Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
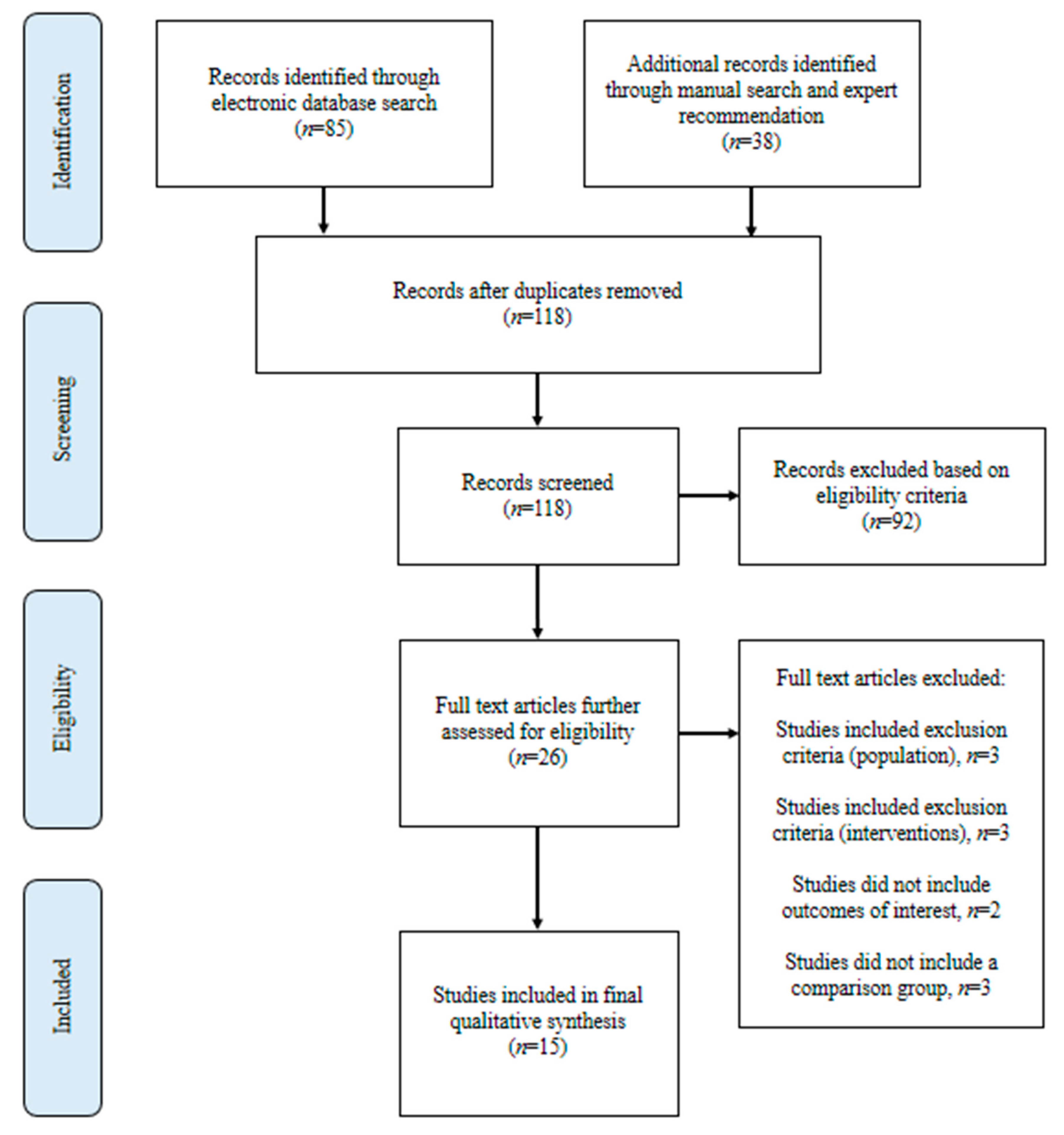
3. Results
3.1. Literature Search
3.2. Study Characteristics
4. Outcomes
4.1. Anthropometrics, Body Composition, and Nutritional Status
4.2. Nutritional Intake
4.3. Functional Status
4.4. QoL
4.5. Response to Cancer Treatment
4.6. Complications and Unplanned Hospitalizations
4.7. Mortality and Survival
4.8. Timing of Nutrition Intervention
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Search Strategy |
|---|
| (Ti,ab((cancer[*1] OR neoplas* OR tumor[*1] OR tumour[*1] OR *carcinoma* OR *sarcoma* OR oncolog*) n/10 (treatment* OR treat* OR chemothera* OR chemo-therap* OR radiat*) n/10 (((diet* OR food OR nutrition*) n/3 (assessment[*1] OR (care n/3 plan) OR plans OR planning OR plan OR counsel* OR council OR diagnos* OR consult* OR (discharg* n/1 education*) OR education* OR evaluation[*1] OR index OR indices OR intervention[*1] OR monitoring[*1] OR “ONS” OR screening[*1] OR therap* OR treatment[*1] OR supplement* OR enteral* OR parental*)) OR “oral nutritional supplement*” OR (parenteral n/1 fluid*) OR ((enteral* OR parenteral* OR intravenous* OR enteric* OR intragastric* OR intestinal* OR intraintestinal* OR tube* OR force*) n/3 (feed OR feeding* OR feeds OR alimentation* OR hyperalimentation*)))) AND ti,ab(((nutrition OR early OR late) n/3 intervention*) OR (standard* n/3 care*)) AND ti,ab,mesh,emb,su,if,au,low,loc,cnt,rg(Australia OR Australian OR Austria OR Austrian OR Belgium OR Belgian OR Bulgaria OR Bulgarian OR Canada OR Canadian OR Croatia OR Croatian OR Cyprus OR Cyprian OR “Czech Republic” OR Czech OR Denmark OR Danish OR Estonia OR Estonian OR “EU-15” OR Finland OR Finnish OR France OR French OR Germany OR German OR Greece OR Greek OR Hungary OR Hungarian OR Iceland OR Icelandic OR Ireland OR Irish OR Italy OR Italian OR Japan OR Japanese OR Latvia OR Latvian OR Lithuania OR Lithuanian OR Luxembourg OR Luxembourgian OR Malta OR Maltese OR Netherlands OR Dutch OR “New Zealand” OR Norway OR Norwegian OR Poland OR Polish OR Portugal OR Portuguese OR Romania OR Romanian OR Slovakia OR Slovakian OR Slovenia OR Slovene OR Spain OR Spanish OR Sweden OR Swedish OR Switzerland OR Swiss OR “United Kingdom” OR British OR “UK” OR “U.K.” OR “United States” OR “US” OR “U.S.” OR “USA” OR “U.S.A.” OR American) AND YR(>=2010) AND la(English)) NOT (dog OR dogs OR cat OR cats OR canine* OR feline* OR porcine* OR pig OR pigs OR piglet* OR cow OR cows OR mice OR mouse OR rat OR rats OR cattle OR veterinar* OR monkey* OR rabbit* OR horse OR horses OR equine* OR zoo OR zoological OR zoology OR zoos OR (animal* n/3 stud*) OR bovine OR geese OR goose OR estuary* OR rodent* OR fish OR fishes OR marine OR dolphin* OR chick OR chicks OR goat OR goats OR ecolog* OR bird* OR sheep* OR zebrafish* OR hamster* OR bat OR bats OR (alternat* n/3 (medicat* OR medicine*)) OR pregnan* OR lactate* OR lactating* OR child* OR adolescen* OR infant* OR infancy OR newborn* OR neonat* OR baby OR babies OR preschool* OR teenage* OR toddler* OR juvenile* OR boy OR boys OR girl* OR pediatric* OR paediatric* OR (“pre” p/0 school*) OR suckling* OR youth OR schoolchild* OR preadolescen* OR ((vitamin* OR mineral*) n/3 supplement*) OR exercis* OR (physical n/3 activ*) OR ((behavior* OR mental*) n/3 health*) OR hospice[*1] OR (palliative n/1 (care OR nursing))) |
References
- The American Cancer Society. The Burden of Cancer. 2019. Available online: https://canceratlas.cancer.org/wp-content/uploads/2019/09/CA3_TheBurdenofCancer.pdf (accessed on 31 August 2020).
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef]
- Attar, A.; Malka, D.; Sabaté, J.M.; Bonnetain, F.; LeComte, T.; Aparicio, T.; Locher, C.; Laharie, D.; Ezenfis, J.; Taïeb, J. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: An AGEO prospective cross-sectional multicenter study. Nutr. Cancer 2012, 64, 535–542. [Google Scholar] [CrossRef]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.I.S.D.S.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Silva, F.R.; de Oliveira, M.G.; Souza, A.S.; Figueroa, J.N.; Santos, C.S. Factors associated with malnutrition in hospitalized cancer patients: A croos-sectional study. Nutr. J. 2015, 14, 123. [Google Scholar] [CrossRef]
- Maasberg, S.; Knappe-Drzikova, B.; Vonderbeck, D.; Jann, H.; Weylandt, K.-H.; Grieser, C.; Pascher, A.; Schefold, J.C.; Pavel, M.; Wiedenmann, B.; et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology 2017, 104, 11–25. [Google Scholar] [CrossRef]
- Planas, M.; Álvarez-Hernández, J.; León-Sanz, M.; Celaya-Pérez, S.; Araujo, K.; de Lorenzo, A.G. Prevalence of hospital malnutrition in cancer patients: A sub-analysis of the PREDyCES® study. Support. Care Cancer 2016, 24, 429–435. [Google Scholar] [CrossRef]
- Platek, M.E.; Popp, J.V.; Possinger, C.S.; Denysschen, C.A.; Horvath, P.; Brown, J.K. Comparison of the prevalence of malnutrition diagnosis in head and neck, gastrointestinal, and lung cancer patients by 3 classification methods. Cancer Nurs. 2011, 34, 410–416. [Google Scholar] [CrossRef]
- Williams, D.G.A.; Ohnuma, T.; Krishnamoorthy, V.; Raghunathan, K.; Sulo, S.; Cassady, B.A.; Hegazi, R.; Wischmeyer, P.E. Postoperative Utilization of Oral Nutritional Supplements in Surgical Patients in US Hospitals. J. Parenter. Enter. Nutr. 2020. [Google Scholar] [CrossRef]
- Wie, G.A.; Cho, Y.A.; Kim, S.Y.; Kim, S.M.; Bae, J.M.; Joung, H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition 2010, 26, 263–268. [Google Scholar] [CrossRef]
- Sesterhenn, A.M.; Szalay, A.; Zimmermann, A.P.; Werner, J.A.; Barth, P.J.; Wiegand, S. Significance of autopsy in patients with head and neck cancer. Laryngo-Rhino-Otologie 2012, 91, 375–380. [Google Scholar]
- Kim, D.H. Nutritional issues in patients with cancer. Intest. Res. 2019, 17, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297.e247–310.e247. [Google Scholar] [CrossRef]
- August, D.A.; Huhmann, M.B. ASPEN clinical guidelines: Nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. J. Parenter. Enter. Nutr. 2009, 33, 472–500. [Google Scholar] [CrossRef]
- Arends, J.J.; Bachmann, P.P.; Baracos, V.V.; Barthelemy, N.N.; Bertz, H.H.; Bozzetti, F.; Fearon, K.C.; Hütterer, E.E.; Isenring, E.E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Sauer, A.C.; Li, J.; Partridge, J.; Sulo, S. Assessing the impact of nutrition interventions on health and nutrition outcomes of community-dwelling adults: A systematic review. Nutr. Diet. Suppl. 2018, 10, 45–57. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef]
- Elia, M.; Van Bokhorst-de van der Schueren, M.A.; Garvey, J.; Goedhart, A.; Lundholm, K.; Nitenberg, G.; Stratton, R. Enteral (oral or tube administration) nutritional support and eicosapentaenoic acid in patients with cancer: A systematic review. Int. J. Oncol. 2006, 28, 5–23. [Google Scholar] [CrossRef][Green Version]
- Blackwood, H.A.; Hall, C.C.; Balstad, T.R.; Solheim, T.S.; Fallon, M.; Haraldsdottir, E.; Laird, B.J. A systematic review examining nutrition support interventions in patients with incurable cancer. Support. Care Cancer 2020, 28, 1877–1889. [Google Scholar] [CrossRef]
- de van der Schueren, M.A.E.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.C.; Leong, L.P.; Lim, S.L. Nutrition intervention approaches to reduce malnutrition in oncology patients: A systematic review. Support. Care Cancer 2016, 24, 469–480. [Google Scholar] [CrossRef]
- Rinninella, E.; Fagotti, A.; Cintoni, M.; Raoul, P.; Scaletta, G.; Quagliozzi, L.; Miggiano, G.A.D.; Scambia, G.; Gasbarrini, A.; Mele, M.C. Nutritional Interventions to Improve Clinical Outcomes in Ovarian Cancer: A Systematic Review of Randomized Controlled Trials. Nutrients 2019, 11, 1404. [Google Scholar] [CrossRef]
- Kiss, N.K.; Krishnasamy, M.; Isenring, E.A. The effect of nutrition intervention in lung cancer patients undergoing chemotherapy and/or radiotherapy: A systematic review. Nutr. Cancer 2014, 66, 47–56. [Google Scholar] [CrossRef]
- Caccialanza, R.; Pedrazzoli, P.; Cereda, E.; Gavazzi, C.; Pinto, C.; Paccagnella, A.; Beretta, G.D.; Nardi, M.; Laviano, A.; Zagonel, V. Nutritional Support in Cancer Patients: A Position Paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J. Cancer 2016, 7, 131–135. [Google Scholar] [CrossRef]
- Aapro, M.; Arends, J.; Bozzetti, F.; Fearon, K.; Grunberg, S.M.; Herrstedt, J.; Hopkinson, J.; Jacquelin-Ravel, N.; Jatoi, A.; Kaasa, S.; et al. Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Ann. Oncol. 2014, 25, 1492–1499. [Google Scholar] [CrossRef]
- Arends, J.J.; Baracos, V.V.; Bertz, H.H.; Bozzetti, F.; Calder, P.P.; Deutz, N.; Erickson, N.N.; Laviano, A.A.; Lisanti, M.M.; Lobo, D.N.D.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wei, J.; Ji, R.; Wang, B.; Xu, X.; Xin, Y.; Jiang, X. Effect of Early Nutrition Intervention on Advanced Nasopharyngeal Carcinoma Patients Receiving Chemoradiotherapy. J. Cancer 2019, 10, 3650–3656. [Google Scholar] [CrossRef] [PubMed]
- Paccagnella, A.; Morello, M.; Da Mosto, M.C.; Baruffi, C.; Marcon, M.L.; Gava, A.; Baggio, V.; Lamon, S.; Babare, R.; Rosti, G.; et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support. Care Cancer 2010, 18, 837–845. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.-F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J.; et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized controlled trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef]
- Cereda, E.; Cappello, S.; Colombo, S.; Klersy, C.; Imarisio, I.; Turri, A.; Caraccia, M.; Borioli, V.; Monaco, T.; Benazzo, M.; et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother. Oncol. 2018, 126, 81–88. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.-M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef]
- Poulsen, G.M.; Pedersen, L.L.; Østerlind, K.; Bæksgaard, L.; Andersen, J.R. Randomized trial of the effects of individual nutritional counseling in cancer patients. Clin. Nutr. 2014, 33, 749–753. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Camilo, M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: Long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 2012, 96, 1346–1353. [Google Scholar] [CrossRef]
- Roca-Rodríguez, M.M.; García-Almeida, J.M.; Lupiañez-Pérez, Y.; Rico, J.M.; Toledo, M.; Alcaide-Torres, J.; Cardona, F.; Medina, J.A.; Tinahones, F.J. Effect of a specific supplement enriched with n-3 polyunsaturated fatty acids on markers of inflammation, oxidative stress and metabolic status of ear, nose and throat cancer patients. Oncol. Rep. 2014, 31, 405–414. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De La Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef]
- Shirai, Y.; Okugawa, Y.; Hishida, A.; Ogawa, A.; Okamoto, K.; Shintani, M.; Morimoto, Y.; Nishikawa, R.; Yokoe, T.; Tanaka, K.; et al. Fish oil-enriched nutrition combined with systemic chemotherapy for gastrointestinal cancer patients with cancer cachexia. Sci. Rep. 2017, 7, 4826. [Google Scholar] [CrossRef] [PubMed]
- Trabal, J.; Leyes, P.; Forga, M.; Maurel, J. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutr. Hosp. 2010, 25, 736–740. [Google Scholar]
- van der Meij, B.S.; Langius, J.A.E.; Smit, E.F.; Spreeuwenberg, M.D.; Von Blomberg, B.M.E.; Heijboer, A.C.; Paul, M.A.; Van Leeuwen, P.A.M. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J. Nutr. 2010, 140, 1774–1780. [Google Scholar] [CrossRef]
- van der Meij, B.S.; Langius, J.A.; Spreeuwenberg, M.D.; Slootmaker, S.M.; Paul, M.A.; Smit, E.F.; Van Leeuwen, P.A.M. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: An RCT. Eur. J. Clin. Nutr. 2012, 66, 399–404. [Google Scholar] [CrossRef]
- Tanaka, N.; Takeda, K.; Kawasaki, Y.; Yamane, K.; Teruya, Y.; Kodani, M.; Igishi, T.; Yamasaki, A. Early Intensive Nutrition Intervention with Dietary Counseling and Oral Nutrition Supplement Prevents Weight Loss in Patients with Advanced Lung Cancer Receiving Chemotherapy: A Clinical Prospective Study. Yonago Acta Med. 2018, 61, 204–212. [Google Scholar] [CrossRef]
- van den Berg, M.G.; Rasmussen-Conrad, E.L.; Wei, K.H.; Lintz-Luidens, H.; Kaanders, J.H.; Merkx, M.A. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br. J. Nutr. 2010, 104, 872–877. [Google Scholar] [CrossRef]
- Murphy, R.A.; Yeung, E.; Mazurak, V.C.; Mourtzakis, M. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br. J. Nutr. 2011, 105, 1469–1473. [Google Scholar] [CrossRef]
- Dewey, A.; Baughan, C.; Dean, T.; Higgins, B.; Johnson, I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. 2007, 2007, Cd004597. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.; Trottenberg, P.; Elsner, F.; Stiel, S.; Haugen, D.F.; Kaasa, S.; Radbruch, L. A systematic review on the role of fish oil for the treatment of cachexia in advanced cancer: An EPCRC cachexia guidelines project. Palliat Med. 2012, 26, 294–304. [Google Scholar] [CrossRef]
- Mazzotta, P.; Jeney, C.M. Anorexia-cachexia syndrome: A systematic review of the role of dietary polyunsaturated Fatty acids in the management of symptoms, survival, and quality of life. J. Pain Symptom Manag. 2009, 37, 1069–1077. [Google Scholar] [CrossRef]
- Colomer, R.; Moreno-Nogueira, J.M.; García-Luna, P.P.; García-Peris, P.; García-De-Lorenzo, A.; Zarazaga, A.; Quecedo, L.; Del Llano, J.; Usán, L.; Casimiro, C. N-3 fatty acids, cancer and cachexia: A systematic review of the literature. Br. J. Nutr. 2007, 97, 823–831. [Google Scholar] [CrossRef]
- de Aguiar Pastore Silva, J.; Emilia de Souza Fabre, M.; Waitzberg, D.L. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clin. Nutr. 2015, 34, 359–366. [Google Scholar] [CrossRef]
- Halpern, M.T.; Yabroff, K.R. Prevalence of outpatient cancer treatment in the United States: Estimates from the Medical Panel Expenditures Survey (MEPS). Cancer Investig. 2008, 26, 647–651. [Google Scholar] [CrossRef]
- Trujillo, E.B.C.K.; Dixon, S.W.; Hill, E.B.; Braun, A.; Lipinski, E.; Platek, M.E.; Vergo, M.T.; Spees, C. Inadequate Nutrition Coverage in Outpatient Cancer Centers: Results of a National Survey. J. Oncol. 2019, 2019, 7462940. [Google Scholar] [CrossRef]
- Congress.gov. H.R.6971—Medical Nutrition Therapy Act of 2020. 2020. Available online: https://www.congress.gov/bill/116th-congress/house-bill/6971?q=%7B%22search%22%3A%5B%22H.R.+6971%22%5D%7D&s=1&r=1 (accessed on 31 August 2020).
- Arensberg, M.; Richards, J.; Benjamin, J.; Kerr, K.; Hegazi, R. Opportunities for Quality Improvement Programs (QIPs) in the Nutrition Support of Patients with Cancer. Healthcare 2020, 8, 227. [Google Scholar] [CrossRef]
- Arensberg, M.B.; Sulo, S.; Drawert, S. Addressing Malnutrition in Cancer Care with Nutrition-Focused Quality Improvement Programs (QIPs) that Support Value-based Payment in the United States. J. Clin. Nutr. Food Sci. 2020, 3, 48–55. [Google Scholar]
| String | Terms |
|---|---|
| Cancer | Cancer, neoplasm, tumor, oncology, carcinoma, sarcoma |
| Treatment | Treatment, chemotherapy, radiation |
| Nutrition | Nutrition, food, diet |
| Intervention | Assessment, care plan, plan, planning, counsel, consult, diagnosis, education, evaluation, index, intervention, monitoring, screening, therapy, treatment, oral nutrition supplement (ONS), enteral, parenteral, intravenous, enteric, intragastric, intestinal, intraintestinal, tube, feeding, feeds |
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Any setting (within last 10 years) | Animal studies |
| >18 years of age | <18 years of age | |
| Diagnosed with cancer | No cancer diagnosis | |
| Receiving or planning to receive active treatment for cancer diagnosis (unless receiving surgery only) | Not receiving or no plans to receive active treatment for cancer diagnosisOnly receiving surgery as a cancer treatment | |
| Any nutritional status (well nourished, malnourished, or at-risk of malnutrition) | Pregnant or lactating females | |
| Studies published within the last 10 years (January 2010 or later) | Studies published before January 2010 | |
| Intervention | Specified nutrition interventions (singly or in combination) for malnourished patients or those at-risk of malnutrition: - Oral nutritional supplements (ONS) - Enteral nutrition - Parenteral nutrition - Dietary counseling/dietary advice - Formalized nutrition discharge education - ONS coupons and literature on ONS-tailored nutritional care plans at discharge - Nutrition education, post-discharge phone calls - Home visits by registered dietitian nutritionist (RDN) | Nutrition interventions to prevent weight gain Non-commercially available or home-prepared ONS Any of the following (alone or in combination with any other interventions, including the specified nutrition interventions): - Vitamin or mineral supplementation or both - Exercise/physical activity - Behavioral/mental health interventions - Alternative medicine |
| Comparison | Specified nutrition intervention(s) vs. no nutrition intervention(s) | No comparison/control group |
| Specified nutrition intervention(s) vs. other specified nutrition intervention(s) | ||
| Specified nutrition intervention(s) vs. standard of care | ||
| Early specified nutrition intervention(s) vs. late intervention(s) | ||
| Duration of Intervention | >1 week | <1 week |
| Outcome | Anthropometrics - Body weight - Body mass index (BMI) | Outcomes other than the specified health and nutrition outcomes |
| Body composition - Muscle mass - Fat mass | ||
| Nutritional status - Results of malnutrition screening/assesment - Energy intake - Protein intake | ||
| Functional status - Muscle strength - Handgrip strength - Physical activity | ||
| Quality of Life (QoL) | ||
| Hospital readmissions/unplanned hospitalizations | ||
| Response to treatment - Treatment tolerance - Treatment interruption - Full completion of treatment protocol | ||
| Emergency Department (ED) visits | ||
| Complications | ||
| Morbidity | ||
| Mortality | ||
| Healthcare costs |
| Study, Year | Design, Sample Size | Population, Country | Cancer Dx, Cancer Tx | Nutrition Status | Nutrition Intervention(s) | Early or Late Intervention(s), Duration | Outcomes of Nutrition Intervention(s) |
|---|---|---|---|---|---|---|---|
| Bourdel-Marchasson, 2014 [30] | RCT 341 | Older adults (70+ years) France | Lymphoma or carcinoma CT | At risk for malnutrition | Counseling + ONS if needed (intervention group) vs. standard care | Early 3-6 months | ↑ Energy intake *ᶲ No difference in weight loss ᶲ No difference in hospitalizations ᶲ No difference in response to cancer treatment ᶲ ↓ Complications (infections) *ᶲ No difference in mortality ᶲ |
| Cereda, 2018 [31] | RCT 159 | Any adults (18+ years) Italy | Head and neck cancer RT or RT plus systemic tx | Any nutrition status | Counseling + ONS (intervention group) vs. counseling only | Early Throughout RT, at 1 month and 3-month follow-up visits after end of RT | ↓ Weight loss *ᶲ ↑ Energy intake *ᶲ ↑ Protein intake *ᶲ ↑ Handgrip strength ᶲ ↑ QoL *ᶲ ↑ Treatment tolerance ᶲ |
| Kim, 2019 [32] | RCT 34 | Any adults (20+ years) Korea | Pancreatic and bile duct cancers CT | Patients with a BMI > 30 kg/m2 were excluded | Counseling + ONS (intervention group) vs. counseling only | Early for 61.8% of participants (initiated study participation in first cycle of CT) 8 weeks | ↑ Nutrition status (measured by PG-SGA) *ᶣ No difference in weight loss ᶲᶣ No difference in skeletal muscle mass ᶲᶣ No difference in FFM ᶲᶣ↑ Fat mass *ᶲᶣ ↑ Energy intake *ᶣ ↑ Protein intake *ᶣ ↑ QoL (fatigue symptoms) ᶣ |
| Meng, 2019 [28] | Prospective cohort study 78 | Adults 18–70 years China | Nasopharyngeal carcinoma CRT | Any nutrition status | Early nutrition intervention (intervention group) vs. late nutrition intervention Intervention for both groups was ONS + EN or PN if needed | Early for participants in the nutrition intervention group; late nutrition intervention group did not receive nutrition support until nutrition-related side effects from treatment developed Nutrition intervention lasted until 3 months after CRT | ↓ Weight loss *ᶲ ↓ BMI change *ᶲ ↑ Treatment tolerance (lower incidence of mucositis) *ᶲ ↓ Treatment breaks (>3 days) *ᶲ ↓ Treatment delays for toxicity *ᶲ ↓ Unplanned hospitalizations *ᶲ |
| Paccagnella, 2010 [29] | Retrospective cohort study 66 | Any adults (18+ years) Italy | Head and neck cancer CRT | Any nutrition status | Individualized counseling + ONS/EN if needed (intervention group) vs. standard care | Early Nutrition intervention lasted until 6 months after CRT | ↓ Weight loss *ᶲ ↑ Treatment tolerance *ᶲ ↓ Treatment delays *ᶲ ↓ Unplanned hospitalizations *ᶲ |
| Poulsen, 2014 [33] | RCT 61 | Any adults (18+ years) Denmark | GI gynecologic, or esophageal cancer CT and/or RT | Any nutrition status | Counseling + ONS-EPA if desired (intervention group) vs. standard care | Early Between 5–12 weeks, follow-up performed 3 months after treatment | ↓ Weight loss *ᶲ ↑ Energy intake *ᶲ ↑ Protein intake *ᶲ No difference in change in FFM ᶲ No difference in change in fat mass ᶲ No difference in QoL ᶲ |
| Ravasco, 2012 [34] | RCT 111 | Any adults (18+ years) Portugal | Colorectal cancer RT followed by surgery + CT | Any nutrition status | Nutrition counseling and education using regular foods (group 1) vs. ONS + usual diet (group 2) vs. usual diet only (group 3) | Early 1.5 months | ↑ Nutrition status (measured by PG-SGA; group 1) *ᶲ ↑ BMI (group 1) *ᶲ↑ Energy intake (group 1) *ᶲ ↑ Protein intake (group 1) *ᶲ ↑ Treatment tolerance (measured by late radiotherapy toxicity; group 1) *ᶲ ↑ QoL (group 1) *ᶲ ↓ Mortality (group 1) *ᶲ Results are from long-term follow-up (range = 4.9–8.2 years) and compared to groups 2 and 3 |
| Roca-Rodriguez, 2014 [35] | RCT 26 | Adults 18–80 years Spain | ENT cancer RT, and CT if needed | Any nutrition status | ONS-EPA (intervention group) vs. isocaloric ONS | Late (14 days after start of RT) 76 days | ↓ BMI decline ᶲ |
| Sanchez-Lara, 2014 [36] | RCT 92 | Adults 18–80 years Mexico | Non-small cell lung cancer CT | Any nutrition status | Diet plus ONS-EPA (intervention group) vs. isocaloric diet only Extra calories from ONS were subtracted from intervention group diet so both groups received an isocaloric diet | Early 8+ weeks | ↓ Weight loss *ᶲ ↑ LBM *ᶲ ↑ Energy intake *ᶲᶣ ↑ Protein intake *ᶲᶣ ↑ QoL (increased global health status; ᶣ improved fatigue and loss of appetite ᶣᶲ) * ↑ Treatment tolerance (less nausea, vomiting, and neuropathy) *ᶲ No difference in tumor response rate ᶲ No difference in overall survival ᶲ↑ PFS ᶲ |
| Shirai, 2017 [37] | Retrospective cohort study 179 | Adults 18–80 years Japan | GI cancer CT | >5% of pre-illness body weight | ONS-EPA (intervention group) vs. no additional nutritional treatment/placebo | Unknown 6 months | ↑ Skeletal muscle mass and LBM *ᶣ No difference in overall survival ᶲ ↑ Treatment tolerance for patients with mGPS of 1 or 2 who received ONS-EPA ᶲ ↑ Prognosis for patients with mGPS of 1 or 2 who received ONS-EPA *ᶲ |
| Trabal, 2010 [38] | RCT 13 | Any adults (18+ years) Spain | Colorectal cancer CT | Excluded patients with severe malnutrition (based on PG-SGA or BMI < 16.5 or >30 kg/m2 Patients withdrawn if they developed malnutrition during the study | Counseling + ONS-EPA (intervention group) vs. counseling only | Early 12 weeks | ↑ Weight *ᶲ ↑ Energy intake ᶲ ↑ Protein intake ᶲ ↑ QoL (improved fatigue, pain, physical function, social function) ᶲ ↑ Treatment tolerance ᶲ |
| van der Meij, 2010 [39] | RCT 40 | Adults 18–80 years The Netherlands | Non-small cell lung cancer CRT | Any nutrition status | ONS-EPA (intervention group) vs. isocaloric ONS | Early 5 weeks | ↓ Weight loss *ᶲ ↓ Loss of FFM *ᶲ No difference in energy intake ᶲ No difference in protein intake ᶲ |
| van der Meij, 2012 [40] | RCT 40 | Adults 18–80 years The Netherlands | Non-small cell lung cancer CRT | Any nutrition status | ONS-EPA (intervention group) vs. isocaloric ONS | Early 5 weeks | ↑ QoL (global health status, physical function, cognitive function, social function) *ᶲ ↑ Physical activity (during weeks 3 and 5) *ᶲ No difference in handgrip strength ᶲ ↑ Treatment tolerance (lower incidence of nausea/vomiting) *ᶲ No difference in treatment delays/dose reduction ᶲ No difference in unplanned hospital admissions ᶲ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, J.; Arensberg, M.B.; Thomas, S.; Kerr, K.W.; Hegazi, R.; Bastasch, M. Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review. Nutrients 2020, 12, 3403. https://doi.org/10.3390/nu12113403
Richards J, Arensberg MB, Thomas S, Kerr KW, Hegazi R, Bastasch M. Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review. Nutrients. 2020; 12(11):3403. https://doi.org/10.3390/nu12113403
Chicago/Turabian StyleRichards, Julie, Mary Beth Arensberg, Sara Thomas, Kirk W. Kerr, Refaat Hegazi, and Michael Bastasch. 2020. "Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review" Nutrients 12, no. 11: 3403. https://doi.org/10.3390/nu12113403
APA StyleRichards, J., Arensberg, M. B., Thomas, S., Kerr, K. W., Hegazi, R., & Bastasch, M. (2020). Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review. Nutrients, 12(11), 3403. https://doi.org/10.3390/nu12113403






