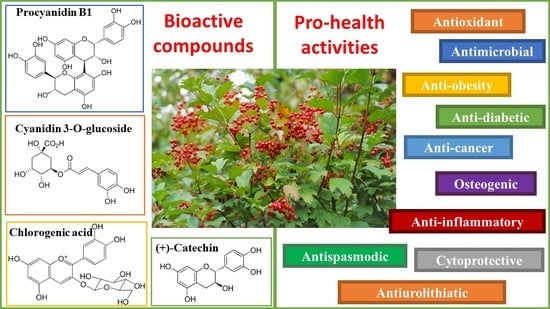
Viburnum opulus L.—A Review of Phytochemistry and Biological Effects
Abstract
:1. Introduction
2. Botanical Characteristics
3. Macronutrients, Minerals, and Dietary Fiber Composition
4. Antioxidative Components
5. Viburnum opulus Health-Promoting Effects
5.1. Antioxidative Effect
5.2. Antimicrobial Activity
5.3. Effect on Carbohydrates Metabolism
5.4. Effect on Lipid Metabolism
5.5. Anti-Inflammatory Potential
5.6. Osteogenic Activity
5.7. Influence on Blood Vessels Activity
5.8. Effect on Urinary System and the Endometriosis
5.9. Anti-Cancer Activity
5.10. Cytoprotective Properties
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karaçelik, A.A.; Küçük, M.; Iskefiyeli, Z.; Aydemir, S.; De Smet, S.; Miserez, B.; Sandra, P. Antioxidant components of Viburnum opulus L. determined by on-line HPLC–UV–ABTS radical scavenging and LC–UV–ESI-MS methods. Food Chem. 2015, 175, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Özrenk, M.; Gϋndoǧdu, N.; Kenskin, N.; Kaya, T. Some physical and chemical characteristics of gilaburu (Viburnum opulus L.) fruits in Erzincan region. Iğdır Univ. J. Inst. Sci. Technol. 2011, 1, 9–14. [Google Scholar]
- Sagdic, O.; Ozturk, I.; Yapar, N.; Yetim, H. Diversity and probiotic potentials of lactic acid bacteria isolated from gilaburu, a traditional Turkish fermented European cranberrybush (Viburnum opulus L.) fruit drink. Food Res. Int. 2014, 64, 537–545. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Ekici, L.; Poyrazoglu, E.S. Phenolic composition of European cranberrybush (Viburnum opulus L.) berries and astringency removal of its commercial juice. Int. J. Food Sci. Technol. 2006, 9205, 1011–1015. [Google Scholar] [CrossRef]
- Akbulut, M.; Calsir, S.; Marakoglu, T.; Coklar, H. Chemical and technological properties of European cranberrybush (Viburnum opulus L.) fruits. Asian J. Chem. 2008, 20, 1875–1885. [Google Scholar]
- Altun, M.L.; Çitoǧlu, G.S.; Yilmaz, B.S.; Özbek, H. Antinociceptive and anti-inflammatory activities of Viburnum opulus. Pharm. Biol. 2009, 47, 653–658. [Google Scholar] [CrossRef]
- Konarska, A.; Domaciuk, M. Differences in the fruit structure and the location and content of bioactive substances in Viburnum opulus and Viburnum lantana fruits. Protoplasma 2018, 255, 25–41. [Google Scholar] [CrossRef]
- Rop, O.; Reznicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant properties of European cranberrybush fruit (Viburnum opulus var. edule). Molecules 2010, 15, 4467–4477. [Google Scholar] [CrossRef]
- Perova, I.B.; Zhogova, A.A.; Cherkashin, A.V.; Éller, K.I.; Ramenskaya, G.V. Biologically active sunstances from European guelder berry fruits. Pharm. Chem. J. 2014, 48, 332–339. [Google Scholar] [CrossRef]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef]
- Soylak, A.; Elci, L.; Saracoglu, S.; Divrikli, U. Chemical analysis of fruit juice of European cranberrybush (Viburnum opulus) from Kayseri-Turkey. Asian J. Chem. 2002, 14, 135–138. [Google Scholar]
- Lachowicz, S.; Oszmianski, J. The influence of addition of cranberrybush juice to pear juice on chemical composition and antioxidant properties. J. Food Sci. Technol. 2018, 55, 3399–3407. [Google Scholar] [CrossRef] [Green Version]
- Al, Ö.; Ülger, H.; Eetekin, T.; Nisari, M.; Susar, H.; Ceylan, D.; Karatoprak, G.Ş. The effect of gilaburu (Viburnum opulus) juice on Ehrlich ascites tumor (EAT) cell culture. Proceedings 2017, 1, 1051. [Google Scholar] [CrossRef] [Green Version]
- Çemtekİn, B.; Kilinç, E.; Karabacak, L.; Dağtekİn, T. Aa evaluationof guelder rose (Viburnum opulus L.) and hawthorn (Crataegus monogyna) concentrates as alternative antioxidant sources to BHT and nitrite in poultry meat model system. Sci. Pap. Ser. D. Anim. Sci. 2019, LXII, 217–227. [Google Scholar]
- Česonienė, L.; Daubaras, R.; Kraujalytė, V.; Venskutonis, P.R.; Šarkinas, A. Antimicrobial activity of Viburnum opulus fruit juices and extracts. J. Verbraucherschutz Leb. 2014, 9, 129–132. [Google Scholar] [CrossRef]
- Erylimaz, M.; Ozbiligin, S.; Ergene, B.; Yilmaz, S.; Altun, M.L.; Saltan, G. Antimicrobial activity of Turkish Viburnum species. Bangladesh J. Bot. 2013, 42, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Bubulica, V.M.; Anghel, I.; Grumezescu, A.M.; Saviuc, C.; Anghel, G.A.; Chifriuc, M.C.; Gheorghe, I.; Lazar, V.; Popescu, A. In vitro evaluation of bactericidal and antibiofilm activity of Lonicera tatarica and Viburnum opulus plant extracts on Staphylococcus strains. Farmacia 2012, 60, 80–91. [Google Scholar]
- Sagdic, O.; Aksoy, A.; Ozkan, G. Evaluation of the antibacterial and antioxidant potentials of cranberry (gilaburu, Viburnum opulus L.) fruit extract. Acta Aliment. 2006, 35, 487–492. [Google Scholar] [CrossRef]
- Turker, H.; Yıldırım, A.B. Screening for antibacterial activity of some Turkish plants against fish pathogens: A possible alternative in the treatment of bacterial infections. Biotechnol. Biotechnol. Equip. 2015, 29, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Majewska, I.; Redzynia, M.; Koziołkiewicz, M. Antidiabetic effect of polyphenolic extracts from selected edible plants as α-amylase, α-glucosidase and PTP1B inhibitors, and β pancreatic cells cytoprotective agents—A comparative study. Curr. Top. Med. Chem. 2015, 15, 2431–2444. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Zakłos-Szyda, M.; Kowalska-Baron, A.; Pietrzyk, N.; Drzazga, A. Evaluation of Viburnum opulus L. Fruit phenolics cytoprotective potential on insulinoma MIN6 cells relevant for diabetes mellitus and obesity. Antioxidants 2020, 9, 433. [Google Scholar] [CrossRef]
- Podsędek, A.; Zakłos-Szyda, M.; Polka, D.; Sosnowska, D. Effects of Viburnum opulus fruit extracts on adipogenesis of 3T3-L1 cells and lipase activity. J. Funct. Foods 2020, 73, 104111. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pietrzyk, N.; Szustak, M.; Podsędek, A. Viburnum opulus L. juice phenolics inhibit mouse 3T3-L1 cells adipogenesis and pancreatic lipase activity. Nutrients 2020, 12, 2003. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Nowak, A.; Pietrzyk, N.; Podsędek, A. Viburnum opulus L. juice phenolic compounds influence osteogenic differentiation in human osteosarcoma Saos-2 cells. Int. J. Mol. Sci. 2020, 21, 4909. [Google Scholar] [CrossRef]
- Kalinkevich, K.; Karandashov, V.E.; Ptitsyn, L.R. In vitro study of the anti-inflammatory activity of some medicinal and edible plants growing in Russia. Russ. J. Bioorganic Chem. 2014, 40, 752–761. [Google Scholar] [CrossRef]
- Chojnacka, K.; Owczarek, K.; Fichna, J.; Sosnowska, D.; Lewandowska, U. Wpływ ekstraktów z liści kaliny koralowej (Viburnum opulus L.) na wzrost ludzkich komórek jelita. Post Fitoter 2019, 20, 10–17. [Google Scholar]
- Kopral, A.T. In vitro evaluation of gilaburu (Viburnum opulus L.) juice on different cell lines. Anadolu J. Educ. Sci. Int. 2019, 9, 549–571. [Google Scholar] [CrossRef] [Green Version]
- Ucar, T.A.; Yildirim, A.B.; Karakas, F.P. Antibacterial and antitumor activities of some wild fruits grown in Turkey. Biotechnol. Biotechnol. Equip. 2012, 26, 2765–2772. [Google Scholar]
- Ilhan, M.; Ergene, B.; Süntar, I.; Özbilgin, S.; Çitoʇlu, G.S.; Demirel, M.A.; Keleş, H.; Altun, L.; Akkol, E.K. Preclinical evaluation of antiurolithiatic activity of Viburnum opulus L. On sodium oxalate-induced urolithiasis rat model. Evidence-based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Erdem, G.; Kesik, V.; Honca, T.; Ozcan, A.; Uguz, S.; Akgvl, E.O.; Aykutlug, O.; Alp, B.F.; Korkmazer, N.; Saldir, M.; et al. Antinephrolithiatic activity of Persea americana (avocado) and Viburnum opulus (guelder rose) against ethylene glycol-induced nephrolithiasis in rats. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Bujor, A.; Miron, A.; Luca, S.V.; Skalicka-Wozniak, K.; Silion, M.; Ancuceanu, R.; Dinu, M.; Girard, C.; Demougeot, C.; Totoson, P. Metabolite profiling, arginase inhibition and vasorelaxant activity of Cornus mas, Sorbus aucuparia and Viburnum opulus fruit extracts. Food Chem. Toxicol. 2019, 133, 1–2. [Google Scholar] [CrossRef]
- Česoniene, L.; Daubaras, R.; Vencloviene, J.; Viškelis, P. Biochemical and agro-biological diversity of Viburnum opulus genotypes. Cent. Eur. J. Biol. 2010, 5, 864–871. [Google Scholar] [CrossRef]
- Polka, D.; Podsedek, A. Phenolics composition and antioxidant capacity of guelder rose fruit, flower and bark extracts. Biotechnol. Food Sci. 2019, 83, 37–46. [Google Scholar]
- Rychlińska, I. Sterols and triterpenes in Viburnum opulus L. leaves. Herba Pol. 2008, 54, 59–65. [Google Scholar]
- Zarifikhosroshahi, M.; Tugba, Z.; Kafkas, E.; Okatan, V. Variation in volatile and fatty acid contents among Viburnum opulus L. fruits growing different locations. Sci. Hortic. 2020, 264, 109160. [Google Scholar] [CrossRef]
- Moldovan, B.; Luminiţa, D.; Chişbora, C.; Cimpoiu, C. Degradation kinetics of anthocyanins from European cranberrybush (Viburnum opulus L.) fruit extracts. effects of temperature, pH and storage solvent. Molecules 2012, 17, 11655–11666. [Google Scholar] [CrossRef]
- Kraujalyte, V.; Venskutonis, P.R.; Pukalskas, A.; Cesoniene, L.; Daubaras, R. Antioxidant properties and polyphenolic compositions of fruits from different European cranberrybush (Viburnum opulus L.) genotypes. Food Chem. 2013, 141, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, O.; Ehrln, J. Phenological variation in fruit characteristics in vertebrate-dispersed plants. Oecologia 1991, 86, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, N.; Ercisli, S.; Gundogdu, M. Evaluation of European cranberrybush (Viburnum opulus L.) genotypes for agro-morphological, biochemical and bioactive characteristics in Turkey. Folia Hortic. 2017, 29, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Ersoy, N.; Ercisli, S.; Akin, M.; Gundogdu, M.; Colak, A.M.; Ben Ayed, R. Agro-morphological and biochemical characteristics of European cranberrybush (Viburnum opulus L.). Compt. Rend. Acad. Bulg. Sci. 2018, 71, 491–499. [Google Scholar]
- Kalyoncu, I.H.; Ersoy, N.; Elidemir, A.Y.; Korali, M.E. Some physico-chemical characteristics and mineral contents of gilaburu (Viburnum opulus L.) fruits in Turkey. Int. J. Agric. Biosyst. Eng. 2013, 7, 424–426. [Google Scholar]
- Ozkan, G.; Ercisli, S.; Ibrahim, H.; Gulce, S. Diversity on fruits of wild grown European cranberrybush from coruh valley in Turkey. Erwerbs-Obstbau 2020, 62, 275–279. [Google Scholar] [CrossRef]
- Ozrenk, K.; Ilhan, G.; Sagbas, H.I.; Karatas, N.; Ercisli, S.; Colak, A.M. Characterization of European cranberrybush (Viburnum opulus L.) genetic resources in Turkey. Sci. Hortic. 2020, 273. [Google Scholar] [CrossRef]
- Moskalets, T.Z.; Moskalets, V.; Vovkohon, A.H.; Knyazyuk, O.V. Fruits of new selection forms and varieties of snowball tree for manufacture of products of therapeutic and prophylactic purpose. Regul. Mech. Biosyst 2019, 10, 432–437. [Google Scholar] [CrossRef]
- Andreeva, T.I.; Komarova, E.N.; Yosubov, M.S.; Korotkova, E. Antioxidant activity of cranberry tree (Viburnum opulus L.) bark extract. Pharm. Chem. J. 2004, 38, 548–550. [Google Scholar] [CrossRef]
- Taşkin, O.; Asik, B.B.; Izli, N. Mineral content of leaves, stalks and fruits of European cranberrybush plant (Viburnum opulus L.). KSÜ Tarım Doğa Derg. 2019, 22, 178–182. [Google Scholar]
- Turek, S.; Cisowski, W. Free and chemically bonded phenolic acids in barks of Viburnum opulus L. and Sambucus nigra L. Acta Pol. Pharm. Drug Res. 2007, 64, 377–383. [Google Scholar]
- Dienaite, L.; Pukalskiene, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Valorization of European cranberry bush (Viburnum opulus L.) berry pomace extracts isolated with pressurized ethanol and water by assessing their phytochemical composition, antioxidant, and antiproliferative activities. Foods 2020, 9, 1413. [Google Scholar] [CrossRef]
- Cam, M.; Hisil, Y.; Kuscu, A. Organic acid, phenolic content and antioxidant capacity of fruit flesh and seedof Viburnum opulus. Chem. Nat. Compd. 2007, 43, 379–380. [Google Scholar] [CrossRef]
- Yunusova, S.G.; Karimova, A.R.; Tsyrlina, E.M.; Yunusov, M.S.; Denisenko, O.N. Change on storage of biological activity of Viburnum opulus seed components. Chem. Nat. Compd. 2004, 40, 349–351. [Google Scholar] [CrossRef]
- Yilmaz, N.; Beyhan, Ö.; Gerçekçioğlu, R.; Kalayci, Z. Determination of fatty acid composition in seed oils of some important berry species and genotypes grown in Tokat Province of Turkey. Afr. J. Biotechnol. 2011, 10, 8070–8073. [Google Scholar]
- Kraujalyte, V.; Leitner, E.; Rimantas, P. Chemical and sensory characterisation of aroma of Viburnum opulus fruits by solid phase microextraction-gas chromatography—Olfactometry. Food Chem. 2012, 132, 717–723. [Google Scholar] [CrossRef]
- Sönmezdağ, A.S.; Sevindik, O.; Kelebek, H.; Selli, S. Aroma compounds of non-alcoholic fermented beverage: Gilaburu juice. EuroBiotech J. 2017, 1, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Yilmaztekin, M.; Sislioglu, K. Changes in volatile compounds and some physicochemical properties of European cranberrybush (Viburnum opulus L.) during ripening through traditional fermentation. J. Food Sci. 2015, 80, C687–C694. [Google Scholar] [CrossRef]
- Udensi, U.K.; Tchounwou, P.B. Potassium homeostasis, oxidative stress, and human disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122. [Google Scholar]
- Yang, C.S.; Ho, C.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing meanings in food science and health science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Çam, M.; Hişil, Y. Comparison of chemical characteristics of fresh and pasteurised juice of gilaburu (Viburnum opulus L.). Acta Aliment. 2007, 36, 381–385. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Maatta, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2 -extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2017, 44, 1–2. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Altun, M.L.; Yilmaz, B.S. HPLC method for the analysis of salicin and chlorogenic acid from Viburnum opulus and V. lantana. Chem. Nat. Compd. 2007, 43, 170–171. [Google Scholar]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozanski, T. The effects of natural iridoids and anthocyanins on selected parameters of liver and cardiovascular system functions. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; de la Luz Cádiz-Gurrea, M.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships between chemical structure and antioxidant activity of isolated phytocompounds from Lemon verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stępień, A.; Aebisher, D.; Bartusik-Aebisher, D. Anticancer properties of Viburnum. Eur. J. Clin. Exp. Med. 2018, 1361, 47–52. [Google Scholar] [CrossRef]
- Bock, K.; Rosendal, S.; Bent, J.; Nielsen, J.; Norm, V. Iridoid allosides from Viburnum opulus. Phytochemistry 1978, 17, 753–757. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.C.; Mold, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Song, R.; Zhao, L.; Yun, Z. Advances in cellular evaluation and standard of antioxidant activity. ChinaBiofilms 2019, 131, 01008. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, M.; Huang, L.; Wang, J.; Gong, L.; Liu, J.; Sun, B. Evaluation of the cellular and animal models for the study of antioxidant activity: A review. J. Food Sci. 2017, 82, 278–288. [Google Scholar] [CrossRef]
- Barak, T.H.; Celep, E.; Yesilada, E. Influence of in vitro human digestion on the bioavailability of phenolic content and antioxidant activity of Viburnum opulus L. (European cranberry) fruit extracts. Ind. Crop. Prod. 2019, 131, 62–69. [Google Scholar] [CrossRef]
- Kraujalis, P.; Kraujaliene, V.; Kazernaviˇ, R.; Venskutonis, P.R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. J. Supercrit. Fluid. 2017, 122, 99–108. [Google Scholar] [CrossRef]
- Erdogan-Orhan, I.; Altun, M.L.; Sever-Yilmaz, B.; Saltan, G. Anti-acetylcholinesterase and antioxidant assets of the major components (salicin, amentoflavone, and chlorogenic acid) and the extracts of Viburnum opulus and Viburnum lantana and their total phenol and flavonoid contents. J. Med. Food 2011, 14, 434–440. [Google Scholar] [CrossRef]
- Polka, D.; Podsędek, A. Comparison of chemical composition and antioxidant capacity of fruit, flower and bark of Viburnum opulus. Plant Foods Hum. Nutr. 2019, 74, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Paşayeva, L.; Arslan, A.K.K.; Kararenk, A.C. Viburnum opulus L. fruit extracts protect human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Proceedings 2019, 40, 5. [Google Scholar] [CrossRef] [Green Version]
- Medina-Gomez, G.; Gray, S.; Vidal-Puig, A. Adipogenesis and lipotoxicity: Role of peroxisome proliferator-activated receptor γ (PPARγ) and PPARγcoactivator-1 (PGC1). Public Health Nutr. 2007, 10, 1132–1137. [Google Scholar] [CrossRef]
- Cumaoǧlu, A.; Rackova, L.; Stefek, M.; Kartal, M.; Maechler, P.; Karasu, Ç. Effects of olive leaf polyphenols against H2O2 toxicity in insulin secreting β-cells. Acta Biochim. Pol. 2011, 58, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.Á.; Fernández-Millán, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2014, 58, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altun, M.; Citoglu, G.S.; Yilmaz, B.S.; Coban, T. Antioxidant properties of Viburnum opulus and Viburnum lantana growing in Turkey. Int. J. Food Sci. Nutr. 2008, 59, 175–180. [Google Scholar] [CrossRef]
- Zayachkivska, O.S.; Gzhegotsky, M.R.; Terletska, O.I.; Lutsyk, D.A.; Yaschenko, A.M.; Dzhura, O.R. Influence of Viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. J. Physiol. Pharmacol. 2006, 57, 155–167. [Google Scholar]
- Eken, A.; Yücel, O.; İpek, İ.; Ayşe, B.; Endİrlİk, B.Ü. An investigation on protective effect of Viburnum opulus L. fruit extract against ischemia/reperfusion-induced oxidative stress after lung transplantation in rats. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 437–444. [Google Scholar]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Ildiz, N.; Baldemir, A.; Altinkaynak, C.; Özdemir, N.; Yilmaz, V.; Ocsoy, I. Self assembled snowball-like hybrid nanostructures comprising Viburnum opulus L. extract and metal ions for antimicrobial and catalytic applications. Enzym. Microb. Technol. 2017, 102, 60–66. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Yetim, H.; Ekici, L.; Ozcan, C.; Ozturk, I.; Tornuk, F.; Karaman, S. Effects of some food juices and additives on some physicochemical, textural, color, microbiological and sensory properties of cemen paste. J. Food Process. Preserv. 2017, 41, 1–12. [Google Scholar] [CrossRef]
- Şapcı, H.; Yılmaz, F.; Vural, C.; Bahtiyari, M.İ.; Benli, H. Antimicrobial and antifungal activity of fabrics dyed with Viburnum opulus and onion skins. Int. J. Second. Metab. 2017, 4, 280–284. [Google Scholar] [CrossRef]
- Yılmaz, F.; Koçak, F.; Özgeriş, F.B.; Şapçı Selamoğlu, H.; Vural, C.; Benli, H.; Bahtiyari, M.İ. Use of Viburnum opulus L.(Caprifoliaceae) in dyeing and antibacterial finishing of cotton. J. Nat. Fibers 2020, 17, 1081–1088. [Google Scholar] [CrossRef]
- Altun, M.L.; Özbek, H.; Çitoǧlu, G.S.; Yilmaz, B.S.; Bayram, I.; CengIz, N. Hepatoprotective and hypoglycemic activities of Viburnum opulus L. Turk. J. Pharm. Sci. 2010, 7, 35–48. [Google Scholar]
- Peng, S.G.; Pang, Y.L.; Zhu, Q.; Kang, J.H.; Liu, M.X.; Wang, Z.; Huang, Y. Chlorogenic acid functions as a novel agonist of PPARγ2 during the differentiation of mouse 3T3-L1 preadipocytes. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycémie control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 1–54. [Google Scholar] [CrossRef]
- Ekbatan, S.S.; Iskandar, M.M.; Sleno, L.; Sabally, K.; Khairallah, J.; Prakash, S.; Kubow, S. Absorption and metabolism of phenolics from digests of polyphenol-rich potato extracts using the Caco-2/HepG2 co-culture system. Foods 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.A.; Sulaiman, A.A.; Alhaddad, H.; Alhadidi, Q. Natural polyphenols: Influence on membrane transporters. J. Intercult. Ethnopharmacol. 2016, 5, 97–104. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid-membrane interactions: Involvement of flavonoid-metal complexes in raft signaling. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of sweet cherry polyphenols on enhanced osteoclastogenesis associated with childhood obesity. Front. Immunol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Hubert, P.A.; Lee, S.G.; Lee, S.K.; Chun, O.K. Dietary polyphenols, berries, and age-related bone loss: A review based on human, animal, and cell studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Sun, T.; Niu, J.G.; He, Z.Q.; Liu, Y.; Wang, F. Amentoflavone protects hippocampal neurons: Anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res. 2015, 10, 1125–1133. [Google Scholar]
- Ovodova, R.G.; Golovchenko, V.V.; Popov, S.V.; Shashkov, A.S.; Ovodov, S.Y. The isolation, preliminary structural studies, and physiological activity of water-soluble polysaccharides from the squeezed berries of the snowball tree Viburnum opulus. Russ. J. Bioorganic Chem. 2000, 26, 54–59. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Garbuz, S.A.; Malfanov, I.L.; Ptitsyn, L.R. Screening of Russian medicinal and edible plant extracts for angiotensin I-converting enzyme (ACE I) inhibitory activity. Russ. J. Bioorganic Chem. 2013, 39, 743–749. [Google Scholar] [CrossRef]
- Tuglu, D.; Yılmaz, E.; Yuvanc, E.; Erguder, I.; Kisa, U.; Bal, F.; Batislam, E. Viburnum opulus: Could it be a new alternative, such as lemon juice, to pharmacological therapy in hypocitraturic stone patients? Arch. Ital. Urol. Androl. 2014, 86, 297–299. [Google Scholar] [CrossRef] [Green Version]
- Kızılay, F.; Ülker, V.; Çelik, O.; Özdemir, T.; Çakmak, Ö.; Can, E.; Nazlı, O. The evaluation of the effectiveness of gilaburu (Viburnum opulus L.) extract in the medical expulsive treatment of distal ureteral stones. Turk. J. Urol. 2019, 45, S63–S69. [Google Scholar] [CrossRef]
- Dag, Z.; Akturk, G.; Filik, L. Acute pancreatitis induced by Viburnum opulus juice in a patient with urolithiasis. Asian Pac. J. Trop. Biomed. 2014, 4, 791. [Google Scholar] [CrossRef] [Green Version]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef]
- Saltan, G.; Süntar, I.; Ozbilgin, S.; Ilhan, M.; Demirel, M.A.; Oz, B.E.; Keleş, H.; Akkol, E.K. Viburnum opulus L.: A remedy for the treatment of endometriosis demonstrated by rat model of surgically-induced endometriosis. J. Ethnopharmacol. 2016, 193, 450–455. [Google Scholar] [CrossRef]
- Ulger, H.; Ertekin, T.; Karaca, O.; Canoz, O.; Nisari, M.; Unur, E.; Elmalı, F. Influence of gilaburu (Viburnum opulus) juice on 1,2-dimethylhydrazine (DMH)-induced colon cancer. Toxicol. Ind. Health 2013, 29, 824–829. [Google Scholar] [CrossRef]
- Ceylan, D.; Aksoy, A.; Ertekin, T.; Yay, A.H.; Nisari, M.; Karatoprak, G.Ş.; Ülger, H. The effects of gilaburu (Viburnum opulus) juice on experimentally induced Ehrlich ascites tumor in mice. J. Cancer Res. Ther. 2018, 14, 314–320. [Google Scholar]
- Zakłos-Szyda, M.; Pawlik, N. The influence of Viburnum opulus polyphenolic compounds on metabolic activity and migration of HeLa and MCF cells. Acta Innov. 2019, 33, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Sarıözkan, S.; Türk, G.; Eken, A.; Bayram, L.Ç.; Baldemir, A.; Doğan, G. Gilaburu (Viburnum opulus L.) fruit extract alleviates testis and sperm damages induced by taxane-based chemotherapeutics. Biomed. Pharmacother. 2017, 95, 1284–1294. [Google Scholar] [CrossRef]




| Organic Acid | Content (mg/100 g) | References |
|---|---|---|
| Malic acid | 578–2090 | [9] |
| Citric acid | 270–1630 | |
| Quinic acid | 52–346 | |
| Shikimic acid | 0–5 | |
| Malic acid | 1083 | [50] |
| Oxalic acid | 81 | |
| Citric acid | 39 | |
| Tartaric acid | 120–144 | [40] |
| Malic acid | 110–135 | |
| Fumaric acid | 10–18 | |
| Succinic acid | 4–6 | |
| Tartaric acid | 113–135 | [41] |
| Malic acid | 108–122 | |
| Fumaric acid | 9–18 | |
| Succinic acid | 3–7 | |
| Tartaric acid | 124–141 | [2] |
| Malic acid | 121–137 | |
| Fumaric acid | 15–16 | |
| Succinic acid | 5 | |
| Acetic acid | 2.6–3.2 |
| Part of Plant | Total Phenolics | Total Flavonoids | Total Anthocyanins | Total Proanthocyanidins | Reference |
|---|---|---|---|---|---|
| Fruit | 753–1460 a | n.a. | 23–45 f | n.a. | [33] |
| 403–733 a | n.a. | n.a. | 201–528 h | [9] | |
| 325.4 a | n.a. | 65 | n.a. | [5] | |
| 680–831 a | 314–489 c | n.a. | n.a. | [8] | |
| 621–987 a | 202–318 c | 15–51 g | n.a. | [40] | |
| 703–911 a | 187–267 c | 6–48 g | n.a. | [41] | |
| 668–856 a | n.a. | 27–53 g | n.a. | [43] | |
| 549–1105 a | n.a. | n.a. | n.a. | [44] | |
| 3730 b | 2010 d | n.a. | 520 i | [34] | |
| 4450 b | n.a. | n.a. | n.a. | [61] | |
| Seed | 1231.0 a | 1032 e | - | n.a. | [48] |
| Bark | 3980 b | 2250 d | - | 1030 i | [34] |
| Flower | 3510 b | 1670 d | - | 220 i | [34] |
| Phenolic Compound | Content (mg/100 g of Fresh Weight) | References | |
|---|---|---|---|
| Hydroxybenzoic acids | Gallic acid | 10.82–11.82 | [2] |
| Vanillic acid | 2.25–2.21 | [2] | |
| Syringic acid | 2.47–3.03 | [2] | |
| Hydroxycinnamic acids | Chlorogenic acid | 2.95–4.43 | [2] |
| 250–580 | [9] | ||
| 203.7 | [4] | ||
| Caffeic acid | 2.63–3.84 | [2] | |
| Coumaric acid | 1.40–1.73 | [2] | |
| Ferulic acid | 4.50–5.59 | [2] | |
| Protocatechuic acid | 2.09–3.63 | [2] | |
| Flavanols | Catechin | 28.50–35.20 | [2] |
| 29.04 | [4] | ||
| Epicatechin | 2.69 | [4] | |
| Procyanidin | 8.28 | [4] | |
| Flavonols | Quercetin | 0.61–0.83 | [2] |
| Quercetin 3-rutinoside | 1.78–2.21 | [2] | |
| 0.9–5.2 | [9] | ||
| 3.69 | [4] | ||
| Quercetin 3-sambubioside | 2.0–10.6 | [9] | |
| Quercetin 3-glucoside | 0.1–3.4 | [9] | |
| 2.61 | [4] | ||
| Quercetin 3-rhamnoside | 0.3–2.0 | [9] | |
| 1.01 | [4] | ||
| Quercetin 3-xyloside | 0.34 | [4] | |
| Quercetin 3-arabinoside | 4.16 | [4] | |
| Isorhamnetin 3-sambubioside | 0.3–3.0 | [9] | |
| Isorhamnetin 3-rutinoside | 0–0.6 | [9] | |
| Anthocyanins | Cyanidin + 2 hexose + pentose | 0–0.36 | [9] |
| Cyanidin + 2 pentose + hexose | 0–2.11 | [9] | |
| Cyanidin + 2 hexose | 0–0.50 | [9] | |
| Cyanidin + 2 pentose + hexose | 0–0.13 | [9] | |
| Cyanidin 3-arabinosyl-glucoside | 0–10.42 | [9] | |
| Unidentified cyanidin glycoside | 0–0.71 | [9] | |
| Cyanidin 3-xylosyl-rutinoside | 0–19.87 | [9] | |
| Cyanidin 3-sambubioside | 0–0.87 | [9] | |
| Cyanidin 3-glucoside | 0.12–12.06 | [9] | |
| 7.23 | [4] | ||
| Cyanidin 3-rutinoside | 0–6.39 | [9] | |
| 0.99 | [4] |
| Phenolic Compound | Content (mg/100 g) | References | |
|---|---|---|---|
| Hydroxycinnamic acids | Chlorogenic acid | 803.9 n.r. | [24] [1] |
| Chlorogenic acid dimer | n.r. | [1] | |
| Neochlorogenic acid | 0.7 | [24] | |
| Cryptochlorogenic acid | 0.4 | [24] | |
| Caffeoylquinic acid derivatives | 12.4 | [24] | |
| Coumaroyl-quinic acid | n.r. | [1] | |
| Flavanols | (+)-Catechin | 65.7 | [24] |
| n.r. | [1] | ||
| (-)-Epicatechin | 13.5 | [24] | |
| n.r. | [1] | ||
| (Epi)catechin derivatives | 18.3 | [24] | |
| Gallocatechin gallate | 3.1 | [24] | |
| Procyanidin B1 | 75.9 | [24] | |
| Procyanidin B2 | 19.9 | [24] | |
| n.r. | [1] | ||
| Procyanidin dimers | 4.0 | [24] | |
| Proanthocyanidin dimer monoglycoside | n.r. | [1] | |
| Procyanidin C1 | 3.3 | [24] | |
| Procyanidin trimers | 17.2 | [24] | |
| n.r. | [1] | ||
| Flavonols | Quercetin 3-vicianoside | 2.0 | [24] |
| Quercetin 3-rutinoside | 1.6 | [24] | |
| n.r. | [1] | ||
| Quercetin 3-rhamnoside | 0.7 | [24] | |
| Quercetin hexose | n.r. | [1] | |
| Quercetin deoxyhexose | n.r. | [1] | |
| Quercetin hexose + pentose | n.r. | [1] | |
| Anthocyanins | Cyanidin 3-sambubioside | 9.3 | [24] |
| Cyanidin 3-glucoside | 13.9 | [24] | |
| Cyanidin 3-rutinoside | 6.8 | [24] |
| Type of Assay | Part of the Plant | Extraction Solvent | Antioxidant Activity Parameter | Reference |
|---|---|---|---|---|
| DPPH● radical scavenging activity | Fruit | 80% methanol with 2% HCl (v/v) | 8.55–9.79 mg ascorbic acid equivalents/g of fruit FW | [8] |
| Fruit | water | IC50 = 0.057 mg of extract/mL | [82] | |
| Fruit | 80% acetone with 0.5% acetic acid (v/v) | IC50 = 0.057 mg of extract/mL | [32] | |
| Fruit | 96% methanol | 103.59 mg BHT equivalents/g of extract | [73] | |
| Fruit | water | 96.74 mg BHT equivalents/g of extract | [73] | |
| Fruit flesh | methanol | EC50 = 24.56 mg/mg DPPH● | [59] | |
| Fruit pomace | acetone | 121.8 mg Trolox equivalents/g of extract DW | [74] | |
| Fruit pomace | ethanol | 106.9 mg Trolox equivalents/g of extract DW | [74] | |
| Fruit pomace | water | 267.4 mg Trolox equivalents/g of extract DW | [74] | |
| Leaf | water | IC50 = 47.18 μg of extract/mL | [82] | |
| Branch | water | IC50 = 0.014 mg of extract/mL | [82] | |
| Seed | methanol | EC50 = 2.35 mg/mg DPPH● | [59] | |
| ABTS+● cation radical scavenging activity | Fruit | 80% methanol with 2% HCl (v/v) | 9.10–11.12 mg ascorbic acid equivalents/g of fruit FW | [8] |
| Fruit | 96% ethanol with 0.2% HCl (v/v) | 7.05 mg ascorbic acid equivalents/g of frozen fruit | [37] | |
| Fruit | 50% ethanol | 643 μmol Trolox equivalents/g of fruit DW | [74] | |
| Fruit | 70% ethanol | 265.7 μmol Trolox equivalents/g of fruit DW | [76] | |
| Fruit | water | 380.36 μmol Trolox equivalents/g of extract | [34] | |
| Fruit juice | - | 31.95–42.38 μmol Trolox equivalents/g of juice | [53] | |
| Fruit pomace | acetone | 376.8 μmol Trolox equivalents/g of extract DW | [74] | |
| Fruit pomace | ethanol | 331.0 μmol Trolox equivalents/g of extract DW | [74] | |
| Fruit pomace | water | 602.3 μmol Trolox equivalents/g of extract DW | [74] | |
| Fruit juice | - | 70.17 μmol Trolox equivalents/mL of juice | [76] | |
| Bark | 70% ethanol | 402.1 μmol Trolox equivalents/g of bark DW | [34] | |
| Bark | water | 1792.16 μmol Trolox equivalents/g of extract | [76] | |
| Flower | 70% ethanol | 161.8 μmol Trolox equivalents/g of flower DW | [34] | |
| Flower | water | 475.95 μmol Trolox equivalents/g of extract | ||
| FRAP—ferric reducing antioxidant power | Fruit | 70% acetone with 0.5% acetic acid | 21.02–34.90 μmol Trolox equivalents/g of fruit FW | [40] |
| Fruit | (v/v) | 23.41–32.70 μmol Trolox equivalents/g of fruit FW | [41] | |
| Fruit | 70% acetone with 0.5% acetic acid | 28.76–36.41 μmol Trolox equivalents/g of fruit FW | [43] | |
| Fruit | (v/v) | 192.9 μmol Trolox equivalents/g of fruit DW | [76] | |
| Fruit | 70% acetone with 0.5% acetic acid | 311.34 μmol Trolox equivalents/g of extract | [34] | |
| Fruit | (v/v) | 0.46 mmol FeSO4 equivalents/g of extract | [73] | |
| Fruit | 70% ethanol | 0.41 mmol FeSO4 equivalents/g of extract | [73] | |
| Fruit juice | water | 64.35 μmol Trolox equivalents/mL of juice | [74] | |
| Fruit juice | 96% methanol | 32.33–35.94 μmol Trolox equivalents/g of juice | [53] | |
| Bark | water | 234.7 μmol Trolox equivalents/g of fruit DW | [76] | |
| Bark | - | 1160.30 μmol Trolox equivalents/g of extract | [34] | |
| Flower | - | 136.5 μmol Trolox equivalents/g of fruit DW | [76] | |
| Flower | 70% ethanol | 463.91 μmol Trolox equivalents/g of extract | [34] | |
| CUPRAC—cupric reducing antioxidant capacity | Fruit | 96% methanol | 208.87 mg ascorbic acid equivalents/g of extract | [73] |
| Fruit | water | 156.49 mg ascorbic acid equivalents/g of extract | [73] | |
| ORAC—oxygen radical absorbance capacity | Fruit | 70% ethanol | 109.3 μmol Trolox equivalents/g of fruit DW | [76] |
| Fruit | 50% ethanol | 1277 μmol Trolox equivalents/g of fruit DW | [74] | |
| Fruit juice | - | 127.37–143.25 μmol Trolox equivalents/g of juice | [53] | |
| Fruit pomace | acetone | 5750 μmol Trolox equivalents/g of extract DW | [74] | |
| Fruit pomace | ethanol | 5320 μmol Trolox equivalents/g of extract DW | [74] | |
| Fruit pomace | water | 8720 μmol Trolox equivalents/g of extract DW | [74] | |
| Bark | 70% ethanol | 1081.7 μmol Trolox equivalents/g of bark DW | [75] | |
| Bark | water | 4386.19 μmol Trolox equivalents/g of extract | [34] | |
| Flower | 70% ethanol | 618.2 μmol Trolox equivalents/g of flower DW | [76] | |
| Flower | water | 4283.41 μmol Trolox equivalents/g of extract | [34] | |
| Nitric oxide scavenging activity | Fruit | 50 mM phosphate buffer, pH 7.0 | 21.89–25.44% of inhibition for 25% fruit extract | [8] |
| Superoxide anion scavenging activity | Fruit | 50 mM phosphate buffer, pH 7.0 | 25.13–28.50% of inhibition for 25% fruit extract | [8] |
| Fruit | 70% ethanol | 897.7 μmol Trolox equivalents/g of fruit DW | [76] | |
| Leaf | water | IC50 = 8.3 mg of extract/mL | [63] | |
| Branch | water | IC50 = 3.7 mg of extract/mL | [63] | |
| Bark | 70% ethanol | 1154.4 μmol Trolox equivalents/g of bark DW | [76] | |
| Flower | 70% ethanol | 911.3 μmol Trolox equivalents/g of flower DW | [76] | |
| Hydroxyl radical scavenging activity | Fruit | 50 mM phosphate buffer, pH 7.0 | 19.40–23.98% of inhibition for 25% fruit extract | [8] |
| Fruit | 70% ethanol | 100.5 μmol Trolox equivalents/g of fruit DW | [76] | |
| Bark | 70% ethanol | 59.1 μmol Trolox equivalents/g of bark DW | [76] | |
| Bark | water | 191.42 μmol Trolox equivalents/g of extract | [34] | |
| Flower | 70% ethanol | 82.3 μmol Trolox equivalents/g of flower DW | [76] | |
| Flower | water | 188.12 μmol Trolox equivalents/g of extract | [34] | |
| DMPD scavenging activity | Fruit | 96% methanol | 52.55 mg Trolox equivalents/g of extract | [73] |
| Fruit | water | 55.00 mg Trolox equivalents/g of extract | [73] | |
| Ferrous ion chelating capacity | Fruit | ethyl acetate | 60.5% of inhibition at 2 mg of extract/mL | [75] |
| Fruit | methanol | 15.0% of inhibition at 2 mg of extract/mL | [75] | |
| Fruit | water | 11.0% of inhibition at 2 mg of extract/mL | ||
| Leaf | ethyl acetate | 21.0% of inhibition at 2 mg of extract/mL | ||
| Leaf | methanol | 13.0% of inhibition at 2 mg of extract/mL | ||
| Leaf | water | 0% of inhibition at 2 mg of extract/mL | ||
| Branch | ethyl acetate | 6.5% of inhibition at 2 mg of extract/mL | ||
| Branch | methanol | 23.0% of inhibition at 2 mg of extract/mL | ||
| Branch | water | 22.5% of inhibition at 2 mg of extract/mL | ||
| Lipid peroxidation in the rat liver homogenate | Fruit | 50 mM phosphate buffer, pH 7.0 | 11.20–13.90% of inhibition for 25% fruit extract | [8] |
| Total antioxidant capacity | Fruit | 96% methanol | 56.89 mg ascorbic acid equivalents/g of extract | [73] |
| Fruit | water | 49.07 mg ascorbic acid equivalents/g of extract | [73] | |
| CVA—cathode voltammetry | Bark | 30% ethanol | K = 101.71 mL/g of extract | [46] |
| 70% ethanol | K = 181.52 mL/g of extract | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajszczak, D.; Zakłos-Szyda, M.; Podsędek, A. Viburnum opulus L.—A Review of Phytochemistry and Biological Effects. Nutrients 2020, 12, 3398. https://doi.org/10.3390/nu12113398
Kajszczak D, Zakłos-Szyda M, Podsędek A. Viburnum opulus L.—A Review of Phytochemistry and Biological Effects. Nutrients. 2020; 12(11):3398. https://doi.org/10.3390/nu12113398
Chicago/Turabian StyleKajszczak, Dominika, Małgorzata Zakłos-Szyda, and Anna Podsędek. 2020. "Viburnum opulus L.—A Review of Phytochemistry and Biological Effects" Nutrients 12, no. 11: 3398. https://doi.org/10.3390/nu12113398
APA StyleKajszczak, D., Zakłos-Szyda, M., & Podsędek, A. (2020). Viburnum opulus L.—A Review of Phytochemistry and Biological Effects. Nutrients, 12(11), 3398. https://doi.org/10.3390/nu12113398