1. Introduction
Eating disorders (EDs) are psychiatric illnesses involving alterations in eating habits and their complications focus on nutritional and body composition disorders [
1]. These EDs range from non-clinical eating disorders such as body image disorders [
2] to severe clinical forms such as bulimia and anorexia nervosa [
3]. EDs are most common in those aged 25 or younger and occur primarily among women and specifically in risk groups such as female dancers, who exhibit high levels of sophistication and are placed in competitive environments, such as dance groups, where they may be at increased risk of EDs [
4]. EDs such as anorexia nervosa and bulimia produce well-known effects on body composition, including a decrease in fat (FM), fat-free mass (FFM), total body water (TBW) [
1], and finally a decrease in bone mineral density [
5,
6].
Studies assessing nutritional status commonly use the following instruments: a clinical interrogation physical examination, anthropometry (skinfolds, BMI, arm circumference, FM, skeletal muscle mass (SMM), etc.) and biochemical analysis (albumin, hemoglobin, transferrin) [
7]. Bioelectrical impedance analysis (BIA) is a common method to assess body composition based on the relationship between total body impedance and total body water. BIA is an inexpensive method used to estimate body composition and nutritional status in both healthy and ill individuals [
8]. The data estimated with BIA through equations are FFM and, by derivation, FM, TBW, and extracellular water (ECW) [
9], but relationships between the biophysical parameters of BIA such as reactance (Xc) and phase angle (PhA) are currently being established in different diseases such as cancer, malnutrition mortality and physical activity [
8,
10,
11].
The use of bioelectric BIA data has gained importance in nutritional studies, and PhA is a direct measure of BIA that is not influenced by closed equations that may affect body composition compartments [
12]. PhA is estimated from resistance (R) and Xc as the arc-tangent (Xc/R × 180°/π) [
12]. These relationships support the hypothesis that PhA is a measure of cell mass, nutritional risk and health [
6]. PhA is a variable of interest in BIA because it is not dependent of height and weight. In addition, these measurements are related to cell membrane function and are an indicator of tissular hydration and nutritional condition [
13]. PhA reflects the electrical property of the tissues, whereby low values are associated with reduced cell unity, and higher values are associated with active cell mass, indicating an adequate state of health [
14,
15]. PhA variability is related to cell composition and function as well as the redistribution of fluids and their changes through the interstitial spaces [
6]. With regard to studies on changes in PhA in diseases such as anorexia nervosa, a decrease in these values has been detected when compared to control subjects [
16].
In summary, the aim of this paper consists of establishing the associations between body composition variables with disordered eating attitudes (DEA) and their predictive capacity (anthropometric and BIA estimated body composition variables) using receiver operating characteristic (ROC) analysis curves to assess DEA in a group of ballet dance students.
4. Discussion
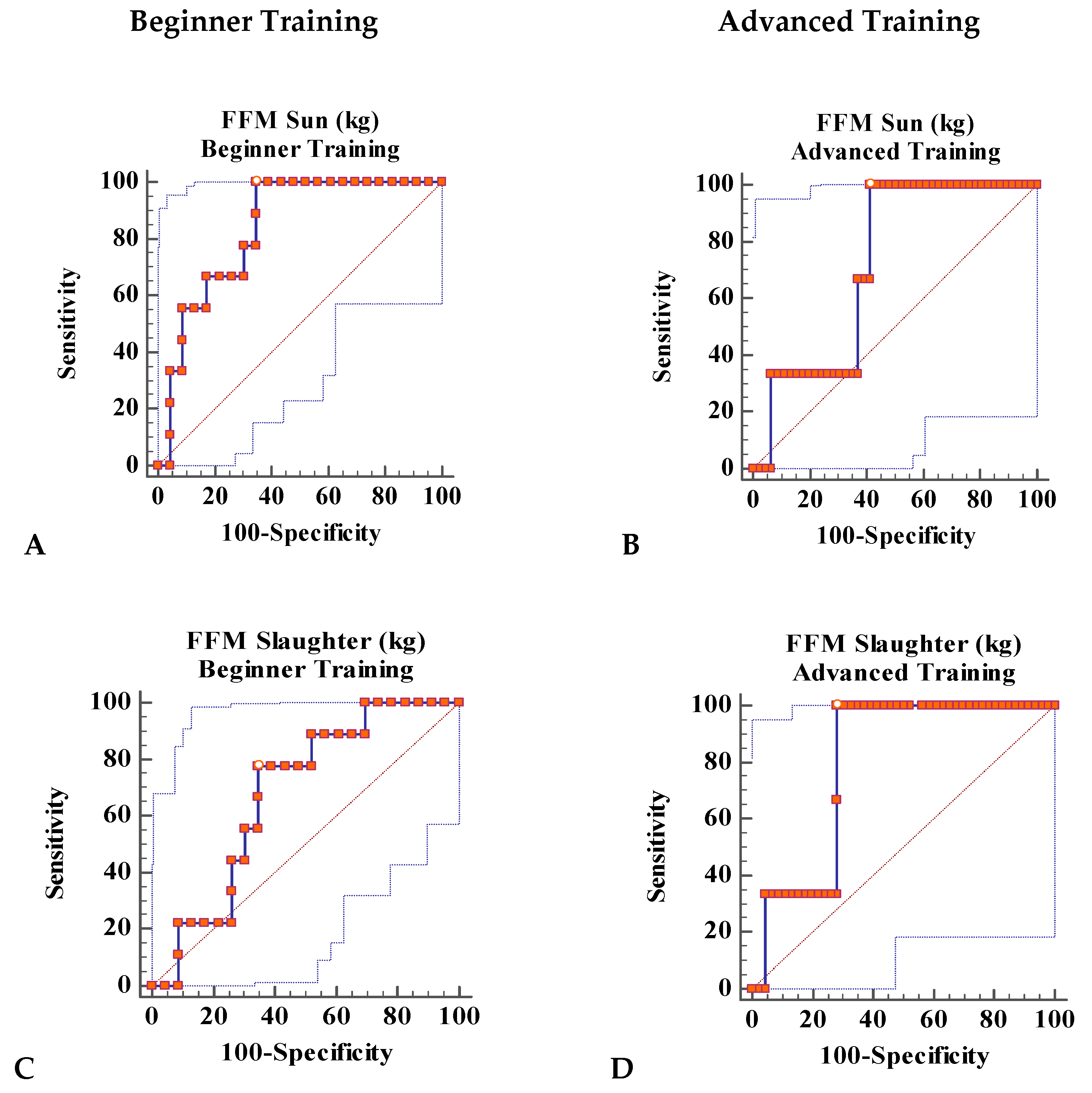
The objective of this study was the analysis of sensitivity and specificity to discriminate DEA from body composition estimates obtained by anthropometry (FM Slaughter and derived FFM and Poortman’s SMM) as well as bioelectric variables (Z, R, Xc and PhA) and their estimates (FFM Sun and derived FM) in relation to the evaluation of their capacity in the identification of DEA.
The novel feature of this study is the relationship between the parameters obtained from bioelectrical impedance including Xc and PhA as excellent predictors of eating disorders, especially in the BT group in comparison to the AT group.
Raw variables (Z, R, Xc and PhA) are increasingly being used in the study of body composition [
6,
16,
28], nutrition [
29,
30,
31], physical activity and training [
11,
32,
33,
34,
35], and in the area of health and disease [
8,
14], etc., because they are not influenced by prediction equations, nor by BMI, FM or FFM as possible confounding variables [
13].
Body composition differences between the BT and AT groups can be attributed initially to age and to the implicit difference in the components of FM and especially in the various components of FFM [
6,
36]. The lower values of the parameter R in the AT group are consistent with higher values of TBW and consequently of intracellular water (ICW), which means that electricity flows more easily through the body and with a minor resistance. The higher values of Xc and PhA in the AT group may indicate lower ECW values and higher ICW values. Cellular integrity has been associated with Xc. In biological conductors, theoretically, higher values are expected in BIA measurements in healthy membranes with better integrity [
28]. In recent years PhA, a raw BIA variable, has been gaining attention because it is an index of the ratio between ECW and ICW, cellular integrity and body cell mass [
37].
The relationship between PhA and BMI has been studied by Koury et al. [
34] who observed a direct association (
r = 0.58). This direct correlation between PhA and BMI (rho = 0.41,
p < 0.05) was only found in the BT group, noting that in the AT group, PhA had an inverse association with body weight and BMI. This would be due to the gain and increase in tissue that occurs with the biological maturation of the youngest girls [
29,
38]. The PhA values in the study groups are 9.6° and 10.5° for the BT and AT groups, respectively, with this difference between groups being clinically important in the female gender [
39] and considering that age is the most important factor in the difference in PhA [
40]. The PhA increases from the earlier years of life through adolescence (18 years), with a mean value of 6.4° for girls 10–15 years of age [
39], which is lower than in our two study groups. In a comparative study by Marra et al. carried out in men, the highest mean PhA value was 7.9° in the dancers, compared to both the anorexic (5.8°) and thin subjects and the controls (6.8°), with these values being much lower than those in our study, even though their study subjects were men [
41]. Another reference study reported PhA values by sex and BMI ranging between 5° and 7° [
39,
42], with these values somewhat low in comparison to ours. These differences may be due to the country and the amount of physical exercise performed, as well as to the use of different measurement instruments [
36].
There are very few data found on the relationships between BIA variables such as PhA in relation to DEA. Only one study found a significant inverse relationship between PhA and a subscale concerning hypochondria [
42]. In our study, non-significant inverse correlations were found in the AT group in all the subscales of the EAT-26, while in the BT group the correlations were direct and significant. It has been proven that parameters such as PhA are sensitive after a period of refeeding in anorexic patients with protein-energy malnutrition, correlating with changes in body weight and BMI [
43]. In another study, data on Xc and PhA are presented only from the perspective of body composition and nutritional changes and their physiological interpretation in anorexic patients [
44].
FM showed different relationships with Xc and PhA between groups, with inverse relationships in the AT group. This finding is in line with the results of Baumgartner [
6]. The associations between PhA and FM are due to and may reflect changes in TBW between FM and FFM associated with an increase in adiposity. The percentage of TBW decreases in FFM and in FM the ECW increases with increasing adiposity [
6].
The relation between PhA and resistance is proportionally inverse, which depends on ICW and ECW. Physically working out, especially causing a muscle mass increase, may lead to an intensification in ICW, which again reduces resistance and consequently leads to a rise in PhA. Xc is directly proportional to PhA and depends on the wholeness of the cell membrane. Well-performed workouts can be a factor in improving cell membrane wholeness through the overcompensation mechanism described earlier. Another component that increases Xc is total cell mass. Workouts can lead to a rise in total cell mass that leads to an intensification in reactance and a consequent rise in PhA. Measurement of the PhA can consequently be an indicator of the effects of training on cellular health and thus on the healthiness of the person. Other authors argue that PhA might be used in clinical practice as a guide to the level of physical activity of the person [
45,
46].
PhA is one of the direct magnitudes obtained by BIA that does not require body weight and height determinations, and it seems to be an objective variable that is a rapid, easy and non-invasive way to deliver information about the nutritional level of participants. The biological meaning of PhA is not fully understood, but it is considered a cellular health benchmark, thus higher PhA values indicate higher cell function. Since PhA is affected by the ratio of intra- to extracellular water, it is accepted that the low values observed in older subjects reflect a decreased skeletal mass and that consequently ICW may be affected by extracellular edema/accumulation with ageing and deficient health. This suggests that PhA is an effective gauge of qualitative changes in body composition and can discriminate between levels of malnutrition. PhA describes the switch between current and voltage delivered, which is believed to be ‘in vivo’ due to the capacitance of tissue interfaces and cell membrane. Therefore, it must be affected by the volume of body cell mass (i.e., the cell compartment where most of the metabolic processes take place), changes in the cell membranes and alterations in the extracellular solutions.
FM does not appear to be a variable that discriminates DEA; however, FFM does discriminate with an AUC of 0.836 for FFM Sun in the BT group and with an AUC of 0.79 for FFM Slaughter in the AT group, both of which are significant. SMM does not seem to be a good discriminating variable either, although it is very similar to FFM. Nonetheless, in a parallel study, the mesomorphic parameter, which indicates the musculoskeletal development of individuals, was an excellent parameter for the discrimination of DEA [
47], also in the BT group.
The AUC values of Xc and PhA were very high, with a sensitivity and specificity to discriminate DEA, but only in the BT group. No similar work was found in the literature for comparison.
Several factors are suggested to contribute to developing DEA, such female gender, being overweight, living in metropolitan areas, misrepresented perception of body weight and body image dissatisfaction [
48,
49].
The ideal social climate for the development of DEA is probably due to several factors, such as the countless hours spent practicing in front of mirrors by ballet dancers, where they and others closely examine their bodies, as well as the seeking of a perfect body shape and perfect dance development, combined with the pressure to obtain inherent thinness in the dance and high performance expectations [
50,
51].