. Metabolism 2016, 65, 874–882. [Google Scholar] [CrossRef]
- Chaoimh, C.N.; Murray, D.M.; Kenny, L.C.; Irvine, A.D.; Hourihane, J.O.; Kiely, M. Cord blood leptin and gains in body weight and fat mass during infancy. Eur. J. Endocrinol. 2016, 175, 403–410. [Google Scholar] [CrossRef][Green Version]
- Simpson, J.; Smith, A.D.A.C.; Fraser, A.; Sattar, N.; Lindsay, R.S.; Ring, S.M.; Tilling, K.; Smith, G.D.; Lawlor, D.A.; Nelson, S.M. Programming of Adiposity in Childhood and Adolescence: Associations with Birth Weight and Cord Blood Adipokines. J. Clin. Endocrinol. Metab. 2016, 102, 499–506. [Google Scholar] [CrossRef]
- Oteng-Ntim, E.; Mononen, S.; Sawicki, O.; Seed, P.T.; Bick, D.; Poston, L. Interpregnancy weight change and adverse pregnancy outcomes: A systematic review and meta-analysis. BMJ Open 2018, 8, e018778. [Google Scholar] [CrossRef]
- Garcia-Flores, J.; Cruceyra, M.; Canamares, M.; Garicano, A.; Nieto, O.; Tamarit, I. Predictive value of fetal hepatic biometry for birth weight and cord blood markers in gestational diabetes. J. Perinatol. 2016, 36, 723–728. [Google Scholar] [CrossRef]
- Druet, C.; Stettler, N.; Sharp, S.; Simmons, R.K.; Cooper, C.; Smith, G.D.; Ekelund, U.; Lévy-Marchal, C.; Jarvelin, M.-R.; Kuh, D.; et al. Prediction of childhood obesity by infancy weight gain: An individual-level meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.G.; Rifas-Shiman, S.L.; Belfort, M.B.; Taveras, E.M.; Oken, E.; Mantzoros, C.; Gillman, M.W. Gestational glucose tolerance and cord blood leptin levels predict slower weight gain in early infancy. J. Pediatr. 2011, 158, 227–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Euclydes, V.L.; Castro, N.P.; Lima, L.R.; De Brito, C.A.; Ribeiro, L.; Simões, F.A.; Requena, G.; Luzia, L.A.; Rondó, P.H.C. Cord blood concentrations of leptin, zinc-alpha2-glycoprotein, and adiponectin, and adiposity gain during the first 3 mo of life. Nutrition 2018, 54, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.B.; Han, S.P.; Zhu, G.Z.; Zhu, C.; Wang, X.J.; Cao, X.G.; Guo, X.-R. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.R.; Geng, T.T.; Gorny, A.W.; Ding, R.; Li, C.; Ley, S.H.; Huang, T. Birth Weight and Risk of Type 2 Diabetes Mellitus, Cardiovascular Disease, and Hypertension in Adults: A Meta-Analysis of 7 646 267 Participants From 135 Studies. J. Am. Heart Assoc. 2018, 7, e008870. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Eriksson, J.G.; Forsén, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Birthweight and mortality in adulthood: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Vollenweider, P.; Bochud, M.; Mooser, V.; Waeber, G.; Marques-Vidal, P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovasc. Diabetol. 2016, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, M.M.; Lassi, Z.S.; Roberts, C.T.; Andraweera, P.H. Cardiovascular risk factors in offspring exposed to gestational diabetes mellitus in utero: Systematic review and meta-analysis. J. Dev. Orig. Health Dis. 2020, 1–18. [Google Scholar] [CrossRef]
- Savino, F.; Rossi, L.; Benetti, S.; Petrucci, E.; Sorrenti, M.; Silvestro, L. Serum reference values for leptin in healthy infants. PLoS ONE 2014, 9, e113024. [Google Scholar] [CrossRef]
- Wang, L.-J.; Mu, S.-C.; Cheng, I.; Chen, Y.-L.; Chen, B.-F.; Jow, G.-M. Decreased leptin concentration in neonates is associated with enhanced postnatal growth during the first year. Kaohsiung J. Med. Sci. 2012, 28, 521–525. [Google Scholar] [CrossRef]
- Kulik-Rechberger, B.; Bury, A.M.; Rakuś-Kwiatosz, A.; Beń-Skowronek, I. Cortisol, leptin and free leptin index (FLI) in newborns in the first days of life and their importance for body weight programming. Ital. J. Pediatr. 2019, 45, 141–146. [Google Scholar] [CrossRef]
- Mann, D.R.; Johnson, A.O.K.; Gimpel, T.; Castracane, V.D. Changes in circulating leptin, leptin receptor, and gonadal hormones from infancy until advanced age in humans. J. Clin. Endocrinol. Metab. 2003, 88, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Prabakaran, D.; Flier, J.S. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J. Clin. Investig. 1998, 101, 1020–1027. [Google Scholar] [CrossRef]
- Udagawa, J.; Hatta, T.; Hashimoto, R.; Otani, H. Roles of leptin in prenatal and perinatal brain development. Congenit. Anom. 2007, 47, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mela, V.; Díaz, F.; Borcel, E.; Argente, Y.J.; Chowen, J.A.; Viveros, M.P. Long Term Hippocampal and Cortical Changes Induced by Maternal Deprivation and Neonatal Leptin Treatment in Male and Female Rats. PLoS ONE 2015, 10, e0137283. [Google Scholar] [CrossRef]
- Sominsky, L.; Ziko, I.; Nguyen, T.-X.; Quach, J.; Spencer, S.J. Hypothalamic effects of neonatal diet: Reversible and only partially leptin dependent. J. Endocrinol. 2017, 234, 41–56. [Google Scholar] [CrossRef]
- Itoh, H.; Yura, S.; Sagawa, N.; Kanayama, N.; Konihi, I. Hamamatsu Birth Cohort for M, Children Study T: Neonatal exposure to leptin reduces glucose tolerance in adult mice. Acta Physiol. 2011, 202, 159–164. [Google Scholar] [CrossRef]
- Boeke, C.E.; Mantzoros, C.S.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Zera, C.A.; Gillman, M.W. Differential associations of leptin with adiposity across early childhood. Obesity 2013, 21, 1430–1437. [Google Scholar] [CrossRef]
- Kettaneh, A.; Heude, B.; Romon, M.; Oppert, J.-M.; Borys, J.-M.; Balkau, B.; Ducimetiere, P.; Charles, M.-A. High plasma leptin predicts an increase in subcutaneous adiposity in children and adults. Eur. J. Clin. Nutr. 2007, 61, 719–726. [Google Scholar] [CrossRef]
- Elsaeed, G.; Mousa, N.; El-Mougy, F.; Hafez, M.; Khodeera, S.; Alhelbawy, M.; Fouda, E.; Elsheikh, S.; Elkaffas, R.; Eldeeb, S.; et al. Monogenic leptin deficiency in early childhood obesity. Pediatr. Obes. 2020, 15, e12574. [Google Scholar] [CrossRef]
- Kohlsdorf, K.; Nunziata, A.; Funcke, J.-B.; Brandt, S.; Von Schnurbein, J.; Vollbach, H.; Lennerz, B.; Fritsch, M.; Greber-Platzer, S.; Fröhlich-Reiterer, E.; et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int. J. Obes. 2018, 42, 1602–1609. [Google Scholar] [CrossRef]
- Volberg, V.; Heggeseth, B.; Harley, K.; Huen, K.; Yousefi, P.; Davé, V.; Tyler, K.; Vedar, M.; Eskenazi, B.; Holland, N. Adiponectin and leptin trajectories in Mexican-American children from birth to 9 years of age. PLoS ONE 2013, 8, e77964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.-J.; Rifas-Shiman, S.L.; Aris, I.M.; Mantzoros, C.; Hivert, M.-F.; Oken, E. Leptin trajectories from birth to mid-childhood and cardio-metabolic health in early adolescence. Metabolism 2019, 91, 30–38. [Google Scholar] [CrossRef]
- Pérez, C.M.; Ortiz, A.P.; Fuentes-Mattei, E.; Velazquez-Torres, G.; Santiago, D.; Giovannetti, K.; Bernabe, R.; Lee, M.-H.; Yeung, S.-C.J. High prevalence of cardiometabolic risk factors in Hispanic adolescents: Correlations with adipocytokines and markers of inflammation. J. Immigr. Minor. Health 2014, 16, 865–873. [Google Scholar] [CrossRef]
- Siegrist, M.; Hanssen, H.; Neidig, M.; Fuchs, M.; Lechner, F.; Stetten, M.; Blume, K.; Lammel, C.; Haller, B.; Vogeser, M.; et al. Association of leptin and insulin with childhood obesity and retinal vessel diameters. Int. J. Obes. 2014, 38, 1241–1247. [Google Scholar] [CrossRef]
- Agostinis-Sobrinho, C.A.; Mendes, E.L.; Moreira, C.; Abreu, S.; Lopes, L.; Oliveira-Santos, J.; Skurvydas, A.; Mota, J.; Santos, R. Association between Leptin, Adiponectin, and Leptin/Adiponectin Ratio with Clustered Metabolic Risk Factors in Portuguese Adolescents: The LabMed Physical Activity Study. Ann. Nutr. Metab. 2017, 70, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.; Loos, R.J.F. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006, 95, 904–908. [Google Scholar] [CrossRef]
- Perng, W.; Hajj, H.; Belfort, M.B.; Rifas-Shiman, S.L.; Kramer, M.S.; Gillman, M.W.; Oken, E. Birth Size, Early Life Weight Gain, and Midchildhood Cardiometabolic Health. J. Pediatr. 2016, 173, 122–130.e121. [Google Scholar] [CrossRef]
- Aris, I.M.; Bernard, J.Y.; Chen, L.-W.; Tint, M.T.; Pang, W.W.; Lim, W.Y.; Soh, S.E.; Saw, S.-M.; Godfrey, K.M.; Gluckman, P.D.; et al. Infant body mass index peak and early childhood cardio-metabolic risk markers in a multi-ethnic Asian birth cohort. Int. J. Epidemiol. 2017, 46, 513–525. [Google Scholar] [CrossRef]
- Aris, I.M.; Rifas-Shiman, S.L.; Li, L.-J.; Kleinman, K.P.; Coull, B.A.; Gold, D.R.; Hivert, M.-F.; Kramer, M.S.; Oken, E. Patterns of body mass index milestones in early life and cardiometabolic risk in early adolescence. Int. J. Epidemiol. 2019, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Vik, T.; Jacobsen, G.; Vatten, L.; Bakketeig, L.S. Pre- and post-natal growth in children of women who smoked in pregnancy. Early Hum. Dev. 1996, 45, 245–255. [Google Scholar] [CrossRef]
- Blake, K.V.; Gurrin, L.C.; Evans, S.F.; Beilin, L.J.; Landau, L.I.; Stanley, F.J.; Newnham, J.P. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum. Dev. 2000, 57, 137–147. [Google Scholar] [CrossRef]
- Huang, J.S.; Lee, T.A.; Lu, M.C. Prenatal programming of childhood overweight and obesity. Matern. Child. Health J. 2007, 11, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, A.F.; Rifas-Shiman, S.L.; Rokoff, L.B.; Hivert, M.-F.; Mantzoros, C.S.; Oken, E. Associations of maternal prenatal smoking with umbilical cord blood hormones: The Project Viva cohort. Metabolism 2017, 72, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Luo, Z.-C.; Dejemli, A.; Delvin, E.; Zhang, J. Maternal Smoking and Metabolic Health Biomarkers in Newborns. PLoS ONE 2015, 10, e0143660. [Google Scholar] [CrossRef]
- Helland, I.B.; Reseland, J.E.; Saugstad, O.D.; Drevon, C.A. Smoking related to plasma leptin concentration in pregnant women and their newborn infants. Acta Paediatr. 2001, 90, 282–287. [Google Scholar] [CrossRef]
- De Oliveira, E.; Moura, E.G.; Santos-Silva, A.P.; Pinheiro, C.R.; Lima, N.S.; Nogueira-Neto, J.F.; Nunes-Freitas, A.L.; Abreu-Villaça, Y.; Passos, M.C.F.; Lisboa, P.C. Neonatal nicotine exposure causes insulin and leptin resistance and inhibits hypothalamic leptin signaling in adult rat offspring. J. Endocrinol. 2010, 206, 55–63. [Google Scholar] [CrossRef]
- Yousefi, M.; Karmaus, W.; Zhang, H.; Ewart, S.; Arshad, H.; Holloway, J.W. The methylation of the LEPR/LEPROT genotype at the promoter and body regions influence concentrations of leptin in girls and BMI at age 18 years if their mother smoked during pregnancy. Int. J. Mol. Epidemiol. Genet. 2013, 4, 86–100. [Google Scholar]
- Khanolkar, A.R.; Byberg, L.; Koupil, I. Parental influences on cardiovascular risk factors in Swedish children aged 5-14 years. Eur. J. Public Health 2011, 22, 840–847. [Google Scholar] [CrossRef]
- Burke, V.; Beilin, L.; Dunbar, D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: A longitudinal study. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 147–157. [Google Scholar] [CrossRef]
- Pravdova, E.; Macho, L.; Fickova, M. Alcohol intake modifies leptin, adiponectin and resistin serum levels and their mRNA expressions in adipose tissue of rats. Endocr. Regul. 2009, 43, 117–125. [Google Scholar] [PubMed]
- Maddalozzo, G.F.; Turner, R.T.; Edwards, C.H.; Howe, K.S.; Widrick, J.J.; Rosen, C.J.; Iwaniec, U.T. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos. Int. 2009, 20, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, F.; Pérez-Cejas, A.; Alemán, M.-R.; González-Reimers, E.; Milena, A.; De La Vega, M.-J.; Martínez-Riera, A.; Gómez-Rodríguez, M.-A. Low serum leptin levels and malnutrition in chronic alcohol misusers hospitalized by somatic complications. Alcohol Alcohol. 2003, 38, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.M.; Fernandez-Sola, J.; Fatjo, F.; Casamitjana, R.; Bataller, R.; Sacanella, E.; Tobias, E.; Badia, E.; Estruch, R. Increased circulating leptin levels in chronic alcoholism. Alcohol. Clin. Exp. Res. 2001, 25, 83–88. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; De Zoete, E.C.; Kok, F.J.; Schaafsma, G.; Hendriks, H.F.J. Effect of moderate alcohol consumption on adipokines and insulin sensitivity in lean and overweight men: A diet intervention study. Eur. J. Clin. Nutr. 2007, 62, 1098–1105. [Google Scholar] [CrossRef]
- Shen, L.; Cordero, J.F.; Wang, J.-S.; Shen, Y.; Li, S.; Liang, L.; Zou, Z.; Li, C. Association between genetically determined leptin and blood lipids considering alcohol consumption: A Mendelian randomisation study. BMJ Open 2019, 9, e026860. [Google Scholar] [CrossRef]
- Hindmarch, C.C.T.; Ferguson, A.V. Physiological roles for the subfornical organ: A dynamic transcriptome shaped by autonomic state. J. Physiol. 2015, 594, 1581–1589. [Google Scholar] [CrossRef]
- Peterson, C.; Huang, S.; Lee, S.A.; Ferguson, A.; Fry, M. The transcriptome of the rat subfornical organ is altered in response to early postnatal overnutrition. Ibro. Rep. 2018, 5, 17–23. [Google Scholar] [CrossRef]
- Reddy, V.D.K.; Jagota, A. Effect of restricted feeding on nocturnality and daily leptin rhythms in OVLT in aged male Wistar rats. Biogerontology 2014, 15, 245–256. [Google Scholar] [CrossRef]
- Neyens, D.; Zhao, H.; Huston, N.J.; Wayman, G.A.; Ritter, R.C.; Appleyard, S.M. Leptin Sensitizes NTS Neurons to Vagal Input by Increasing Postsynaptic NMDA Receptor Currents. J. Neurosci. 2020, 40, 7054–7064. [Google Scholar] [CrossRef]
- Mietlicki-Baase, E.G.; Olivos, D.R.; Jeffrey, B.A.; Hayes, M.R. Cooperative interaction between leptin and amylin signaling in the ventral tegmental area for the control of food intake. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1116–E1122. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Brooks, V.L. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: Role of oestrogen. J. Physiol. 2014, 593, 1633–1647. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shi, Z.; Cassaglia, P.A.; Brooks, V.L. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension 2013, 61, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, J.C.; Hu, Y.; Lu, H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 1997, 46, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Carmo, J.M.D.; Da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Hall, D.; Subramanian, M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. Geroscience 2019, 41, 13–24. [Google Scholar] [CrossRef]
- Mitchell, S.E.; Nogueiras, R.; Morris, A.; Tovar, S.; Grant, C.; Cruickshank, M.; Rayner, D.V.; Dieguez, C.; Williams, L.M. Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J. Physiol. 2009, 587, 3573–3585. [Google Scholar] [CrossRef]
- Pan, W.W.; Myers, M.G., Jr. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 2018, 19, 95–105. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valenti, V.; Bilbao, I.; De La Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2019, 11, 2664. [Google Scholar] [CrossRef]
- Simonds, S.E.; Pryor, J.T.; Ravussin, E.; Greenway, F.L.; Dileone, R.; Allen, A.M.; Bassi, J.; Elmquist, J.K.; Keogh, J.M.; Henning, E.; et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014, 159, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Ekmen, N.; Helvaci, A.; Gunaldi, M.; Sasani, H.; Yildirmak, S.T. Leptin as an important link between obesity and cardiovascular risk factors in men with acute myocardial infarction. Indian Heart J. 2016, 68, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H.; Lennon, L.T.; Sattar, N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? J. Am. Coll. Cardiol. 2011, 58, 1870–1877. [Google Scholar] [CrossRef]
- Franks, P.W.; Brage, S.; Luan, J.; Ekelund, U.; Rahman, M.; Farooqi, I.S.; Halsall, I.; O’Rahilly, S.; Wareham, N.J. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes. Res. 2005, 13, 1476–1484. [Google Scholar] [CrossRef]
- Tsai, J.-P. The association of serum leptin levels with metabolic diseases. Ci Ji Yi Xue Za Zhi 2017, 29, 192–196. [Google Scholar] [CrossRef]
- Sun, Q.; van Dam, R.M.; Meigs, J.B.; Franco, O.H.; Mantzoros, C.S.; Hu, F.B. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: A prospective study. Diabetes 2010, 59, 611–618. [Google Scholar] [CrossRef]
- Faulkner, J.L.; de Chantemele, E.J.B. Sex Differences in Mechanisms of Hypertension Associated With Obesity. Hypertension 2018, 71, 15–21. [Google Scholar] [CrossRef]
- Kasacka, I.; Piotrowska, Z.; Niezgoda, M.; Lebkowski, W. Differences in leptin biosynthesis in the stomach and in serum leptin level between men and women. J. Gastroenterol. Hepatol. 2019, 34, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Sieminska, L.; Wojciechowska, C.; Foltyn, W.; Kajdaniuk, D.; Kos-Kudla, B.; Marek, B.; Nasiek, M.; Nowak, M.; Strzelczyk, J.; Zemczak, A. The relation of serum adiponectin and leptin levels to metabolic syndrome in women before and after the menopause. Endokrynol. Pol. 2006, 57, 15–22. [Google Scholar]
- Sylvia, K.E.; Lorenz, T.K.; Heiman, J.R.; Demas, G.E. Physiological predictors of leptin vary during menses and ovulation in healthy women. Reprod. Biol. 2018, 18, 132–136. [Google Scholar] [CrossRef]
- Dragano, N.R.; Haddad-Tovolli, R.; Velloso, L.A. Leptin, Neuroinflammation and Obesity. Front. Horm. Res. 2017, 48, 84–96. [Google Scholar] [PubMed]
| Time | Physiological Factors in Association with Leptin | Ref. |
|---|---|---|
| Gestational Period |
| [18,23,31,32,33] [23,33] [13] [18,26,27,28] |
| Neonatal to early life |
| [17,23,33,34,36,43] [79,80] [61,73] [65] [74] [13] [67] [44,45,46,49,50,51,77,78] |
| Adulthood |
| [102,103,104,106,107] [115] [14,15,16,107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.G.; Lee, B.J.; Jeong, J.K. Temporal Leptin to Determine Cardiovascular and Metabolic Fate throughout the Life. Nutrients 2020, 12, 3256. https://doi.org/10.3390/nu12113256
Kim JG, Lee BJ, Jeong JK. Temporal Leptin to Determine Cardiovascular and Metabolic Fate throughout the Life. Nutrients. 2020; 12(11):3256. https://doi.org/10.3390/nu12113256
Chicago/Turabian StyleKim, Jae Geun, Byung Ju Lee, and Jin Kwon Jeong. 2020. "Temporal Leptin to Determine Cardiovascular and Metabolic Fate throughout the Life" Nutrients 12, no. 11: 3256. https://doi.org/10.3390/nu12113256
APA StyleKim, J. G., Lee, B. J., & Jeong, J. K. (2020). Temporal Leptin to Determine Cardiovascular and Metabolic Fate throughout the Life. Nutrients, 12(11), 3256. https://doi.org/10.3390/nu12113256






