Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty
Abstract
:1. Introduction
1.1. Frailty and Cognitive Impairment in Older Adults with Diabetes Mellitus
1.2. Frailty
1.3. Cognitive Impairment
1.4. Interaction between Frailty and Cognitive Impairment
2. Mechanistic Insights into Diabetes, Frailty, and Cognitive Impairment
2.1. Insulin Resistance
2.2. Arteriosclerosis and Brain White Matter Lesions
2.3. Chronic Inflammation, Oxidative Stress, and Mitochondrial Dysfunction
2.4. Hyperglycemia and Hypoglycemia
2.5. Physical Inactivity
3. Nutrition and Nutrients in Older Adults with Diabetes Mellitus
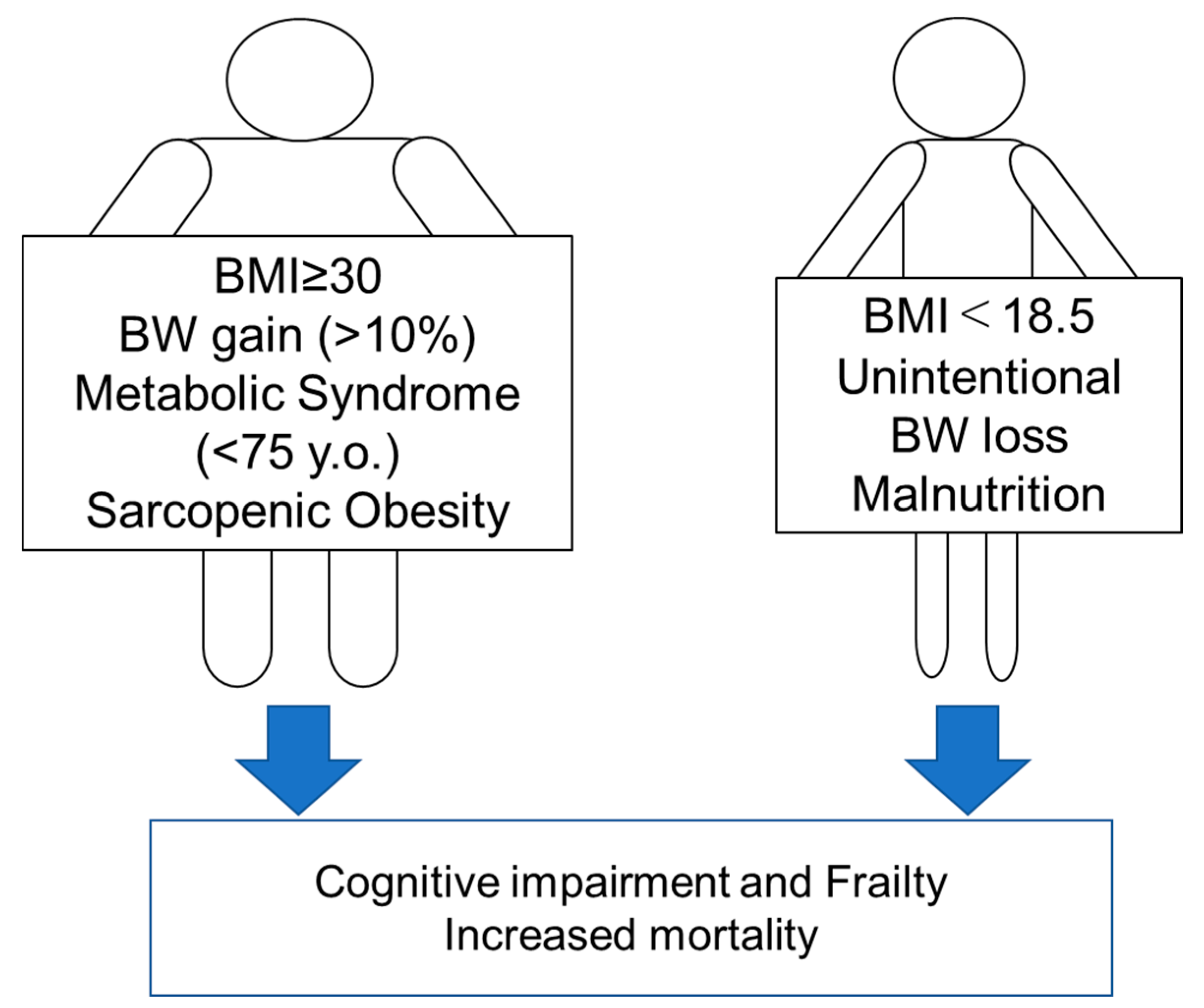
3.1. Obesity: Setting an Appropriate BMI Range
3.2. Metabolic Syndrome
3.3. Sarcopenic Obesity
3.4. Malnutrition
3.5. Changes in Body Weight
4. Appropriate Energy and Macro/Micronutrient Intake in Older Adults with Diabetes
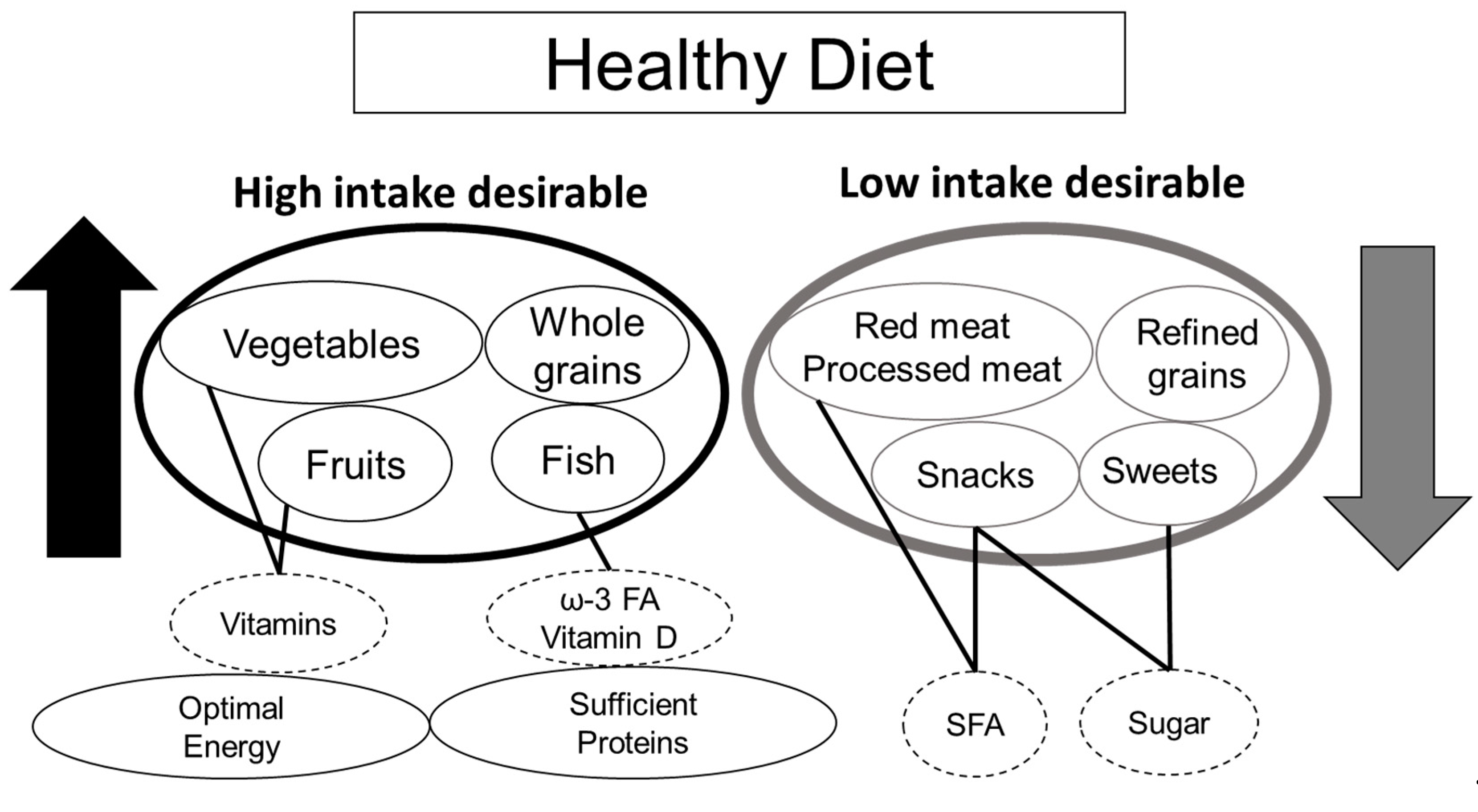
4.1. Energy Intake
4.2. Proteins
4.3. Vitamins
4.3.1. Vitamin D
4.3.2. Other Vitamins
4.4. Fatty Acids
4.5. Mediterranean Diet and Healthy Diet
5. Strategy Shift in Dietary Management of Older Adults with Diabetes
5.1. Age-Related Differences in Nutritional Intake on Health Outcomes
5.2. Strategy Shift in Dietary Management from Metabolic Syndrome/Obesity to Frailty
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pensions at a Glance 2019: OECD and G20 Indicators; OECD Publishing: Paris, France, 2019.
- Charvat, H.; Goto, A.; Goto, M.; Inoue, M.; Heianza, Y.; Arase, Y.; Sone, H.; Nakagami, T.; Song, X.; Qiao, Q.; et al. Impact of population aging on trends in diabetes prevalence: A meta-regression analysis of 160,000 Japanese adults. J. Diabetes Investig. 2015, 6, 533–542. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Golden, S.H.; Cefalu, W.T. Diabetes and aging: Unique considerations and goals of care. Diabetes Care 2017, 40, 440–443. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 12. Older adults: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43, S152–S162. [Google Scholar] [CrossRef] [Green Version]
- Committee Report: Glycemic targets for elderly patients with diabetes: Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes. J. Diabetes Investig. 2017, 8, 126–128. [CrossRef] [PubMed] [Green Version]
- Managing Older People with Type 2 Diabetes Global Guideline. International Diabetes Federation. Available online: https://www.idf.org/e-library/guidelines/78-global-guideline-for-managing-older-people-with-type-2-diabetes.html (accessed on 21 September 2020).
- Wong, E.; Backholer, K.; Gearon, E.; Harding, J.; Freak-Poli, R.; Stevenson, C.; Peeters, A. Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013, 1, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; McBurnie, M.A.; Newman, A.; Tracy, R.P.; Kop, W.J.; Hirsch, C.H.; Gottdiener, J.; Fried, L.P. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2002, 162, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.K.; Zheng, Z.; Xu, X.; Ma, C.; Chan, P. The prevalence and incidence of frailty in Pre-diabetic and diabetic community-dwelling older population: Results from Beijing longitudinal study of aging II (BLSA-II). BMC Geriatr. 2017, 17, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.C. Sarcopenia, frailty, and diabetes in older adults. Diabetes Metab. J. 2016, 40, 182–189. [Google Scholar] [CrossRef]
- Yanase, T.; Yanagita, I.; Muta, K.; Nawata, H. Frailty in elderly diabetes patients. Endocr. J. 2018, 65, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Xu, W.; Ou, Y.N.; Cao, X.P.; Tan, M.S.; Tan, L.; Yu, J.T. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019, 55, 100944. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Schneider, A.L.; Biessels, G.J.; Touradji, P.; Hill-Briggs, F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: A meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc. 2014, 20, 278–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, A.M.; Sharrett, A.R.; Schneider, A.L.; Coresh, J.; Albert, M.; Couper, D.; Griswold, M.; Gottesman, R.F.; Wagenknecht, L.E.; Windham, B.G.; et al. Diabetes in midlife and cognitive change over 20 years: A cohort study. Ann. Intern. Med. 2014, 161, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Whitmer, R.A. The epidemiology of adiposity and dementia. Curr. Alzheimer Res. 2007, 4, 117–122. [Google Scholar] [CrossRef]
- Dik, M.G.; Jonker, C.; Comijs, H.C.; Deeg, D.J.; Kok, A.; Yaffe, K.; Penninx, B.W. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care 2007, 30, 2655–2660. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, E.C.; Nation, D.A. Importance of treatment status in links between type 2 diabetes and alzheimer’s disease. Diabetes Care 2019, 42, 972–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebadi, S.A.; Darvish, P.; Fard, A.J.; Lima, B.S.; Ahangar, O.G. Hypoglycemia and cognitive function in diabetic patients. Diabetes Metab. Syndr. 2018, 12, 893–896. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Karter, A.J.; Yaffe, K.; Quesenberry, C.P., Jr.; Selby, J.V. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009, 301, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Abbatecola, A.M.; Rizzo, M.R.; Barbieri, M.; Grella, R.; Arciello, A.; Laieta, M.T.; Acampora, R.; Passariello, N.; Cacciapuoti, F.; Paolisso, G. Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. Neurology 2006, 67, 235–240. [Google Scholar] [CrossRef]
- Rawlings, A.M.; Sharrett, A.R.; Mosley, T.H.; Ballew, S.H.; Deal, J.A.; Selvin, E. Glucose peaks and the risk of dementia and 20-year cognitive decline. Diabetes Care 2017, 40, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Umegaki, H.; Iimuro, S.; Shinozaki, T.; Araki, A.; Sakurai, T.; Iijima, K.; Ohashi, Y.; Ito, H. Risk factors associated with cognitive decline in the elderly with type 2 diabetes: Pooled logistic analysis of a 6-year observation in the Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2012, 12 (Suppl. 1), 110–116. [Google Scholar] [CrossRef]
- Haroon, N.N.; Austin, P.C.; Shah, B.R.; Wu, J.; Gill, S.S.; Booth, G.L. Risk of dementia in seniors with newly diagnosed diabetes: A population-based study. Diabetes Care 2015, 38, 1868–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marseglia, A.; Wang, H.X.; Rizzuto, D.; Fratiglioni, L.; Xu, W. Participating in mental, social, and physical leisure activities and having a rich social network reduce the incidence of diabetes-related dementia in a cohort of swedish older adults. Diabetes Care 2019, 42, 232–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, G.E.; Park, Y.G.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Lee, M.K.; Kim, B.; Hong, O.K.; Kwon, H.S. BMI, weight change, and dementia risk in patients with new-onset type 2 diabetes: A nationwide cohort study. Diabetes Care 2019, 42, 1217–1224. [Google Scholar] [CrossRef]
- Araki, A.; Yoshimura, Y.; Sakurai, T.; Umegaki, H.; Kamada, C.; Iimuro, S.; Ohashi, Y.; Ito, H. Low intakes of carotene, vitamin B(2), pantothenate and calcium predict cognitive decline among elderly patients with diabetes mellitus: The Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2017, 17, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.; Fallah, N.; Rockwood, M.R.; Rockwood, K. Transitions in cognitive status in relation to frailty in older adults: A comparison of three frailty measures. J. Nutr. Health Aging 2011, 15, 863–867. [Google Scholar] [CrossRef]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Walters, K. Frailty as a predictor of alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 881–888. [Google Scholar] [CrossRef]
- Han, E.S.; Lee, Y.; Kim, J. Association of cognitive impairment with frailty in community-dwelling older adults. Int. Psychogeriatr. 2014, 26, 155–163. [Google Scholar] [CrossRef]
- Gale, C.R.; Ritchie, S.J.; Cooper, C.; Starr, J.M.; Deary, I.J. Cognitive ability in late life and onset of physical frailty: The Lothian Birth Cohort 1936. J. Am. Med. Dir. Assoc. 2017, 65, 1289–1295. [Google Scholar] [CrossRef]
- Thein, F.S.; Li, Y.; Nyunt, M.S.Z.; Gao, Q.; Wee, S.L.; Ng, T.P. Physical frailty and cognitive impairment is associated with diabetes and adversely impact functional status and mortality. Postgrad. Med. 2018, 130, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Kozaki, K.; Kuzuya, M.; Matsui, Y.; Satake, S. Chapter 2 Frailty concepts. Geriatr. Gerontol. Int. 2020, 20 (Suppl. 1), 14–19. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.E.; Walker, M. Genetics of insulin resistance and the metabolic syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Umegaki, H. Sarcopenia and diabetes: Hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. J. Diabetes Investig. 2015, 6, 623–624. [Google Scholar] [CrossRef]
- Neergaard, J.S.; Dragsbaek, K.; Christiansen, C.; Nielsen, H.B.; Brix, S.; Karsdal, M.A.; Henriksen, K. Metabolic syndrome, insulin resistance, and cognitive dysfunction: Does your metabolic profile affect your brain? Diabetes 2017, 66, 1957–1963. [Google Scholar] [CrossRef] [Green Version]
- Kong, S.H.; Park, Y.J.; Lee, J.Y.; Cho, N.H.; Moon, M.K. Insulin resistance is associated with cognitive decline among older koreans with normal baseline cognitive function: A prospective community-based cohort study. Sci. Rep. 2018, 8, 650. [Google Scholar] [CrossRef] [Green Version]
- Benedict, C.; Grillo, C.A. Insulin resistance as a therapeutic target in the treatment of Alzheimer’s disease: A state-of-the-art review. Front. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Dvoretskiy, S.; Lieblein-Boff, J.C.; Jonnalagadda, S.; Atherton, P.J.; Phillips, B.E.; Pereira, S.L. Exploring the association between vascular dysfunction and skeletal muscle mass, strength and function in healthy adults: A systematic review. Nutrients 2020, 12, 715. [Google Scholar] [CrossRef] [Green Version]
- Sfyri, P.; Matsakas, A. Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: Emphasis on apolipoprotein E deficiency and peripheral arterial disease. J. Biomed. Sci. 2017, 24, 42. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Araki, A. Diabetes mellitus and white matter hyperintensity. Geriatr. Gerontol. Int. 2015, 15 (Suppl. 1), 34–42. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Kimbara, Y.; Yamaoka, T.; Sato, K.; Tsuboi, Y.; Kodera, R.; Chiba, Y.; Mori, S.; Fujiwara, Y.; Tokumaru, A.M.; et al. White matter hyperintensity in elderly patients with diabetes mellitus is associated with cognitive impairment, functional disability, and a high glycoalbumin/glycohemoglobin ratio. Front. Aging Neurosci. 2017, 9, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjari Moghaddam, H.; Ghazi Sherbaf, F.; Aarabi, M.H. Brain microstructural abnormalities in type 2 diabetes mellitus: A systematic review of diffusion tensor imaging studies. Front. Neuroendocrinol. 2019, 55, 100782. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Shimoji, K.; Ishikawa, J.; Tachibana, A.; Kodera, R.; Oba, K.; Toyoshima, K.; Chiba, Y.; Tokumaru, A.M.; Araki, A. Associations between sarcopenia and white matter alterations in older adults with diabetes mellitus: A diffusion tensor imaging study. J. Diabetes Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of chronic inflammation in aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [Green Version]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin resistance and oxidative stress in the brain: What’s new? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praticò, D.; Clark, C.M.; Liun, F.; Rokach, J.; Lee, V.Y.; Trojanowski, J.Q. Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Arch. Neurol. 2002, 59, 972–976. [Google Scholar] [CrossRef] [Green Version]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [Green Version]
- Lejri, I.; Agapouda, A.; Grimm, A.; Eckert, A. Mitochondria-and oxidative stress-targeting substances in cognitive decline-related disorders: From molecular mechanisms to clinical evidence. Oxid. Med. Cell. Longev. 2019, 2019, 9695412. [Google Scholar] [CrossRef] [Green Version]
- Cox, D.J.; Kovatchev, B.P.; Gonder-Frederick, L.A.; Summers, K.H.; McCall, A.; Grimm, K.J.; Clarke, W.L. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care 2005, 28, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Kodl, C.T.; Seaquist, E.R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008, 29, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.H.; Rodríguez-Mañas, L.; Morley, J.E.; Sinclair, A.J. Hypoglycemia in older people—A less well recognized risk factor for frailty. Aging Dis. 2015, 6, 156–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, J. Management of hypoglycemia in older adults with type 2 diabetes. Postgrad. Med. 2019, 131, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, S.W.; Aoyama, K.; Chen, Y.; Garnier, P.; Matsumori, Y.; Gum, E.; Liu, J.; Swanson, R.A. Hypoglycemic neuronal death and cognitive impairment are prevented by poly (ADP-ribose) polymerase inhibitors administered after hypoglycemia. J. Neurosci. 2003, 23, 10681–10690. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, S. Neurologic damage in hypoglycemia. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 126, pp. 513–532. [Google Scholar]
- Ogama, N.; Sakurai, T.; Kawashima, S.; Tanikawa, T.; Tokuda, H.; Satake, S.; Miura, H.; Shimizu, A.; Kokubo, M.; Niida, S.; et al. Association of glucose fluctuations with sarcopenia in older adults with type 2 diabetes mellitus. J. Clin. Med. 2019, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019, 4, e124952. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K. The diseasome of physical inactivity—And the role of myokines in muscl—Fat cross talk. J. Physiol. 2009, 587, 5559–5568. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Miyazawa, I.; Kadota, A.; Miura, K.; Okamoto, M.; Nakamura, T.; Ikai, T.; Maegawa, H.; Ohnishi, A. Twelve-year trends of increasing overweight and obesity in patients with diabetes: The Shiga Diabetes Clinical Survey. Endocr. J. 2018, 65, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H.; Guh, D.P. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shu, X.O.; Gao, Y.T.; Yang, G.; Matthews, C.E.; Li, Q.; Li, H.; Jin, F.; Zheng, W. Anthropometric predictors of coronary heart disease in Chinese women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 734–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Bes-Rastrollo, M.; Bulló, M.; Corella, D.; Fito, M.; Ros, E.; Lamuela-Raventós, R.M.; Rekondo, J.; et al. Obesity indexes and total mortality among elderly subjects at high cardiovascular risk: The PREDIMED study. PLoS ONE 2014, 9, e103246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araki, A.; Iimuro, S.; Sakurai, T.; Umegaki, H.; Iijima, K.; Nakano, H.; Oba, K.; Yokono, K.; Sone, H.; Yamada, N.; et al. Long-term multiple risk factor interventions in Japanese elderly diabetic patients: The Japanese Elderly Diabetes Intervention Trial—study design, baseline characteristics and effects of intervention. Geriatr. Gerontol. Int. 2012, 12 (Suppl. 1), 7–17. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Tanaka, S.; Iimuro, S.; Akanuma, Y.; Ohashi, Y.; Yamada, N.; Araki, A.; Ito, H.; Sone, H.; Japan Diabetes Complications Study Group and the Japanese Elderly Diabetes Intervention Trial Group. Body mass index and mortality among Japanese patients with type 2 diabetes: Pooled analysis of the Japan diabetes complications study and the Japanese elderly diabetes intervention trial. J. Clin. Endocrinol. Metab. 2014, 99, 2692–2696. [Google Scholar] [CrossRef] [Green Version]
- Uretsky, S.; Messerli, F.H.; Bangalore, S.; Champion, A.; Cooper-Dehoff, R.M.; Zhou, Q.; Pepine, C.J. Obesity paradox in patients with hypertension and coronary artery disease. Am. J. Med. 2007, 120, 863–870. [Google Scholar] [CrossRef]
- Schaap, L.A.; Koster, A.; Visser, M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol. Rev. 2013, 35, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, D.; Yoshida, T.; Watanabe, Y.; Yamada, Y.; Kimura, M.; Kyoto-Kameoka Study Group. A U-Shaped relationship between the prevalence of frailty and body mass index in community-dwelling japanese older adults: The Kyoto-Kameoka Study. J. Clin. Med. 2020, 9, 1367. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Graciani, A.; Guallar-Castillón, P.; López-García, E.; Rodríguez-Mañas, L.; Rodríguez-Artalejo, F. Diabetes and risk of frailty and its potential mechanisms: A prospective cohort study of older adults. J. Am. Med. Dir. Assoc. 2015, 16, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Gregg, E.W.; Lin, J.; Bardenheier, B.; Chen, H.; Rejeski, W.J.; Zhuo, X.; Hergenroeder, A.L.; Kritchevsky, S.B.; Peters, A.L.; Wagenknecht, L.E.; et al. Impact of intensive lifestyle intervention on disability-free life expectancy: The Look AHEAD Study. Diabetes Care 2018, 41, 1040–1048. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Oda, E. Metabolic syndrome: Its history, mechanisms, and limitations. Acta Diabetol. 2012, 49, 89–95. [Google Scholar] [CrossRef] [PubMed]
- National Nutrition and Health Survey 2018. The Japanese Ministry of Health, Labor and Welfare. 2020. Available online: https://www.mhlw.go.jp/stf/newpage_08789.html (accessed on 21 September 2020).
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillier, T.A.; Rizzo, J.H.; Pedula, K.L.; Cauley, J.A.; Schwartz, A.V.; Ensrud, K.E.; Browner, W.S. Increased mortality associated with the metabolic syndrome in older women with diabetes. Diabetes Care 2005, 28, 2258–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monami, M.; Lamanna, C.; Balzi, D.; Bartalucci, F.; Melani, C.; Masotti, G.; Marchionni, N.; Mannucci, E. Metabolic syndrome and cardiovascular mortality in older type 2 diabetic patients: A longitudinal study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 646–649. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Iimuro, S.; Araki, A.; Umegaki, H.; Ohashi, Y.; Yokono, K.; Ito, H. Age-associated increase in abdominal obesity and insulin resistance, and usefulness of AHA/NHLBI definition of metabolic syndrome for predicting cardiovascular disease in Japanese elderly with type 2 diabetes mellitus. Gerontology 2010, 56, 141–149. [Google Scholar] [CrossRef]
- Blaum, C.S.; West, N.A.; Haan, M.N. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Iimuro, S.; Sakamaki, K.; Umegaki, H.; Araki, A.; Ohashi, Y.; Ito, H.; Japanese Elderly Diabetes Intervention Trial Study Group. Risk factors for a 6-year decline in physical disability and functional limitations among elderly people with type 2 diabetes in the Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2012, 12 (Suppl. 1), 117–126. [Google Scholar] [CrossRef]
- Laudisio, A.; Bandinelli, S.; Gemma, A.; Ferrucci, L.; Incalzi, R.A. Metabolic syndrome and functional ability in older age: The InCHIANTI study. Clin. Nutr. 2014, 33, 626–633. [Google Scholar] [CrossRef]
- Yaffe, K.; Haan, M.; Blackwell, T.; Cherkasova, E.; Whitmer, R.A.; West, N. Metabolic syndrome and cognitive decline in elderly Latinos: Findings from the Sacramento Area Latino Study of Aging study. J. Am. Geriatr. Soc. 2007, 55, 758–762. [Google Scholar] [CrossRef]
- Komulainen, P.; Lakka, T.A.; Kivipelto, M.; Hassinen, M.; Helkala, E.L.; Haapala, I.; Nissinen, A.; Rauramaa, R. Metabolic syndrome and cognitive function: A population-based follow-up study in elderly women. Dement. Geriatr. Cogn. Disord. 2007, 23, 29–34. [Google Scholar] [CrossRef]
- Yogi-Morren, D.; Galioto, R.; Strandjord, S.E.; Kennedy, L.; Manroa, P.; Kirwan, J.P.; Kashyap, S.; Gunstad, J. Duration of type 2 diabetes and very low density lipoprotein levels are associated with cognitive dysfunction in metabolic syndrome. Cardiovasc. Psychiatry Neurol. 2014, 2014, 656341. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Choi, K.M. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirani, V.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Cumming, R.G. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing 2017, 46, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.; Seibel, M.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Hirani, V. Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: The Concord Health and Ageing in Men Project. J. Bone Miner. Res. 2017, 32, 575–583. [Google Scholar] [CrossRef]
- Scott, D.; Chandrasekara, S.D.; Laslett, L.L.; Cicuttini, F.; Ebeling, P.R.; Jones, G. Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5–10 years in community-dwelling older adults. Calcif. Tissue Int. 2016, 99, 30–42. [Google Scholar] [CrossRef]
- Low, S.; Goh, K.S.; Ng, T.P.; Ang, S.F.; Moh, A.; Wang, J.; Ang, K.; Subramaniam, T.; Sum, C.F.; Lim, S.C. The prevalence of sarcopenic obesity and its association with cognitive performance in type 2 diabetes in Singapore. Clin. Nutr. 2020, 39, 2274–2281. [Google Scholar] [CrossRef]
- Liu, G.X.; Chen, Y.; Yang, Y.X.; Yang, K.; Liang, J.; Wang, S.; Gan, H.T. Pilot study of the Mini Nutritional Assessment on predicting outcomes in older adults with type 2 diabetes. Geriatr. Gerontol. Int. 2017, 17, 2485–2492. [Google Scholar] [CrossRef]
- Sanz, París, A.; García, J.M.; Gómez-Candela, C.; Burgos, R.; Martín, Á.; Matía, P. Malnutrition prevalence in hospitalized elderly diabetic patients. Nutr. Hosp. 2013, 28, 592–599. [Google Scholar]
- Turnbull, P.J.; Sinclair, A.J. Evaluation of nutritional status and its relationship with functional status in older citizens with diabetes mellitus using the mini nutritional assessment (MNA) tool—a preliminary investigation. J. Nutr. Health Aging 2002, 6, 185–189. [Google Scholar]
- Alfonso-Rosa, R.M.; Del Pozo-Cruz, B.; Del Pozo-Cruz, J.; Del Pozo-Cruz, J.T.; Sañudo, B. The relationship between nutritional status, functional capacity, and health-related quality of life in older adults with type 2 diabetes: A pilot explanatory study. J. Nutr. Health Aging 2013, 17, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malara, A.; Sgrò, G.; Caruso, C.; Ceravolo, F.; Curinga, G.; Renda, G.F.; Spadea, F.; Garo, M.; Rispoli, V. Relationship between cognitive impairment and nutritional assessment on functional status in Calabrian long-term-care. Clin. Intervs. Aging 2014, 9, 105–110. [Google Scholar]
- Verlaan, S.; Ligthart-Melis, G.C.; Wijers, S.L.J.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. High prevalence of physical frailty among community-dwelling malnourished older adults—A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2017, 18, 374–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Dympna, G.; Heymsfield, S.; Wang, Z.M. 12 Skeletal Muscle Markers. The Role of Protein and Amino Acids in Sustaining and Enhancing Performance. In Committee on Military Nutrition Research: Activity Report; Institute of Medicine Committee on Military Nutrition Research; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.K.; Woodward, M.; Wang, D.; Ohkuma, T.; Warren, B.; Sharrett, A.R.; Williams, B.; Marre, M.; Hamet, P.; Harrap, S.; et al. The risks of cardiovascular disease and mortality following weight change in adults with diabetes: Results from ADVANCE. J. Clin. Endocrinol. Metab. 2020, 105, 152–162. [Google Scholar] [CrossRef]
- Houston, D.K.; Neiberg, R.H.; Miller, M.E.; Hill, J.O.; Jakicic, J.M.; Johnson, K.C.; Gregg, E.W.; Hubbard, V.S.; Pi-Sunyer, X.; Rejeski, W.J.; et al. Physical function following a long-term lifestyle intervention among middle aged and older adults with type 2 diabetes: The Look AHEAD Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1552–1559. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Kamada, C.; Takahashi, K.; Kaimoto, T.; Iimuro, S.; Ohashi, Y.; Araki, A.; Umegaki, H.; Sakurai, T.; Ito, H.; et al. Relations of nutritional intake to age, sex and body mass index in Japanese elderly patients with type 2 diabetes: The Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2012, 12 (Suppl. 1), 29–40. [Google Scholar] [CrossRef]
- Omura, T.; Tamura, Y.; Yamaoka, T.; Yoshimura, Y.; Sakurai, T.; Umegaki, H.; Kamada, C.; Iimuro, S.; Ohashi, Y.; Ito, H.; et al. Assessing the association between optimal energy intake and all-cause mortality in older patients with diabetes mellitus using the Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2020, 20, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yoshida, D.; Honda, T.; Hata, J.; Shibata, M.; Hirakawa, Y.; Furuta, Y.; Kishimoto, H.; Ohara, T.; Kitazono, T.; et al. Prevalence and mortality of sarcopenia in a community-dwelling older Japanese population: The Hisayama Study. J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, J.; Kim, Y.; Won, C.W.; Kim, Y.J. Calorie intake and cognitive function in the elderly: Data from the Korean Frailty and Aging Cohort Study (KFACS). J. Nutr. Health Aging 2019, 23, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Schoufour, J.D.; Franco, O.H.; Kiefte-de Jong, J.C.; Trajanoska, K.; Stricker, B.; Brusselle, G.; Rivadeneira, F.; Lahousse, L.; Voortman, T. The association between dietary protein intake, energy intake and physical frailty: Results from the Rotterdam Study. Br. J. Nutr. 2019, 121, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, D.; Yoshida, T.; Nanri, H.; Watanabe, Y.; Date, H.; Itoi, A.; Goto, C.; Ishikawa-Takata, K.; Sagayama, H.; Ebine, N.; et al. Association between the prevalence of frailty and doubly labeled water-calibrated energy intake among community-dwelling older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Isanejad, M.; Sirola, J.; Rikkonen, T.; Mursu, J.; Kröger, H.; Qazi, S.L.; Tuppurainen, M.; Erkkilä, A.T. Higher protein intake is associated with a lower likelihood of frailty among older women, Kuopio OSTPRE-Fracture Prevention Study. Eur. J. Nutr. 2020, 59, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, R.; Tange, C.; Tomida, M.; Nishita, Y.; Kato, Y.; Yuki, A.; Ando, F.; Shimokata, H.; Arai, H. Dietary factors associated with the development of physical frailty in community-dwelling older adults. J. Nutr. Health Aging 2019, 23, 89–95. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Milano-Teixeira, L.; Rodrigues, B.; Bacurau, R.; Marzetti, E.; Uchida, M. Relative protein intake and physical function in older adults: A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 1330. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; Franssen, R.; Dullemeijer, C.; van Dronkelaar, C.; Kyung Kim, H.; Ispoglou, T.; Zhu, K.; Prince, R.L.; van Loon, L.J.C.; de Groot, L.C. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: Individual participant data and meta-analysis of RCT’s. J. Nutr. Health Aging 2017, 21, 994–1001. [Google Scholar] [CrossRef]
- Hanach, N.I.; McCullough, F.; Avery, A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: A systematic review and meta-analysis. Adv. Nutr. 2019, 10, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahi, B.; Morais, J.A.; Gaudreau, P.; Payette, H.; Shatenstein, B. Energy and protein intakes and their association with a decline in functional capacity among diabetic older adults from the NuAge cohort. Eur. J. Nutr. 2016, 55, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Katagiri, A.; Ishibashi, S.; Abe, R.; Saito, Y.; Murase, T.; Yamashita, H.; Yajima, Y.; Ito, H.; Ohashi, Y.; et al. Effects of lifestyle modifications on patients with type 2 diabetes:the Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three year-interim report. Horm. Metab. Res. 2002, 34, 509–515. [Google Scholar] [CrossRef]
- Yamaoka, T.; Araki, A.; Tamura, Y.; Tanaka, S.; Fujihara, K.; Horikawa, C.; Aida, R.; Kamada, C.; Yoshimura, Y.; Moriya, T.; et al. Association between low protein intake and mortality in patients with type 2 diabetes. Nutrients 2020, 12, 1629. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, W.; Zhang, D. Association between dietary protein intake and cognitive function in adults aged 60 years and older. J. Nutr. Health Aging 2020, 24, 223–229. [Google Scholar] [CrossRef]
- Roberts, R.O.; Roberts, L.A.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; O’Connor, H.M.; Knopman, D.S.; Petersen, R.C. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J. Alzheimers Dis. 2012, 32, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauche, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Fernandes de Abreu, D.A.; Eyles, D.; Féron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S265–S277. [Google Scholar] [CrossRef]
- Patel, P.; Shah, J. Role of Vitamin D in Amyloid clearance via LRP-1 upregulation in Alzheimer’s disease: A potential therapeutic target? J. Chem. Neuroanat. 2017, 85, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Blizzard, L.; Fell, J.; Ding, C.; Winzenberg, T.; Jones, G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin. Endocrinol. (Oxf.) 2010, 73, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Lee, J.Y.; Kim, D.H. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: A systematic review and dose-response meta-analysis. BMC Geriatr. 2018, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Bolzetta, F.; Stubbs, B.; Noale, M.; Vaona, A.; Demurtas, J.; Celotto, S.; Cester, A.; Maggi, S.; Koyanagi, A.; Cereda, E.; et al. Low-dose vitamin D supplementation and incident frailty in older people: An eight year longitudinal study. Exp. Gerontol. 2018, 101, 1–6. [Google Scholar] [CrossRef]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2018, 14, 4. [Google Scholar] [CrossRef]
- Cangussu, L.M.; Nahas-Neto, J.; Orsatti, C.L.; Bueloni-Dias, F.N.; Nahas, E.A. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: A randomized, double-blind, placebo-controlled clinical trial. Osteoporos. Int. 2015, 26, 2413–2421. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Goodwill, A.M.; Szoeke, C.A. Systematic review and meta-analysis of the effect of low vitamin D on cognition. J. Am. Geriatr. Soc. 2017, 65, 2161–2168. [Google Scholar] [CrossRef]
- Byrn, M.A.; Adams, W.; Penckofer, S.; Emanuele, M.A. Vitamin D supplementation and cognition in people with type 2 diabetes: A randomized control trial. J. Diabetes Res. 2019, 2019, 5696391. [Google Scholar] [CrossRef] [PubMed]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, E.; Canada, T. Dietary Folate and Vitamin B12 Intake and cognitive decline among community-dwelling older persons. Nutr. Clin. Pract. 2006, 21, 188–189. [Google Scholar] [CrossRef]
- Nelson, C.; Wengreen, H.J.; Munger, R.G.; Corcoran, C.D. Dietary folate and vitamins B-12 and B-6 not associated with incident Alzheimer’s disease. J. Alzheimer Dis. 2006, 9, 435–443. [Google Scholar]
- Wengreen, H.J.; Munger, R.G.; Corcoran, C.D.; Zandi, P.; Hayden, K.M.; Fotuhi, M.; Skoog, I.; Norton, M.C.; Tschanz, J.; Breitner, J.C.; et al. Antioxidant intake and cognitive function of elderly men and women: The Cache County Study. J. Nutr. Health Aging 2007, 11, 230–237. [Google Scholar] [PubMed]
- Devore, E.E.; Kang, J.H.; Stampfer, M.J.; Grodstein, F. The association of antioxidants and cognition in the Nurses’ Health Study. Am. J. Epidemiol. 2013, 177, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Forbes, S.C.; Holroyd-Leduc, J.M.; Poulin, M.J.; Hogan, D.B. Effect of nutrients, dietary supplements and vitamins on cognition: A Systematic review and meta-analysis of randomized controlled trials. Can. Geriatr. J. 2015, 18, 231–245. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.W.; Kim, H.S.; Han, J.H.; Bae, J.B.; Oh, D.J.; Han, J.W.; Kim, K.W. Efficacy of vitamins on cognitive function of non-demented people: A systematic review and meta-analysis. Nutrients 2020, 12, 1168. [Google Scholar] [CrossRef] [Green Version]
- Kwok, T.; Lee, J.; Ma, R.C.; Wong, S.Y.; Kung, K.; Lam, A.; Ho, C.S.; Lee, V.; Harrison, J.; Lam, L. A randomized placebo controlled trial of vitamin B12 supplementation to prevent cognitive decline in older diabetic people with borderline low serum vitamin B12. Clin. Nutr. 2017, 36, 1509–1515. [Google Scholar] [CrossRef]
- Dass, A.S.; Narayana, S.; Venkatarathnamma, P.N. Effect of Vitamin E and omega 3 fatty acids in type 2 diabetes mellitus patients. J. Adv. Pharm. Technol. Res. 2018, 9, 32–36. [Google Scholar] [PubMed]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bays, H.E.; Ballantyne, C.M.; Braeckman, R.A.; Stirtan, W.G.; Soni, P.N. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: Effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am. J. Cardiovasc. Drugs 2013, 13, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: Meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018, 3, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Godin, J.; Cahill, L.; Rockwood, K. Association of fatty acid consumption with frailty and mortality among middle-aged and older adults. Nutrition 2020, 70, 110610. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Hutchins-Wiese, H.L.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.J.; Durham, H.A.; Kenny, A.M. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. Nutr. Health Aging 2013, 17, 76–80. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of KAMOGAWA-DM cohort study. J. Clin. Biochem. Nutr. 2020, 66, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.W.; Hou, W.S.; Li, M.; Tang, Z.Y. Omega-3 fatty acids and risk of cognitive decline in the elderly: A meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2016, 28, 165–166. [Google Scholar] [CrossRef]
- Brainard, J.S.; Jimoh, O.F.; Deane, K.H.O.; Biswas, P.; Donaldson, D.; Maas, K.; Abdelhamid, A.S.; Hooper, L.; PUFAH Group. Omega-3, omega-6, and polyunsaturated fat for cognition: Systematic review and meta-analysis of randomized trials. J. Am. Med. Dir. Assoc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Stampfer, M.J.; Breteler, M.M.; Rosner, B.; Kang, J.H.; Okereke, O.; Hu, F.B.; Grodstein, F. Dietary fat intake and cognitive decline in women with type 2 diabetes. Diabetes Care 2009, 32, 635–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cukierman-Yaffe, T.; Bosch, J.; Diaz, R.; Dyal, L.; Hancu, N.; Hildebrandt, P.; Lanas, F.; Lewis, B.S.; Marre, M.; Yale, J.F.; et al. Effects of basal insulin glargine and omega-3 fatty acid on cognitive decline and probable cognitive impairment in people with dysglycaemia: A substudy of the ORIGIN trial. Lancet Diabetes Endocrinol. 2014, 2, 562–572. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; Gialluisi, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Mediterranean diet and mortality in the elderly: A prospective cohort study and a meta-analysis. Br. J. Nutr. 2018, 120, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Pizato, N.; da Mata, F.; Figueiredo, A.; Ito, M.; Pereira, M.G. Mediterranean diet and musculoskeletal-functional outcomes in community-dwelling older people: A systematic review and meta-analysis. J. Nutr. Health Aging 2018, 22, 655–663. [Google Scholar] [CrossRef]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean diet reduces incident frailty risk: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2018, 66, 783–788. [Google Scholar] [CrossRef]
- Tepper, S.; Sivashensky, A.A.; Shahar, D.R.; Geva, D.; Cukierman-Yaffe, T. The association between Mediterranean diet and the risk of falls and physical function indices in older type 2 diabetic people varies by age. Nutrients 2018, 10, 767. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Garcia, E.; Hagan, K.A.; Fung, T.T.; Hu, F.B.; Rodríguez-Artalejo, F. Mediterranean diet and risk of frailty syndrome among women with type 2 diabetes. Am. J. Clin. Nutr. 2018, 107, 763–771. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci. Rep. 2017, 7, 41317. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [Green Version]
- Mattei, J.; Bigornia, S.J.; Sotos-Prieto, M.; Scott, T.; Gao, X.; Tucker, K.L. The Mediterranean diet and 2-year change in cognitive function by status of type 2 diabetes and glycemic control. Diabetes Care 2019, 42, 1372–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fard, N.R.P.; Amirabdollahian, F.; Haghighatdoost, F. Dietary patterns and frailty: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Risk Reduction of Cognitive Decline and Dementia WHO Guidelines. WHO. 2019. Available online: https://www.who.int/mental_health/neurology/dementia/guidelines_risk_reduction/en/ (accessed on 21 September 2020).
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Iimuro, S.; Yoshimura, Y.; Umegaki, H.; Sakurai, T.; Araki, A.; Ohashi, Y.; Iijima, K.; Ito, H.; Japanese Elderly Diabetes Intervention Trial Study Group. Dietary pattern and mortality in Japanese elderly patients with type 2 diabetes mellitus: Does a vegetable- and fish-rich diet improve mortality? An explanatory study. Geriatr. Gerontol. Int. 2012, 12 (Suppl. 1), 59–67. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Mañas, L.; Rodríguez-Sánchez, B.; Carnicero, J.A.; Rueda, R.; García-Garcia, F.J.; Pereira, S.L.; Sulo, S. Impact of nutritional status according to GLIM criteria on the risk of incident frailty and mortality in community-dwelling older adults. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Satake, S.; Senda, K.; Hong, Y.J.; Miura, H.; Endo, H.; Sakurai, T.; Kondo, I.; Toba, K. Validity of the Kihon Checklist for assessing frailty status. Geriatr. Gerontol. Int. 2016, 16, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, K.; Araki, A.; Tamura, Y.; Iritani, O.; Ogawa, S.; Kozaki, K.; Ebihara, S.; Hanyu, H.; Arai, H.; Kuzuya, M.; et al. Development of the Dementia Assessment Sheet for Community-based Integrated Care System 8-items, a short version of the Dementia Assessment Sheet for Community-based Integrated Care System 21-items, for the assessment of cognitive and daily functions. Geriatr. Gerontol. Int. 2018, 18, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, K.; Araki, A.; Tamura, Y.; Ishikawa, J.; Kodera, R.; Oba, K.; Chiba, Y.; Awata, S. Use of Dementia Assessment Sheet for Community-based Integrated Care System 8-items (DASC-8) for the screening of frailty and components of comprehensive geriatric assessment. Geriatr. Gerontol. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Machida, S.; Matsumoto, N.; Shibagaki, Y.; Sakurada, T. Age modifies the association of dietary protein intake with all-cause mortality in patients with chronic kidney disease. Nutrients 2018, 10, 1744. [Google Scholar] [CrossRef] [Green Version]
- Otto, M.C.; Padhye, N.S.; Bertoni, A.G.; Jacobs, D.R., Jr.; Mozaffarian, D. Everything in moderatio—dietary diversity and quality, central obesity and risk of diabetes. PLoS ONE 2015, 10, e0141341. [Google Scholar]
- de Oliveira Otto, M.C.; Anderson, C.A.M.; Dearborn, J.L.; Ferranti, E.P.; Mozaffarian, D.; Rao, G.; Wylie-Rosett, J.; Lichtenstein, A.H.; American Heart Association Behavioral Change for Improving Health Factors Committee of the Council on Lifestyle and Cardiometabolic Health and Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; et al. Dietary diversity: Implications for obesity prevention in adult populations: A science advisory from the American Heart Association. Circulation 2018, 38, e160–e168. [Google Scholar] [CrossRef]
- Osuka, Y.; Kojima, N.; Yoshida, Y.; Kim, M.; Won, C.W.; Suzuki, T.; Kim, H. Exercise and/or dietary varieties and incidence of frailty in community-dwelling older women: A 2-year cohort study. J. Nutr. Health Aging 2019, 23, 425–430. [Google Scholar] [CrossRef]
- Sato, E.; Suzukamo, Y.; Miyashita, M.; Kazuma, K. Development of a diabetes diet-related quality-of-life scale. Diabetes Care 2004, 27, 1271–1275. [Google Scholar] [CrossRef] [Green Version]
- Pilemann-Lyberg, S.; Thorsteinsson, B.; Snorgaard, O.; Zander, M.; Vestergaard, H.; Røder, M.E. Severe hypoglycaemia during treatment with sulphonylureas in patients with type 2 diabetes in the Capital Region of Denmark. Diabetes Res. Clin. Pract. 2015, 110, 202–207. [Google Scholar] [CrossRef]
- Geller, A.I.; Shehab, N.; Lovegrove, M.C.; Kegler, S.R.; Weidenbach, K.N.; Ryan, G.J.; Budnitz, D.S. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern. Med. 2014, 174, 678–686. [Google Scholar] [CrossRef]
- Namba, M.; Iwakura, T.; Nishimura, R.; Akazawa, K.; Matsuhisa, M.; Atsumi, Y.; Satoh, J.; Yamauchi, T.; Japan Diabetes Society (JDS) Committee for Surveys on Severe Hypoglycemia. The current status of treatment-related severe hypoglycemia in Japanese patients with diabetes mellitus: A report from the committee on a survey of severe hypoglycemia in the Japan Diabetes Society. J. Diabetes Investig. 2018, 9, 642–656. [Google Scholar] [CrossRef] [Green Version]
- Schloot, N.C.; Haupt, A.; Schütt, M.; Badenhoop, K.; Laimer, M.; Nicolay, C.; Reaney, M.; Fink, K.; Holl, R.W. Risk of severe hypoglycemia in sulfonylurea-treated patients from diabetes centers in Germany/Austria: How big is the problem? Which patients are at risk? Diabetes Metab. Res. Rev. 2016, 32, 316–324. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 12. Older Adults: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. 1), S139–S147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, E.M.; Mander, A.G.; Ames, D.; Kotowicz, M.A.; Carne, R.P.; Brodaty, H.; Woodward, M.; Boundy, K.; Ellis, K.A.; Bush, A.I.; et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 2013, 36, 2981–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorcely, B.; Nitis, J.; Schwartzbard, A.; Newman, J.; Goldberg, I.; Sum, M.A. Case Report: Euglycemic diabetic ketoacidosis presenting as chest pain in a patient on a low carbohydrate diet. Curr. Diabetes Rev. 2020. [CrossRef] [PubMed]
- Yabe, D.; Iwasaki, M.; Kuwata, H.; Haraguchi, T.; Hamamoto, Y.; Kurose, T.; Sumita, K.; Yamazato, H.; Kanada, S.; Seino, Y. Sodium-glucose co-transporter-2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: A randomized, open-label, 3-arm parallel comparative, exploratory study. Diabetes Obes. Metab. 2017, 19, 739–743. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients 2020, 12, 3367. https://doi.org/10.3390/nu12113367
Tamura Y, Omura T, Toyoshima K, Araki A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients. 2020; 12(11):3367. https://doi.org/10.3390/nu12113367
Chicago/Turabian StyleTamura, Yoshiaki, Takuya Omura, Kenji Toyoshima, and Atsushi Araki. 2020. "Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty" Nutrients 12, no. 11: 3367. https://doi.org/10.3390/nu12113367
APA StyleTamura, Y., Omura, T., Toyoshima, K., & Araki, A. (2020). Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients, 12(11), 3367. https://doi.org/10.3390/nu12113367




