Multinutrients for the Treatment of Psychiatric Symptoms in Clinical Samples: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studies Included
2.2. Participants
2.3. Interventions
2.4. Comparators/Control
2.5. Outcome Measures
2.6. Search Strategies for Identification of Studies
2.6.1. Electronic Searches
2.6.2. Other Sources
2.7. Selection of Studies
2.8. Data Extraction
2.9. Assessment of Methodological Quality of Included Studies
2.10. Data Synthesis and Measures of Treatment Effect
2.11. Missing Data
2.12. Assessment of the Quality of the Effect Estimate
3. Results
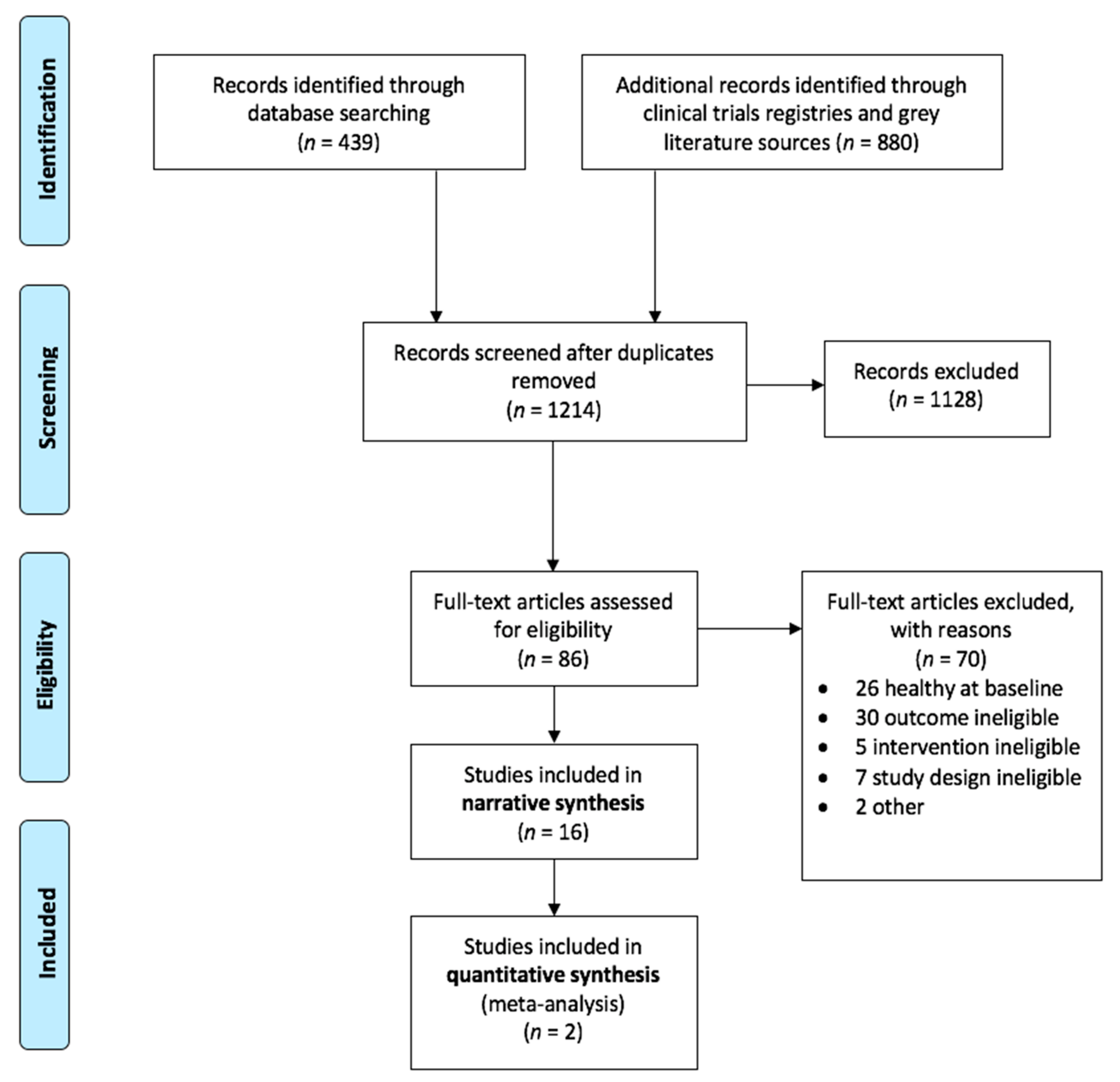
3.1. Study Selection and Inclusion
3.2. Formula Ingredients
3.3. Psychiatric Categories
3.3.1. Depression
3.3.2. Post-Natural Disaster Stress
3.3.3. Antisocial Behaviors
3.3.4. Behavioral Issues in Dementia
3.3.5. ADHD
3.3.6. Autism
4. Discussion
4.1. Populations and Outcomes Studied
4.2. Dose and Range of the Ingredients
4.3. The Use of Medications
4.4. Robustness and Breadth of Methodology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Details Regarding Search Terms Used
References
- Jacka, F.N.; Reavley, N.J.; Jorm, A.F.; Toumbourou, J.W.; Lewis, A.J.; Berk, M. Prevention of common mental disorders: What can we learn from those who have gone before and where do we go next? Aust. N. Z. J. Psychiatry 2013, 47, 920–929. [Google Scholar] [CrossRef]
- Jacka, F.N.; Kremer, P.J.; Berk, M.; De Silva-Sanigorski, A.M.; Moodie, M.; Leslie, E.R.; Pasco, J.A.; Swinburn, B.A. A prospective study of diet quality and mental health in adolescents. PLoS ONE 2011, 6, e24805. [Google Scholar] [CrossRef]
- Jacka, F.N.; Pasco, J.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ’SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2017, 1–14. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Broadley, M.R. Historical variation in the mineral composition of edible horticultural products. J. Hortic. Sci. Biotechnol. 2005, 80, 660–667. [Google Scholar] [CrossRef]
- Bellaloui, N.; Reddy, K.N.; Zablotowicz, R.M.; Abbas, H.K.; Abel, C.A. Effects of glyphosate application on seed iron and root ferric (III) reductase in soybean cultivars. J. Agric. Food Chem. 2009, 57, 9569–9574. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compost. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO(2) depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Cryan, J.F.; Stanton, C. Gut Microbes and Brain Development Have Black Box Connectivity. Biol. Psychiatry 2018, 83, 97–99. [Google Scholar] [CrossRef]
- McNally, L.; Bhagwagar, Z.; Hannestad, J. Inflammation, glutamate, and glia in depression: A literature review. CNS Spectr. 2008, 13, 501–510. [Google Scholar] [CrossRef]
- Oddy, W.H.; Allen, K.L.; Trapp, G.S.A.; Ambrosini, G.L.; Black, L.J.; Huang, R.C.; Rzehak, P.; Runions, K.C.; Pan, F.; Beilin, L.J.; et al. Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain Behav. Immun. 2018. [Google Scholar] [CrossRef] [Green Version]
- Ames, B.N.; Elson-Schwab, I.; Silver, E. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased Km): Relevance to genetic disease and polymorphisms. Am. J. Clin. Nutr. 2002, 75, 616–658. [Google Scholar] [CrossRef] [Green Version]
- Toker, L.; Agam, G. Mitochondrial dysfunction in psychiatric morbidity: Current evidence and therapeutic prospects. Neuropsychiatr. Dis. Treat. 2015, 11, 2441–2447. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, B.J.; Rucklidge, J.J.; McLeod, K.; Romijn, A. The emerging field of nutritional mental health: Inflammation, the microbiome, oxidative stress, and mitochondrial function. Clin. Psychol. Sci. 2015, 3, 964–980. [Google Scholar] [CrossRef] [Green Version]
- Rucklidge, J.J.; Johnstone, J.; Kaplan, B.J. Magic bullet thinking-why do we continue to perpetuate this fallacy? Br. J. Psychiatry 2013, 203, 154. [Google Scholar] [CrossRef] [Green Version]
- Sarris, J.; Mischoulon, D.; Schweitzer, I. Adjunctive nutraceuticals with standard pharmacotherapies in bipolar disorder: A systematic review of clinical trials. Bipolar Disord. 2011, 13, 454–465. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am. J. Psychiatry 2016, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, B.J.; Crawford, S.G.; Field, C.J.; Simpson, J.S. Vitamins, minerals, and mood. Psychol. Bull. 2007, 133, 747–760. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Sarris, J.; Rosenbaum, S.; Teasdale, S.; Berk, M.; Yung, A.R. The effects of vitamin and mineral supplementation on symptoms of schizophrenia: A systematic review and meta-analysis. Psychol. Med. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucklidge, J.J.; Johnstone, J.; Kaplan, B.J. Nutrient supplementation approaches in the treatment of ADHD. Expert Rev. Neurother. 2009, 9, 461–476. [Google Scholar] [CrossRef]
- Benton, D. The impact of diet on anti-social, violent and criminal behaviour. Neurosci. Biobehav. Rev. 2007, 31, 752–774. [Google Scholar] [CrossRef]
- Mertz, W. A balanced approach to nutrition for health: The need for biologically essential minerals and vitamins. J. Am. Diet. Assoc. 1994, 94, 1259–1262. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Frampton, C.M.; Gorman, B.; Boggis, A. Vitamin-mineral treatment of attention-deficit hyperactivity disorder in adults: Double-blind randomised placebo-controlled trial. Br. J. Psychiatry 2014, 204, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucklidge, J.J.; Eggleston, M.J.; Johnstone, J.M.; Darling, K.; Frampton, C.M. Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: A fully blinded, randomized, placebo-controlled trial. J. Child Psychol. Psychiatry 2018, 59, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Pike, V.; Zlotkin, S. Excess micronutrient intake: Defining toxic effects and upper limits in vulnerable populations. Ann. N. Y. Acad. Sci. 2019, 1446, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Benton, D. To establish the parameters of optimal nutrition do we need to consider psychological in addition to physiological parameters? Mol. Nutr. Food Res. 2013, 57, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Katz, M.; Adar Levine, A.; Kol-Degani, H.; Kav-Venaki, L. A compound herbal preparation (CHP) in the treatment of children with ADHD: A randomized controlled trial. J. Atten. Disord. 2010, 14, 281–291. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Morgenstern, H.; Altman, D.G.; Berlin, J.; Chang, S.; McCulloch, P.; Sun, X.; Moher, D. Consensus-based recommendations for investigating clinical heterogeneity in systematic reviews. BMC Med. Res. Methodol. 2013, 13, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G. Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; Higgins, J.P.T., Green, S., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 187–241. [Google Scholar]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef]
- Schunemann, H.; Brozek, J.; Guyatt, G.; Oxman, A.; GRADE Handbook. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. [Updated October 2013]. 2013. Available online: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed on 5 February 2019).
- Ryan, R.; Synnot, A.; Hill, S. Describing results. In Cochrane Consumers and Communication Group; Cochrane Consumers and Communication: London, UK, 2016. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.-A.; Goldstein-Shirley, J.; Robinson, J.; Casey, S. The effects of a multi-modal intervention trial of light, exercise, and vitamins on women’s mood. Women Health 2001, 34, 93–112. [Google Scholar] [CrossRef]
- Zaalberg, A.; Nijman, H.; Bulten, E.; Stroosma, L.; Staak, C. Effects of nutritional supplements on aggression, rule-breaking, and psychopathology among young adult prisoners. Aggress Behav. 2010, 36. [Google Scholar] [CrossRef]
- Schoenthaler, S.J.; Amos, S.; Doraz, W.; Kelly, M.A.; Muedeking, G.D.; Wakefield, J.A. The effect of randomized vitamin-mineral supplementation on violent and non-violent antisocial behavior among incarcerated juveniles. J. Nutr. Environ. Med. 1997, 7. [Google Scholar] [CrossRef]
- Sinn, N.; Bryan, J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J. Dev. Behav. Pediatr. 2007, 28, 82–91. [Google Scholar] [CrossRef]
- Adams, J.B.; Holloway, C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J. Altern. Complement. Med. 2004, 10, 1033–1039. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011, 11, 111. [Google Scholar] [CrossRef]
- Berk, M.; Turner, A.; Malhi, G.S.; Ng, C.H.; Cotton, S.M.; Dodd, S.; Samuni, Y.; Tanious, M.; McAulay, C.; Dowling, N. A randomised controlled trial of a mitochondrial therapeutic target for bipolar depression: Mitochondrial agents, N-acetylcysteine, and placebo. BMC Med. 2019, 17, 18. [Google Scholar]
- Mech, A.W.; Farah, A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: A randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2016, 77, 668–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.E.; Tiozzo, E.; Melillo, A.B.; Leonard, S.; Chen, L.; Mendez, A.; Woolger, J.M.; Konefal, J. The effect of methylated vitamin B complex on depressive and anxiety symptoms and quality of life in adults with depression. ISRN Psychiatry 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Stough, C.; Bousman, C.; Mischoulon, D.; Murphy, J.; Macdonald, P.; Adams, L.; Nazareth, S.; Oliver, G. Nutraceuticals for major depressive disorder-more is not merrier: An 8-week double-blind, randomised, controlled trial. J. Affect. Disord. 2019, 245, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, B.J.; Rucklidge, J.J.; Romijn, A.R.; Dolph, M. A randomised trial of nutrient supplements to minimise psychiatric illness after a natural disaster. Psychiatry Res. 2015, 228. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Andridge, R.; Gorman, B.; Blampied, N.; Gordon, H.; Boggis, A. Shaken but unstirred? Effects of micronutrients on stress and trauma after an earthquake: RCT evidence comparing formulas and doses. Hum. Psychopharmacol. Clin. Exp. 2012, 27. [Google Scholar] [CrossRef]
- Gesch, C.B.; Hammond, S.M.; Hampson, S.E.; Eves, A.; Crowder, M.J. Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners Randomised, placebo-controlled trial. Br. J. Psychiatry 2002, 181, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Pardini, M.; Serrati, C.; Guida, S.; Mattei, C.; Abate, L.; Massucco, D.; Sassos, D.; Amore, M.; Krueger, F.; Cocito, L. Souvenaid reduces behavioral deficits and improves social cognition skills in frontotemporal dementia: A proof-of-concept study. Neurodegener. Dis. 2015, 15, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Åsberg, M.A. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. Psychol. Assess. 1996. [Google Scholar] [CrossRef]
- Locke, B.; Putman, P. Center for epidemiological studies depression scale. 1971. [Google Scholar]
- Duru, G.; Fantino, B. The clinical relevance of changes in the Montgomery–Asberg Depression Rating Scale using the minimum clinically important difference approach. Curr. Med. Res. Opin. 2008, 24, 1329–1335. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Howard, R.; Phillips, P.; Johnson, T.; O’Brien, J.; Sheehan, B.; Lindesay, J.; Bentham, P.; Burns, A.; Ballard, C.; Holmes, C. Determining the minimum clinically important differences for outcomes in the DOMINO trial. Int. J. Geriatr. Psychiatry 2011, 26, 812–817. [Google Scholar] [CrossRef]
- Hall, R.C. Global assessment of functioning: A modified scale. Psychosomatics 1995, 36, 267–275. [Google Scholar] [CrossRef]
- Shaffer, D.; Gould, M.; Brasic, J.; Ambrosini, P.; Fisher, P.; Bird, H.; Aluwahlia, S. A children’s global assessment scale (CGAS). Arch. Gen. Psychiatry 1983, 40, 1228–1231. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Kaplan, B.J. Broad-spectrum micronutrient formulas for the treatment of psychiatric symptoms: A systematic review. Expert Rev. Neurother. 2013, 13, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Blampied, M.; Bell, C.; Gilbert, C.; Rucklidge, J.J. Broad spectrum micronutrient formulas for the treatment of symptoms of depression, stress, and/or anxiety: A systematic review. Expert Rev. Neurother. 2020, 20, 351–371. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Fernstrom, J.D. Control of brain neurotransmitter synthesis by precursor availability and nutritional state. Biochem. Pharmacol. 1976, 25, 1691–1696. [Google Scholar] [CrossRef]
- Marriage, B.; Clandinin, M.T.; Glerum, D.M. Nutritional cofactor treatment in mitochondrial disorders. J. Am. Diet. Assoc. 2003, 103, 1029–1038. [Google Scholar] [CrossRef]
- Stevens, A.J.; Purcell, R.V.; Darling, K.A.; Eggleston, M.J.; Kennedy, M.A.; Rucklidge, J.J. Human gut microbiome changes during a 10 week randomised control trial for micronutrient supplementation in children with attention deficit hyperactivity disorder. Sci. Rep. 2019, 9, 10128. [Google Scholar] [CrossRef]
- Ames, B.N. A role for supplements in optimizing health: The metabolic tune-up. Arch. Biochem. Biophys. 2004, 423, 227–234. [Google Scholar] [CrossRef]
- Snaith, R.; Harrop, F.; Newby, T.D.; Teale, C. Grade scores of the Montgomery—Åsberg depression and the clinical anxiety scales. Br. J. Psychiatry 1986, 148, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Orthomolecular psychiatry: Varying the concentrations of substances normally present in the human body may control mental disease. J. Nutr. Environ. Med. 1995, 5, 187–198. [Google Scholar] [CrossRef]
- Chang, J.J.; Mack, W.J.; Saver, J.L.; Sanossian, N. Magnesium: Potential roles in neurovascular disease. Front. Neurol. 2014, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Welch, K.; Ramadan, N.M. Mitochondria, magnesium and migraine. J. Neurol. Sci. 1995, 134, 9–14. [Google Scholar] [CrossRef]
- Ahsan, S.K. Magnesium in health and disease. J. Pak. Med. Assoc. 1998, 48, 246–249. [Google Scholar]
- Mousain-Bosc, M.; Roche, M.; Rapin, J.; Bali, J.-P. Magnesium VitB6 intake reduces central nervous system hyperexcitability in children. J. Am. Coll. Nutr. 2004, 23, 545S–548S. [Google Scholar] [CrossRef]
- Mousain-Bosc, M.; Roche, M.; Polge, A.; Pradal-Prat, D.; Rapin, J.; Bali, J. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. I. Attention deficit hyperactivity disorders. Magnes. Res. 2006, 19, 46–52. [Google Scholar]
- Kelishadi, R.; Hashemipour, M.; Adeli, K.; Tavakoli, N.; Movahedian-Attar, A.; Shapouri, J.; Poursafa, P.; Rouzbahani, A. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Russo, A. Decreased zinc and increased copper in individuals with anxiety. Nutr. Metab. Insights 2011, 4, 1–5. [Google Scholar] [CrossRef]
- Solati, Z.; Jazayeri, S.; Tehrani-Doost, M.; Mahmoodianfard, S.; Gohari, M.R. Zinc monotherapy increases serum brain-derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: A double-blind, randomized, placebo-controlled trial. Nutr. Neurosci. 2015, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Hirate, M.; Tamano, H.; Oku, N. Release of glutamate and GABA in the hippocampus under zinc deficiency. J. Neurosci. Res. 2003, 72, 537–542. [Google Scholar] [CrossRef]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig. Dis. Sci. 2013, 58, 77–87. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Simpson, J.S.A.; Ferre, R.C.; Gorman, C.P.; McMullen, D.M.; Crawford, S.G. Effective mood stabilization with a chelated mineral supplement: An open-label trial in bipolar disorder. J. Clin. Psychiatry 2001, 62, 936–944. [Google Scholar] [CrossRef]
- Haase, H.; Overbeck, S.; Rink, L. Zinc supplementation for the treatment or prevention of disease: Current status and future perspectives. Exp. Gerontol. 2008, 43, 394–408. [Google Scholar] [CrossRef]
- Tanaka, E. Clinically important pharmacokinetic drug–drug interactions: Role of cytochrome P450 enzymes. J. Clin. Pharm. Ther. 1998, 23, 403–416. [Google Scholar] [CrossRef]
- Mehl-Madrona, L.; Mainguy, B. Adjunctive treatment of psychotic disorders with micronutrients. The J. Altern. Complement. Med. 2017, 23, 526–533. [Google Scholar] [CrossRef]
- Gately, D.; Kaplan, B.J. Database analysis of adults with bipolar disorder consuming a micronutrient formula. Clin. Med. Insights Psychiatry 2009, 2, 3–16. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.J.; Ealam, B.; Beaglehole, B.; Mulder, R.T. An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The J. Altern. Complement. Med. 2019, 25, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Kazdin, A.E. Addressing the treatment gap: A key challenge for extending evidence-based psychosocial interventions. Behav. Res. Ther. 2017, 88, 7–18. [Google Scholar] [CrossRef]
- Wise, J. Only half of patients referred for talking therapies enter treatment. BMJ 2014, 348, g295. [Google Scholar] [CrossRef]






| Reference | Intervention-Daily Dose | Sample Size | Sample Characteristics | Study Length | Outcomes | Results |
|---|---|---|---|---|---|---|
| Berk et al., 2019 [44] ACTRN12612000830897 | Combined Treatment (CT): N-acetylcysteine (NAC) 2000 mg, Acetyl L-carnitine (ALC) 1000 mg, Ubiquinone (Co Q10) 200 mg, magnesium 64 mg (as orotate 500 mg), calcium ascorbate dehydrate 242 mg (equiv ascorbic acid 200 mg), cholecalciferol 12.5μg (equiv Vit D3 250 IU), α-tocopherol 60 IU (equiv natural Vit E 50 IU), alpha lipoic acid 150 mg, Retinyl palmitate 900 μg (equiv Vit A 3000 IU), Vit H 600 μg, thiamine hydrochloride 100 mg, riboflavin 100 mg, nicotinamide 200 mg, calcium pantothenate 100 mg, pyridoxine hydrochloride 100 mg, folic acid 800 μg, and cyanocobalamin (Vit B12) 800 μg or: NAC 2000 mg Plus: usual medication | n = 148 47 CT 52 NAC 49 placebo based on analysis | Adults with bipolar disorder (Diagnostic and Statistical Manual (DSM)-IV-TR) with current depressive episode based on Montgomery-Asberg Depression Rating Scale (MADRS) >/= 20; intervention was adjunctive to usual medication; multisite study | 16 weeks | Primary: Montgomery-Asberg Depression Rating Scale (MADRS) Secondary: Beck Depression Rating Scale (BDRS); Young Mania Rating Scale (YMRS), Clinical Global Impression-Improvement (CGI-I) and CGI-Severity (CGI-S) subscales; Social and Occupational Functioning Assessment Scale (SOFAS), Longitudinal Interval Follow-Up Evaluation - Range of Impaired Functioning Tool (LIFE-RIFT), and Quality of Life Enjoyment, and Satisfaction Questionnaire Short Form (Q-LES-Q) | Negative: no between group differences at study end (week 16) Positive: at 4 weeks post-continuation (week 20; n = 32 for CT; n = 37 for placebo) improvements were significantly greater in the CT group compared to placebo on the MADRS (d = 0.53), BDRS (d = 0.50), CGI-I (d = −0.43), SOFAS (d = −0.55), LIFE-RIFT (d = 0.53). Authors unclear on whether improvement reflects delayed benefit or upon withdrawal from intervention. |
| Brown et al., 2001 [38] | B1 50 mg, B6 50 mg, B2 50 mg, B9 400 µg, Se 200 µg, Vit D 400 IU Plus: 20 min walk outside, 5 days/week, with 60% target heart rate increase; and increased exposure to light | n = 104 53 intervention 51 placebo | Adult women, with mild to moderate depressive symptoms >/= 16 based on the Center for Epidemiology Studies Depression Scale | 8 weeks | Primary: Center for Epidemiology Studies Depression Scale (CESD-D) Secondary: Profile of Mood States (POMS), Depression-Happiness Scale (DHS), Rosenberg Self-Esteem Scale (RSE), General Well-Being Schedule (GWB) | Positive: intervention group improved significantly more than placebo group in mood CESD-D (d = −0.32*); DHS (d = 0.33); self-esteem, RSE: (d = −0.38*); and general sense of well-being, GWB (d = 0.23). *lower score = improvement |
| Lewis et al., 2013 [46] | Max Stress B: B1 1 mg, B2 1.6 mg, B3 30 mg, B5 3.3 mg, B6 3 mg, B9 1000 μg, B12 263 µg, B7 334 µg, PABA, Biotin, Inositol | n = 60 30 intervention 30 placebo | Adults with major depressive disorder (MDD) or a related depressive disorder (DSM-IV-TR definition) and elevated level of homocysteine (>10 μmol/L) | 8 weeks | Primary: Beck Depression Inventory-II (BDI) Secondary: Beck Anxiety Index (BAI); Quality of life from the Medical Outcomes Study Short Form 36 (SF-36) | Unclear: improvements are reported by the authors for the intervention group compared to placebo in depression on the BDI, anxiety on the BAI, and overall mental health on the SF-36; however, authors also report, “effect for time by randomization was nonsignificant,” suggesting no between-group differences |
| Mech et al., 2016 [45] NCT02709668 | EnLyte®: B9 citrated folic acid 1 mg, folinic acid 2.5mg, l-methylfolate magnesium 7 mg, B1 25 μg, Flavin adenine dinucleotide 25 μg, Pyridoxal 5′-phosphate 25 μg, B12 50 μg, Nicotinamide adenine dinucleotide (NADH) 25 μg, Trimethyl glycine 500 μg, AminoFerr TM: 1.5 mg, Vit C 25 mg, l-threonic acid 1 mg, Sharp PS® Gold: phosphatidylserine-omega-3 conjugated 20 mg | n = 282 159 intervention 123 placebo | Adults with MDD (DSM-5 definition) and positive for either methylenetetra-hydrofolate (MTHFR) C677T or A1298C polymorphism | 8 weeks | Primary: MADRS | Unclear: improvements are reported by authors for depression on the MADRS (d = −0.81) in intervention group compared to placebo, however, between-group comparisons and M(SD) are not included in the paper; lower homocysteine in intervention group (d = −0.88). |
| Sarris et al., 2019 [47] ACTRN12613001300763 and 12613001299796 | S-adenosyl methionine (SAMe) 800 mg, folinic acid 500 μg, Vit B12 200 μg, Omega-3 fatty acid concentrate (EPA-esters 1000 mg, DHA-esters 656 mg), 5-HTP 200 mg, zinc picolinate elemental 30 mg, Vit B6 100 mg, Vit C 60 mg, magnesium amino acid chelate, elemental 40 mg, Vit E 40 IU Plus: current SSRI | n = 113 56 intervention 57 placebo | Adults with MDD who are inadequately responsive to current MDD medication and >/=18 on MADRS or >/=14 if not medicated; multisite study | 8 weeks | Primary: MADRS Secondary: Beck Depression Inventory-II (BDI-II), Hamilton Anxiety Rating Scale (HAMA), Short Form Survey-12 (SF-12), Leeds Sleep Evaluation Questionnaire (LSEQ), CGI-I & CGI-S | Negative: placebo superior to nutraceutical combination in reducing MADRS scores (d = 0.21); response rates: 51% for the placebo and 40% for the active intervention; remission rates: 43% and 34% for placebo and active groups, respectively; no differences on other measures |
| Outcomes | Results | № of Participants (Studies) | Certainty of the Evidence (GRADE) |
|---|---|---|---|
| Depression: Clinical Improvement (Depression) Assessed with: MADRS, CES-D, BDI Follow up: range 8–20 weeks | Five studies investigated the impact of multinutrients on depressive symptoms [38,44,45,46,47]. In two studies the benefit is unclear: one study [45] showed what appears to be a clinically significant effect in a population with both depression and an MTHFR genetic variant (MD = −10.7, MID = 1.6–1.9) but provided no between-group p-value; the second study [46] included a population with elevated homocysteine and reported benefit on the BDI, but statistical data did not suggest between-group differences. Another study [38] reported a statistically, but not clinically significant effect (MD = 3.1, p = 0.004, MID = 4). Two studies [44,47] did not show a clinically or statistically significant effect at the primary outcome endpoint. | 707 (5 RCTs) | ⨁⨁◯◯ LOW a, b |
| Post-Natural Disaster Depression, Anxiety, Stress, Assessed with: DASS Follow up: range 4–6 weeks | Two studies investigated the effect of multinutrients on post-natural disaster (flood, earthquake) symptoms of depression, anxiety, and stress [48,49]. Both studies compared similar multinutrient formulations to active controls. Within group improvements were observed in both studies across all treatment groups. The flood study observed greater improvement over time with multinutrients compared to vitamin D on measures of anxiety (d = 1.08) and stress (d = 0.88), but not for depression. While there were no significant between group differences between two different doses of multinutrients and the B-complex with minerals in the earthquake study, with all three groups improving, more participants were rated as treatment responders with the multinutrient intervention. | 147 (2 RCTs) | ⨁⨁◯◯ LOW b, g, h |
| Antisocial Behavior (Antisocial) Assessed with: Number of disciplinary incidents per 1000 person/days, reports of serious offenses, violent rule infarctions reported by prison staff, SDAS, GHQ 28 Follow up: range 2 weeks to 9 months | Three studies measured the effect of multinutrients on antisocial or offending behavior measured as disciplinary incidents in incarcerated populations [39,40,50]. Two studies that were sufficiently homogeneous to meta-analyze [39,50], reported greater improvements in the multinutrient group vs placebo, but provided insufficient data to perform between group comparisons. The third study [40] investigated the effect of multinutrient supplementation on the number of violent rule infarctions in a population of incarcerated individuals aged 13–17. Multinutrients demonstrated a decrease in mean rule violations per subject of 2.85, compared to 1.63 in the placebo arm, a difference which was statistically significant (p = 0.005). MID is unclear. | 455 (3 RCTs) | ⨁⨁◯◯ LOW d, f, g |
| Behavioral issues in Dementia (Dementia) Assessed with: NPI, CGI-S Follow up: 12 weeks | For the outcome of behavioral issues in the context of dementia, one study measured the effect of a multinutrient vs placebo using the CGI-S and NPI [51]. Using the CGI-S, this study suggests a statistically and clinically significant effect of multinutrient supplementation in this population (MD = −1.15, p < 0.01, MID = −1.1). However, using the NPI instrument, the study suggests a statistically, but not clinically significant effect (MD = −4.70. p < 0.01, MID = −8.2). | 26 (1 RCT) | ⨁⨁◯◯ LOW e |
| ADHD: Global/Symptomatic Improvement (ADHD) Assessed with: CGI-I, CGI-ADHD, CPRS, CAARS Follow up: range 8–15 weeks | Three studies investigated the impact of multinutrients on global and symptom improvement in patients with ADHD [26,27,41]. One study showed benefit for two pooled groups (multinutrients plus PUFA and PUFA-alone) compared to placebo, but did not find group differences between the multinutrients plus PUFA group compared to the PUFA alone group [41]. Two studies were sufficiently homogenous and were combined in meta-analyses [26,27]. The results showed clinically and statistically significant improvements on global functioning SMD = −0.49, p = 0.001, clinically and statistically significant improvements on clinician-rated global scores (MD = −0.58, p = 0.001, MID = −0.5) and ADHD scores (MD = −0.54, p = 0.002, MID = −0.5). Pooled analysis of clinician-rated symptom scores showed a statistically significant improvement for inattention (MD = 1.53, p = 0.05), but not for hyperactivity or total scores. No effect was observed for pooled observer-rated ADHD scores. ADHD symptom improvement was statistically and clinically significant in the adult study when outcome was measured by participant-report (MD = 6.71, p = 0.009, MID = 5.9). | 260 (3 RCTs) | ⨁⨁◯◯ LOW c, d |
| Autism Assessed with: Parent Global Impression Follow up: 12 weeks | Two studies investigated clinical improvement in autism [42,43], both used the Parent Global Impression (PGI) scale. In children with autism, multinutrients demonstrated a statistically and clinically significant difference compared to placebo on the PGI sleep subscale (MD = 1.1, p = 0.03, MID = 0.5). In children and adults with autism, multinutrients demonstrated a statistically, but not clinically, significant difference in PGI ratings (MD = 0.33, p < 0.01, MID = 0.5). While the studies were adequately homogenous for pooling, confidence intervals were not consistently reported, which precluded meta-analysis. | 124 (2 RCTs) | ⨁⨁◯◯ LOW b, d |
| Reference | Intervention Daily Dose | Sample Size | Sample Characteristics | Study Length | Outcomes | Results |
|---|---|---|---|---|---|---|
| Kaplan et al., 2015 [48] ANZCTR 12613001051730 | EMPowerplus (EMP+)TM: 4 capsules containing Vit A 384 μg, Vit C 133.2 mg, Vit D 320 IU, Vit E 53.6 mg, B1 4 mg, B2 3.2 mg, B3 20 mg, B5 4.8 mg, B6 8 mg, B9 320 µg, B12 293.2 µg, Biotin 240 µg, Ca 293.2 mg, Fe 3.2 mg, P 186.8 mg, I 45.2 µg, Mg 133.2 mg, Zn 10.8 mg, Se 45.2 µg, Cu 1.6 mg, Mn 2.0 mg, Cr 138.8 µg, Mo 32.0 µg, K 53.2 mg, plus a proprietary blend of Phenylalanine, L-methionine, Citrus bioflavonoids, Germanium sesquioxide, Nickel, Vanadium, Grape seed, L-glutamine, Inositol, Choline bitartrate and Ginkgo biloba or B-complex: B1 50 mg, B2 20 mg, B3 50 mg, B5 50 mg, B6 20 mg, B7 300 µg, Folate 400 µg, B12 500 µg, Intrinsic factor 20 mg, or Vit D 1000 IU | n = 56 All active: 18 Micronutrient 17 Vit D 21 B-Complex | Adults with elevated symptoms of depression, anxiety or stress whose homes were damaged by a flood | 6 weeks | Primary: Depression Anxiety and Stress Scale (DASS): Total Secondary: DASS Depression, Anxiety and Stress subscales; Modified Clinical Global Impression- Improvement (CGI-I): Mood, Anxiety, Stress subscales completed by the participants | Positive: The micronutrient and B-complex groups experienced significant declines in psychological symptoms compared with vitamin D alone. Micronutrient vs vitamin D: DASS: total (d = 0.94); depression (d = 0.64); anxiety (d = 1.08), stress (d = 0.88), as reported by authors. B-complex vs vitamin D:DASS: total (d = 0.81); depression (d = 0.58); anxiety (d = 0.89), stress (d = 0.76), as reported by authors. No significant differences between micronutrient and B-complex. |
| Rucklidge et al., 2012 [49] ANZCTR12611000460909 | CNETM (equivalent to EMP+TM as above) as a “low dose” (4 capsules) or a “high dose” (8 capsules) or 1 tablet of BeroccaTM containing Vit A 100 IU, Vit C 1000 mg, B1 15 mg, B2 15 mg, B3 50 mg, B6 10 mg, B9 400 µg, B12 10 µg, B7 150 µg, B5 23 mg, Ca 50 mg, Mg 50 mg, Zn 10 mg, Na 260 mg, Vit K 5 mg | n = 91 All active: 30 Berocca 31 CNE 4 capsules daily 30 CNE 8 capsules daily | Adults experiencing heightened anxiety or stress 2–3 months post-earthquake | 4 weeks | Primary: DASS: Total Secondary: DASS subscales; Impact of Events Scale (IES); Perceived Stress Scale (PSS); Traumatic Exposure Severity Scale (TESS), modified CGI-I: Mood, Anxiety, Stress subscales completed by the participants | Positive: All three active treatment groups experienced significant reduction in psychological symptoms. |
| Reference | Intervention Daily Dose | Sample Size | Sample Characteristics | Study Length | Outcomes | Results |
|---|---|---|---|---|---|---|
| Gesch et al., 2002 [50] | ForcevalTM Vit A 750 µg, Vit D 10 µg, B1 1.2 mg, B2 1.6 mg, B3 18 mg, B5 4 mg, B6 2 mg, B9 400 µg, B12 3 µg, Vit C 60 mg, Vit E 10 mg, Vit K 120 µg, Vit H 100 µg, Ca 100 mg, Fe 12 mg, Cu 2 mg, Mg 30 mg, Zn 15 mg, I 140 µg, Mn 3 mg, K 4 mg, P 44 mg, Se 50 µg, Cr 200 µg, Mo 250 µg; ALA 1260 mg, GLA 160 mg, EPA 80 mg, DHA 44 mg | n = 172 82 active 90 placebo | adult prisoners (>18 years) | 2 weeks to 9 months 142 days average | Primary: Number of disciplinary incidents per 1000 prison days; Secondary: Reports of serious offenses | Positive: Authors report the average reduction in disciplinary incidents was 35.1% for the active group compared to 6.7% for placebo group; data were insufficient in the paper to calculate effect sizes; authors also report reduction in serious offenses in active group, but not placebo group |
| Schoenthaler et al., 1997 [40] | Vit A 900 μg, B1 3.6 mg, B2 3.9 mg, B3 48 mg; B5 15 mg, B6 30 mg, B7 90 μg, B9 400 μg, B12 7.2 μg, Vit C 120 mg, Vit D 5 μg, Vit E 45 mg, Ca 122 mg, Fe 8 mg, K 700 mg, Iodine 0.150 mg, Mg 59 mg, Zn 11 mg, Se 55 μg, Cu 0.9 mg, Mn 2.3 mg, Chromium 35 μg, Mo 45 μg, Inositol 40 mg, Choline 40 mg, Guarana 87.78 mg, Caffeine 44 mg, p-amino benzoic acid 50 mg | n = 62 32 active 30 placebo | incarcerated youth (13–17 years) | 12 weeks | Primary: Violent rule infractions reported by prison staff | Positive: 28% fewer rule infractions: both violent (d = 0.52), and non-violent (d = 0.70) in those who received the supplement than those who received placebo |
| Zaalberg et al., 2010 [39] | Vit A 875 µg, B1 1.2 mg, B2 1.6 mg, B3 18 mg, B5 4 mg, B6 2 mg, B9 400 µg, B12 3 µg, Vit H 100 µg, Vit C 60 mg, Vit D3 5 µg, Vit E 10 mg, Ca 100 mg, Mg 100 mg, P 52 mg, Zn 15 mg, Fe 12 mg, Mn 3 mg, Cu 2 mg, K 4 mg, I 140 µg, Se 50 µg, Cr 200 µg, Mo 250 µg; DHA 400 mg, EPA 400 mg, GLA 100 mg, and 2 capsules primrose oil | n = 221 115 active 106 placebo | adult male prisoners (18–25 years) across 8 Dutch prisons | 1–3 months | Primary: Number of disciplinary incidents per 1000 prison days; Secondary: Social Dysfunction and Aggression Scale, General Health Questionnaire-28 | Positive: Authors report 34% fewer aggressive and rule-breaking incidents vs 14% increase in the placebo group. Data were insufficient to calculate effect sizes. Negative: No group differences on self-reports of aggression or psychological well-being |
| Reference | Intervention Daily Dose | Sample Size | Sample Characteristics | Study Length | Outcomes | Results |
|---|---|---|---|---|---|---|
| Pardini et al., 2015 [51] | SouvenaidTM: EPA 300 mg, DHA 1200 mg, Phospholipids 106 mg, Choline 400 mg, uridine-mono-phosphate 625 mg, Vit E 40 mg, Vit C 80 mg, Se 60 µg, B12 3 µg, B6 1 mg, B9 400 µg | n = 26 13 active 13 placebo, crossover design | adults (50–65 years) with diagnosis of behavioral variant of frontotemporal dementia | 12 weeks | Primary: Neuropsychiatric Inventory (NPI); Secondary: Frontal Assessment Battery (FAB), Clinical Global Impression-Severity (CGI-S), the Reading the Mind in the Eyes Test (RMET) | Positive: authors report reduced agitation, apathy, disinhibition, and irritability on the NPI; improvement on the CGI-S; an increase in Theory of Mind skills for those on active treatment; reversal of improvement when taken off active; insufficient data provided to calculate effect sizes Negative: no impact on executive functioning on the FAB |
| Reference | Intervention Daily Dose | Sample Size | Sample Characteristics | Study Length | Outcomes | Results |
|---|---|---|---|---|---|---|
| Rucklidge et al., 2018 [27] ANZCTRN12613000896774 | Daily Essential Nutrients: Vit A 384 IU, Vit C 40 mg, Vit D 200 IU, Vit E 24 IU, Vit K 8 µg, B1 4 mg, B2 1.2 mg, B3 6 mg, B6 4.67 mg, B9 50 μg, B12 60 μg, B7 72 μg, B5 2 mg, Ca 88 mg, Fe 0.92 mg, P 56 mg, I 13.6 μg, Mg 40 mg, Zn 3.2 mg, Se 13.6 μg, Cu 0.48 mg, Mn 0.64 mg, Cr 41.6 μg, Mo 9.6 μg, P 16 mg. Proprietary blend: Choline bitartrate, Alpha-lipoic acid, Inositol, Acetylcarnitine (as acetyl-L-carnitine hydrochloride), Grape seed extract, Ginkgo biloba leaf extract, Methionine (as L-methionine hydrochloride), Cysteine (as N-acetyl-L-cysteine), Germanium sesquioxide (as chelate), Boron, Vanadium, Lithium orotate, Nickel. Other ingredients: Cellulose glycine 45 mg, Citric acid 26.814 mg, Magnesium stearate 24 mg, Silicon dioxide 20 mg | n = 93 47 active 46 placebo | Children (7–12 years) with ADHD | 10 weeks | Primary: Conners Parent/Teacher Rating Scale-Revised (CPRS) Diagnostic and Statistical Manual (DSM)-IV Attention Deficit Hyperactivity Disorder (ADHD) Symptoms Total Secondary: Clinical Global Impression-Improvement (CGI-I); CGI-I-ADHD; CGI-I-Mood; Children’s-Global Assessment Scale-(C-GAS); Clinician ADHD-Rating Scale (RS)-IV Symptoms Total; Child Mania Rating Scale-Parent (CMRS-P); Clinician ADHD-RS-IV; Parent Strengths and Difficulties Questionnaire (SDQ)-Total Problem Score; Parent SDQ-Conduct Problem Score; Teacher SDQ-Total Problem score; Teacher SDQ-Conduct Problem Score Teacher Behavior Rating Inventory of Executive Function (BRIEF), Behavioural Regulation; Index Teacher BRIEF-Emotional Control subscale | Positive: CGI-I overall (d = 0.46) CGI-I-ADHD (d = 0.53); CGI-I-Mood (d = 0.51); C-GAS (d = 0.48); Parent SDQ-Conduct Problem Score (d = 0.52); Teacher BRIEF-Emotional Control Subscale (d = 0.66) Negative: Clinician ADHD-RS-IV Symptoms Total, CPRS DSM-IV ADHD Symptoms Total; CMRS-P; Clinician ADHD-RS-IV Conners Teacher Rating Scale-DSM-IV Total; Parent SDQ-Total Problem Score; Teacher SDQ-Total Problem Score; Teacher SDQ-Conduct Problem Score Teacher BRIEF-Behavioural Regulation Index |
| Rucklidge et al., 2014 [26] ANZCTR12609000308291 | EMP+: Vit A 5760 IU, Vit C 600 mg, Vit D 1440 IU, Vit E 360 IU, B1 18 mg, B2 13.5 mg, B3 90 mg, B5 21.6 mg, B6 36 mg, B9 1440 µg, B12 900 µg, Biotin 1080 µg, Pantothenic acid 21.6 mg, Ca 1320 mg, Fe 13.74 mg, P 840mg, I 204 µg, Mg 600 mg, Zn 48 mg, Se 204 µg, Cu 7.2 mg, Mn 9.6 mg, Cr 624 µg, Mo 144 µg, K 240 mg, Germanium sesquioxide 20.7 mg, B 2400 µg, V 1194 µg, Ni 29.4 µg, Choline bitartrate 540 mg, DL-phenylalanine 360 mg, Citrus bioflavonoids 240 mg, Inositol 180 mg, Glutamine 180 mg, L-methionine 60 mg, Gingko biloba 36 mg, grape seed extract 45 mg | n = 80 42 active 38 placebo | Adults with ADHD | 8 weeks | Primary: Conners Adult ADHD Rating Scale (CAARS), self or observer version Secondary: CGI-I-Overall Impression; CGI-I-ADHD; Global Assessment of Functioning (GAF); MADRS; Self-report: CAARS, DSM-IV ADHD symptoms total; CAARS inattention, hyperactivity/impulsivity; Observer: CAARS DSM-IV ADHD symptoms total; CAARS, inattention, hyp/imp; Clinician: CAARS DSM-IV ADHD symptoms total; inattention, hyperactivity/impulsivity | Positive: CAARS DSM-IV ADHD symptoms, self-report (d = 0.61); CAARS ADHD symptoms, Observer (d = 0.59); CGI-I-ADHD (d = 0.53); CGI-I-Overall (d = 0.57); CAARS, self-report inattention (d = 0.62), hyperactivity/impulsivity (d = 0.47); CAARS, observer, hyperactivity/impulsivity (d = 0.67); GAF (d = 0.46) Negative: CAARS DSM-IV ADHD symptoms, clinician; MADRS; CAARS, observer, inattention; CAARS, clinician inattention, hyperactivity/impulsivity |
| Sinn & Bryan, 2007 [41] | Blackmores Chewable Multivitamins & Minerals for Kids (Nutrients): Vit 175 IU, B1 700 µg, B2 1.1 mg, B3 12 mg, B5 2.7 mg, B6 1.3 mg, B9 100 µg, B12 1.5 µg, Vit C 60 mg, D3 100 IU, Vit E 6 IU, Vit H 50 µg, Ca 33.9 mg, Fe 7.5 mg, Mg 8.32 mg, Mn 77 µg, Cu 178.6 µg, K 118 µg Plus: Polyunsaturated fatty acid (PUFA): eye q™: EPA 93 mg, DHA 29 mg, GLA 10 mg, Vit E 1.8 mg or eye q™ alone | n = 87 36 active: (nutrients + PUFA) 29 PUFA 22 placebo | Children with ADHD | 15 weeks | Primary: Conners Parent and Teacher Rating Scales (CPRS), ADHD Index Secondary: CPRS subscales: cognitive problems/inattention, hyperactivity; Global scales for restless/impulsive, emotional liability, total; DSM-IV inattentive; hyperactive/impulsive, total; Oppositional; Anxious/Shy; Perfectionism; Social Problems; Psychosomatic | Negative: At 15 weeks, the PUFA group combined with the PUFA + nutrients group showed significant improvements over placebo for parent ratings of inattention, hyperactivity, and global ADHD indices on the CPRS. PUFA + nutrients compared to PUFA alone showed no group differences; authors concluded PUFA was the primary mechanism of improvement. However, the PUFA + nutrients group was not compared to placebo. No changes reported on the teachers rating scales. |
| Reference | Intervention Daily Dose | Sample Size | Sample Characteristics | Study Length | Outcomes | Results |
|---|---|---|---|---|---|---|
| Adams et al., 2011 [41] NCT01225198 | Vit A 1000 IU, Vit C 600 mg, Vit D3 300 IU, Vit E 150 IU, B1 20 mg, B2 20 mg, B3 25 mg, B5 15 mg, B6 40 mg, B9 100 µg, B12 500 µg, Folinic acid 550 µg, Vit H 150 µg, Choline 250 mg, Inositol 100 mg, Mixed carotenoids 3.6 mg, Coenzyme Q10 50 mg, n-acetyl cysteine 50 mg, Ca 100 mg, Cr 70 µg, I 100 µg, Li 500 µg, Mg 100 mg, Mn 3 mg, Mo 150 µg, K 50 mg, Se 22 µg, S 500 mg, Zn 12 mg | n = 104 53 active 51 placebo | children and adults (3–58 years) with autism spectrum disorder | 12 weeks | Parent Global Impressions (PGI)-Revised and subscales: Expressive Language Receptive language Play Cognition Sleep Sociability Eye Contact Hyperactivity Tantrumming | Positive: PGI-Revised subscales: Overall (d = 0.46) Tantrumming (d = 0.51); Receptive language (d = 0.40) Hyperactivity (d = 0.37) Negative: Expressive language, play, cognition, sleep, sociability, eye contact |
| Adams and Holloway, 2004 [42] | Vit A (7560, 10,584 IU), B1 (20, 30 mg), B2 (25, 25 mg), B3 (25, 35 mg), B5 (45, 25 mg), B6 (30, 30 mg), B9 (800, 800 µg), B12 (1200, 1600 µg), B7 (100, 150 µg), Choline (50, 60 mg), Inositol (50, 60 mg), Vit C (650, 800 mg), Mixed bioflavonoids (200, 400 mg), Vit D3 (150, 150 IU), Vit E (175, 250 IU), Ca (175, 200 mg), Ca D-glucarate (0, 75 mg), Cr (75, 100 µg), Mg (175, 200 mg), Mn (3, 3 mg), Mo (0, 75 µg), K (75, 75 mg), Se (70, 85 µg), Si (0, 3 mg), S (175, 300 mg), Zn (15, 20 mg), N-acetyl cysteine (25, 50 mg), Alpha lipoic acid (0, 25 mg) | n = 20 11 active 9 placebo | children (3–8 years) with autism spectrum disorder | 12 weeks | (PGI) subscales: Sleep Gastrointestinal symptoms Expressive Language Receptive language Play Cognition Sleep Sociability Eye Contact Hyperactivity | Positive: PGI-Revised subscales: sleep and gastrointestinal problems; insufficient data to calculate effect sizes Negative: Expressive and receptive language, play, cognition, sociability, eye contact, hyperactivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnstone, J.M.; Hughes, A.; Goldenberg, J.Z.; Romijn, A.R.; Rucklidge, J.J. Multinutrients for the Treatment of Psychiatric Symptoms in Clinical Samples: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3394. https://doi.org/10.3390/nu12113394
Johnstone JM, Hughes A, Goldenberg JZ, Romijn AR, Rucklidge JJ. Multinutrients for the Treatment of Psychiatric Symptoms in Clinical Samples: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020; 12(11):3394. https://doi.org/10.3390/nu12113394
Chicago/Turabian StyleJohnstone, Jeanette M., Andrew Hughes, Joshua Z. Goldenberg, Amy R. Romijn, and Julia J. Rucklidge. 2020. "Multinutrients for the Treatment of Psychiatric Symptoms in Clinical Samples: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 12, no. 11: 3394. https://doi.org/10.3390/nu12113394
APA StyleJohnstone, J. M., Hughes, A., Goldenberg, J. Z., Romijn, A. R., & Rucklidge, J. J. (2020). Multinutrients for the Treatment of Psychiatric Symptoms in Clinical Samples: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 12(11), 3394. https://doi.org/10.3390/nu12113394





