Food Addiction and Psychosocial Adversity: Biological Embedding, Contextual Factors, and Public Health Implications
Abstract
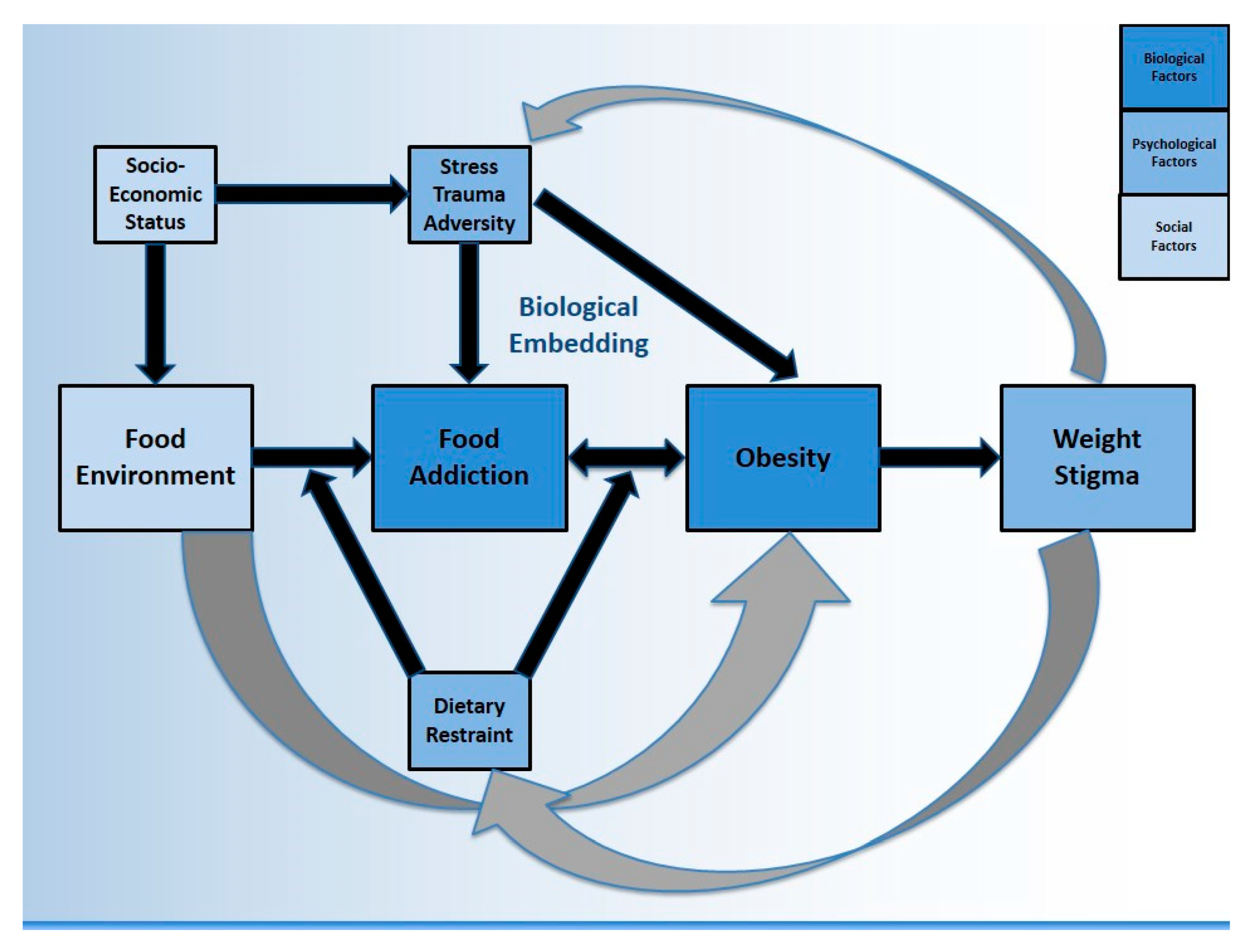
:1. Background
1.1. The Biopsychosocial Model & Other Foundational Theories
1.2. Food Addiction & Eating Disorders
2. Food Addiction Neuroscience & Social Context
2.1. Food Environment as a Driver of Food Addiction & Obesity
2.2. Socioeconomic Status
3. Biological Embedding of Stress, Trauma, & Adversity
3.1. Epigenetic Mechanisms of Biological Embedding
4. Stress & Obesity
4.1. Stress & Addictions
5. Psychological Correlates of Food Addiction & Obesity
5.1. Dietary Restraint
5.2. Weight Stigma
6. What Does It Mean for Public Health?
6.1. Food Policy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Popkin, B.M. Nutritional Patterns and Transitions. Popul. Dev. Rev. 1993, 19, 138–157. [Google Scholar] [CrossRef]
- Hall, K.D. Did the Food Environment Cause the Obesity Epidemic? Obesity 2018, 26, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A. How can drug addiction help us understand obesity? Nat. Neurosci. 2005, 8, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Bohon, C.; Small, D. Relation Between Obesity and Blunted Striatal Response to Food Is Moderated by TaqIA A1 Allele. Science 2008, 322, 449–452. [Google Scholar] [CrossRef] [Green Version]
- Saules, K.K.; Carr, M.M.; Herb, K.M. Overeating, Overweight, and Substance Use: What Is the Connection? Curr. Addict. Rep. 2018, 5, 232–242. [Google Scholar] [CrossRef]
- Gold, M.S. From bedside to bench and back again: A 30-year saga. Physiol. Behav. 2011, 104, 157–161. [Google Scholar] [CrossRef]
- Avena, N.M.; Hoebel, B.G. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience 2003, 122, 17–20. [Google Scholar] [CrossRef]
- Hoebel, B.G.; Avena, N.M.; Rada, P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr. Opin. Pharmacol. 2007, 7, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Hoebel, B.G. Brain neurotransmitters in food and drug reward. Am. J. Clin. Nutr. 1985, 42, 1133–1150. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Avena, N.M.; Hoebel, B.G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005, 134, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience 2008, 156, 865–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiss, D.A.; Avena, N.; Rada, P. Sugar Addiction: From Evolution to Revolution. Front. Psychiatry 2018, 9, 545. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kwok, S.; Mayes, L.C.; Potenza, M.N.; Rutherford, H.J.; Strathearn, L. Early adverse experience and substance addiction: Dopamine, oxytocin, and glucocorticoid pathways. Ann. N. Y. Acad. Sci. 2017, 1394, 74–91. [Google Scholar] [CrossRef] [Green Version]
- Peña, C.; Neugut, Y.D.; Calarco, C.A.; Champagne, F.A. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur. J. Neurosci. 2014, 39, 946–956. [Google Scholar] [CrossRef]
- Wei, N.-L.; Quan, Z.-F.; Zhao, T.; Yu, X.-D.; Xie, Q.; Zeng, J.; Ma, F.-K.; Wang, F.; Tang, Q.-S.; Wu, H.; et al. Chronic stress increases susceptibility to food addiction by increasing the levels of DR2 and MOR in the nucleus accumbens. Neuropsychiatr. Dis. Treat. 2019, 15, 1211–1229. [Google Scholar] [CrossRef]
- MacKay, J.C.; Kent, P.; James, J.S.; Cayer, C.; Merali, Z. Ability of palatable food consumption to buffer against the short- and long-term behavioral consequences of social defeat exposure during juvenility in rats. Physiol. Behav. 2017, 177, 113–121. [Google Scholar] [CrossRef]
- Grimm, J.W.; Hyde, J.; Glueck, E.; North, K.; Ginder, D.; Jiganti, K.; Hopkins, M.; Sauter, F.; MacDougall, D.; Hovander, D. Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats. Appetite 2019, 139, 50–58. [Google Scholar] [CrossRef]
- Cohen, M.; Jing, D.; Yang, R.R.; Tottenham, N.; Lee, F.S.; Casey, B. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. USA 2013, 110, 18274–18278. [Google Scholar] [CrossRef] [Green Version]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Levis, S.C.; Bentzley, B.S.; Molet, J.; Bolton, J.L.; Perrone, C.R.; Baram, T.Z.; Mahler, S.V. On the early life origins of vulnerability to opioid addiction. Mol. Psychiatr. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al’Absi, M. The influence of stress and early life adversity on addiction: Psychobiological mechanisms of risk and resilience. Int. Rev. Neurobiol. 2020, 152, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Wiss, D.A.; Brewerton, T.D. Adverse Childhood Experiences and Adult Obesity: A Systematic Review of Plausible Mechanisms and Meta-Analysis of Cross-Sectional Studies. Physiol. Behav. 2020, 223, 112964. [Google Scholar] [CrossRef]
- Hemmingsson, E. Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Curr. Obes. Rep. 2018, 7, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Cammack, A.L.; Gazmararian, J.A.; Suglia, S.F. History of child maltreatment and excessive dietary and screen time behaviors in young adults: Results from a nationally representative study. Prev. Med. 2020, 139, 106176. [Google Scholar] [CrossRef]
- Nunes-Neto, P.R.; Köhler, C.A.; Schuch, F.B.; Solmi, M.; Quevedo, J.; Maes, M.; Murru, A.; Vieta, E.; McIntyre, R.S.; McElroy, S.L.; et al. Food addiction: Prevalence, psychopathological correlates and associations with quality of life in a large sample. J. Psychiatr. Res. 2018, 96, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Bloomfield, M.A.; McCutcheon, R.A.; Kempton, M.; Freeman, T.P.; Howes, O. The effects of psychosocial stress on dopaminergic function and the acute stress response. Elife 2019, 8, e46797. [Google Scholar] [CrossRef]
- Dillon, D.G.; Holmes, A.J.; Birk, J.L.; Brooks, N.; Lyons-Ruth, K.; Pizzagalli, D.A. Childhood Adversity Is Associated with Left Basal Ganglia Dysfunction During Reward Anticipation in Adulthood. Biol. Psychiat. 2009, 66, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Osadchiy, V.; Mayer, E.A.; Bhatt, R.; Labus, J.S.; Gao, L.; Kilpatrick, L.A.; Liu, C.; Tillisch, K.; Naliboff, B.; Chang, L.; et al. History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obes. Sci. Pract. 2019, 5, 416–436. [Google Scholar] [CrossRef] [Green Version]
- Heilig, M.; Epstein, D.H.; Nader, M.A.; Shaham, Y. Time to connect: Bringing social context into addiction neuroscience. Nat. Rev. Neurosci. 2016, 17, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Baughman, K.; Logue, E.; Sutton, K.; Capers, C.; Jarjoura, D.; Smucker, W. Biopsychosocial characteristics of overweight and obese primary care patients: Do psychosocial and behavior factors mediate sociodemographic effects? Prev. Med. 2003, 37, 129–137. [Google Scholar] [CrossRef]
- Qu, Y.; Galván, A.; Fuligni, A.J.; Telzer, E.H. A Biopsychosocial Approach to Examine Mexican American Adolescents’ Academic Achievement and Substance Use. RSF Russell Sage Found. J. Sci. 2018, 4, 84–97. [Google Scholar] [CrossRef]
- Krieger, N. Theories for social epidemiology in the 21st century: An ecosocial perspective. Int. J. Epidemiol. 2001, 30, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.H., Jr. The Life Course as Developmental Theory. Child. Dev. 1998, 69, 1–12. [Google Scholar] [CrossRef]
- Hertzman, C. The biological embedding of early experience and its effects on health in adulthood. Ann. N. Y. Acad. Sci. 1999, 896, 85–95. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Kuh, D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Int. J. Epidemiol. 2002, 31, 285–293. [Google Scholar] [CrossRef]
- Lerner, R.M. The Place of Learning within the Human Development System: A Developmental Contextual Perspective. Hum. Dev. 1995, 38, 361–366. [Google Scholar] [CrossRef]
- Overton, W.F.; Lerner, R.M. Fundamental Concepts and Methods in Developmental Science: A Relational Perspective. Res. Hum. Dev. 2014, 11, 63–73. [Google Scholar] [CrossRef]
- Lerner, R.M.; Johnson, S.K.; Buckingham, M.H. Relational Developmental Systems-Based Theories and the Study of Children and Families: Lerner and Spanier (1978) Revisited. J. Fam. Theor. Rev. 2015, 7, 83–104. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, E.M.; Grilo, C.M.; Gearhardt, A.N. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin. Psychol. Rev. 2016, 44, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Racine, S.E.; Burt, A.S.; Iacono, W.G.; McGue, M.; Klump, K.L. Dietary restraint moderates genetic risk for binge eating. J. Abnorm. Psychol. 2011, 120, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiss, D.; Brewerton, T. Separating the Signal from the Noise: How Psychiatric Diagnoses Can Help Discern Food Addiction from Dietary Restraint. Nutrients 2020, 12, 2937. [Google Scholar] [CrossRef]
- Meule, A.; von Rezori, V.; Blechert, J. Food Addiction and Bulimia Nervosa. Eur. Eat. Disord. Rev. 2014, 22, 331–337. [Google Scholar] [CrossRef]
- Meule, A.; Gearhardt, A.N. Ten Years of the Yale Food Addiction Scale: A Review of Version 2.0. Curr. Addict. Rep. 2019, 6, 218–228. [Google Scholar] [CrossRef]
- Fernandez-Aranda, F.; Karwautz, A.; Treasure, J. Food addiction: A transdiagnostic construct of increasing interest. Eur. Eat. Disord. Rev. 2018, 26, 536–540. [Google Scholar] [CrossRef]
- Romero, X.; Agüera, Z.; Granero, R.; Sánchez, I.; Riesco, N.; Jiménez-Murcia, S.; Gisbert-Rodriguez, M.; Sánchez-González, J.; Casalé, G.; Baenas, I.; et al. Is food addiction a predictor of treatment outcome among patients with eating disorder? Eur Eat. Disord Rev. 2019, 27, 700–711. [Google Scholar] [CrossRef]
- Brewerton, T.D. Food addiction as a proxy for eating disorder and obesity severity, trauma history, PTSD symptoms, and comorbidity. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2017, 22, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.M.; Flint, A.J.; Field, A.E.; Austin, B.S.; Rich-Edwards, J.W. Abuse victimization in childhood or adolescence and risk of food addiction in adult women. Obesity 2013, 21, E775–E781. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.M.; Flint, A.J.; Roberts, A.L.; Agnew-Blais, J.; Koenen, K.C.; Rich-Edwards, J.W. Posttraumatic Stress Disorder Symptoms and Food Addiction in Women by Timing and Type of Trauma Exposure. JAMA Psychiatry 2014, 71, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, C.; Innamorati, M.; Lamis, D.A.; Farina, B.; Pompili, M.; Contardi, A.; Fabbricatore, M. Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child. Abus. Negl. 2016, 58, 180–190. [Google Scholar] [CrossRef]
- Holgerson, A.A.; Clark, M.M.; Ames, G.E.; Collazo-Clavell, M.L.; Kellogg, T.A.; Graszer, K.M.; Kalsy, S.A.; Grothe, K. Association of Adverse Childhood Experiences and Food Addiction to Bariatric Surgery Completion and Weight Loss Outcome. Obes. Surg. 2018, 28, 3386–3392. [Google Scholar] [CrossRef]
- Warren, C.S.; Lindsay, A.R.; White, E.K.; Claudat, K.; Velasquez, S.C. Weight-related concerns related to drug use for women in substance abuse treatment: Prevalence and relationships with eating pathology. J. Subst. Abus. Treat. 2013, 44, 494–501. [Google Scholar] [CrossRef]
- Wiss, D.A.; Waterhous, T.S. Nutrition Therapy for Eating Disorders, Substance Use Disorders, and Addictions. In Eating Disorders, Addictions and Substance Use Disorders, Research, Clinical and Treatment Perspectives; Springer: Berlin/Heidelberg, Germany, 2014; pp. 509–532. ISBN 9783642453779. [Google Scholar]
- Khalil, R.B.; Sleilaty, G.; Richa, S.; Seneque, M.; Iceta, S.; Rodgers, R.; Alacreu-Crespo, A.; Maimoun, L.; Lefebvre, P.; Renard, E.; et al. The Impact of Retrospective Childhood Maltreatment on Eating Disorders as Mediated by Food Addiction: A Cross-Sectional Study. Nutrients 2020, 12, 2969. [Google Scholar] [CrossRef]
- Cassin, S.E.; Sijercic, I.; Montemarano, V. Psychosocial Interventions for Food Addiction: A Systematic Review. Curr. Addict. Rep. 2020, 7, 9–19. [Google Scholar] [CrossRef]
- Hebebrand, J.; Albayrak, Ö.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menzies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Ifland, J.R.; Preuss, H.G.; Marcus, M.T.; Rourke, K.M.; Taylor, W.C.; Burau, K.; Jacobs, W.S.; Kadish, W.; Manso, G. Refined food addiction: A classic substance use disorder. Med. Hypotheses 2009, 72, 518–526. [Google Scholar] [CrossRef]
- Ifland, J.; Preuss, H.G.; Marcus, M.T.; Rourke, K.M.; Taylor, W.; Wright, T.H. Clearing the Confusion around Processed Food Addiction. J. Am. Coll. Nutr. 2015, 34, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.J. Is it time to consider the “food use disorder? ” Appetite 2017, 115, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, R.J.; Francis, H.M.; Attuquayefio, T.; Gupta, D.; Yeomans, M.R.; Oaten, M.J.; Davidson, T. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R. Soc. Open Sci. 2020, 7, 191338. [Google Scholar] [CrossRef] [Green Version]
- Dohle, S.; Diel, K.; Hofmann, W. Executive functions and the self-regulation of eating behavior: A review. Appetite 2018, 124, 4–9. [Google Scholar] [CrossRef]
- Steward, T.; Mestre-Bach, G.; Vintró-Alcaraz, C.; Lozano-Madrid, M.; Agüera, Z.; Fernández-Formoso, J.A.; Granero, R.; Jiménez-Murcia, S.; Vilarrasa, N.; García-Ruiz-de-Gordejuela, A.; et al. Food addiction and impaired executive functions in women with obesity. Eur. Eat. Disord. Rev. 2018, 26, 574–584. [Google Scholar] [CrossRef]
- Chao, S.-H.; Liao, Y.-T.; Chen, V.C.-H.; Li, C.-J.; McIntyre, R.S.; Lee, Y.; Weng, J.-C. Correlation between brain circuit segregation and obesity. Behav. Brain Res. 2018, 337, 218–227. [Google Scholar] [CrossRef]
- Ronan, L.; Alexander-Bloch, A.; Fletcher, P.C. Childhood Obesity, Cortical Structure, and Executive Function in Healthy Children. Cereb. Cortex 2019, 30, 2519–2528. [Google Scholar] [CrossRef]
- Laurent, J.S.; Watts, R.; Adise, S.; Allgaier, N.; Chaarani, B.; Garavan, H.; Potter, A.; Mackey, S. Associations Among Body Mass Index, Cortical Thickness, and Executive Function in Children. JAMA Pediatr. 2020, 174, 170. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Wu, Q.; Liu, Y.; Guo, C. Obesity is associated with poor working memory in women, not men: Findings from a nationally representative dataset of U.S. adults. Eat. Behav. 2019, 35, 101338. [Google Scholar] [CrossRef]
- Sattler, K.M.; Deane, F.P.; Tapsell, L.; Kelly, P.J. Gender differences in the relationship of weight-based stigmatisation with motivation to exercise and physical activity in overweight individuals. Health Psychol. Open 2018, 5, 205510291875969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardabassi, V.; Tomasetto, C. Weight status or weight stigma? Obesity stereotypes—Not excess weight—Reduce working memory in school-aged children. J. Exp. Child. Psychol. 2019, 189, 104706. [Google Scholar] [CrossRef] [PubMed]
- Kiyici, S.; Koca, N.; Sigirli, D.; Aslan, B.B.; Guclu, M.; Kisakol, G. Food Addiction Correlates with Psychosocial Functioning More Than Metabolic Parameters in Patients with Obesity. Metab. Syndr. Relat. Disord. 2020, 18, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, K.R.; Weinstock, J.; McGrath, A.B. The Clinical Significance of Food Addiction. J. Addict. Med. 2020, 14, e153–e159. [Google Scholar] [CrossRef] [PubMed]
- VanderBroek-Stice, L.; Stojek, M.K.; Beach, S.; vanDellen, M.R.; MacKillop, J. Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite 2017, 112, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Wolz, I.; Granero, R.; Fernández-Aranda, F. A comprehensive model of food addiction in patients with binge-eating symptomatology: The essential role of negative urgency. Compr. Psychiatry 2017, 74, 118–124. [Google Scholar] [CrossRef]
- Meule, A.; de Zwaan, M.; Müller, A. Attentional and motor impulsivity interactively predict ‘food addiction’ in obese individuals. Compr. Psychiatry 2017, 72, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, A.L.; Gardiner, E.; Loxton, N.J. Investigating the relationship between reward sensitivity, impulsivity, and food addiction: A systematic review. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2020, 28, 368–384. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Guerrieri, R.; Havermans, R.; Roefs, A.; Jansen, A. The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. Int. J. Obes. 2009, 33, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Trifilieff, P.; Martinez, D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 2014, 76, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Savage, S.W.; Zald, D.H.; Cowan, R.L.; Volkow, N.D.; Marks-Shulman, P.A.; Kessler, R.M.; Abumrad, N.N.; Dunn, J.P. Regulation of novelty seeking by midbrain dopamine D2/D3 signaling and ghrelin is altered in obesity. Obesity 2014, 22, 1452–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kekic, M.; McClelland, J.; Bartholdy, S.; Chamali, R.; Campbell, I.C.; Schmidt, U. Bad Things Come to Those Who Do Not Wait: Temporal Discounting Is Associated with Compulsive Overeating, Eating Disorder Psychopathology and Food Addiction. Front. Psychiatry 2019, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.; Vainik, U.; Garcia-Garcia, I.; Dagher, A. Overlapping Neural Endophenotypes in Addiction and Obesity. Front. Endocrinol. 2017, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moloney, G.M.; van Oeffelen, W.E.P.A.; Ryan, F.J.; van de Wouw, M.; Cowan, C.; Claesson, M.J.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Differential gene expression in the mesocorticolimbic system of innately high- and low-impulsive rats. Behav. Brain Res. 2019, 364, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C.; MacKillop, J.; Weafer, J.; Hernandez, K.M.; Gao, J.; Palmer, A.A.; Wit, H. de Genetic analysis of impulsive personality traits: Examination of a priori candidates and genome-wide variation. Psychiatry Res. 2018, 259, 398–404. [Google Scholar] [CrossRef]
- Morland, K.; Wing, S.; Roux, A.; Poole, C. Neighborhood characteristics associated with the location of food stores and food service places. Am. J. Prev. Med. 2002, 22, 23–29. [Google Scholar] [CrossRef]
- Black, J.L.; Macinko, J. Neighborhoods and obesity. Nutr. Rev. 2008, 66, 2–20. [Google Scholar] [CrossRef]
- Carroll-Scott, A.; Gilstad-Hayden, K.; Rosenthal, L.; Peters, S.M.; McCaslin, C.; Joyce, R.; Ickovics, J.R. Disentangling neighborhood contextual associations with child body mass index, diet, and physical activity: The role of built, socioeconomic, and social environments. Soc. Sci. Med. 2013, 95, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Cubbin, C.; Oh, S. A systematic review of neighbourhood economic context on child obesity and obesity-related behaviours. Obes. Rev. 2019, 20, 420–431. [Google Scholar] [CrossRef]
- Claassen, M.; Klein, O.; Bratanova, B.; Claes, N.; Corneille, O. A systematic review of psychosocial explanations for the relationship between socioeconomic status and body mass index. Appetite 2019, 132, 208–221. [Google Scholar] [CrossRef] [Green Version]
- Darmon, N.; Drewnowski, A. Does social class predict diet quality? Am. J. Clin. Nutr. 2008, 87, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleary, S.A.; Ettienne, R. The relationship between food parenting practices, parental diet and their adolescents’ diet. Appetite 2019, 135, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.S.; Ge, B.; Petroski, G.; Kruse, R.L.; McElroy, J.A.; Koopman, R.J. Socioeconomic Status and Other Factors Associated with Childhood Obesity. J. Am. Board Fam. Med. 2018, 31, 514–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, S.; Santaularia, N.; Berge, J.; Larson, N.; Neumark-Sztainer, D. Is the childhood home food environment a confounder of the association between child maltreatment exposure and adult body mass index? Prev. Med. 2018, 110, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.B.; Shashank, A.; Gasevic, D.; Schuurman, N.; Poirier, P.; Teo, K.; Rangarajan, S.; Yusuf, S.; Lear, S.A. The Local Food Environment and Obesity: Evidence from Three Cities. Obesity 2019, 28, 40–45. [Google Scholar] [CrossRef]
- Cooksey-Stowers, K.; Schwartz, M.B.; Brownell, K.D. Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States. Int. J. Environ. Res. Public Health 2017, 14, 1366. [Google Scholar] [CrossRef] [Green Version]
- Drewnowski, A.; Buszkiewicz, J.; Aggarwal, A.; Rose, C.; Gupta, S.; Bradshaw, A. Obesity and the Built Environment: A Reappraisal. Obesity 2019, 28, 22–30. [Google Scholar] [CrossRef]
- Chirinos, D.A.; Garcini, L.M.; Seiler, A.; Murdock, K.W.; Peek, K.; Stowe, R.P.; Fagundes, C. Psychological and Biological Pathways Linking Perceived Neighborhood Characteristics and Body Mass Index. Ann. Behav. Med. 2018, 53, 827–838. [Google Scholar] [CrossRef]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal Diagrams for Epidemiologic Research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef]
- Sobal, J.; Stunkard, A.J. Socioeconomic Status and Obesity: A Review of the Literature. Psychol. Bull. 1989, 105, 260–275. [Google Scholar] [CrossRef]
- McLaren, L. Socioeconomic Status and Obesity. Epidemiol Rev. 2007, 29, 29–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wilde, J.; Eilander, M.; Middelkoop, B. Effect of neighbourhood socioeconomic status on overweight and obesity in children 2–15 years of different ethnic groups. Eur. J. Public Health 2019, 29, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Creger, T.; Howard, V.; Judd, S.E.; Harrington, K.F.; Fontaine, K.R. Association of community food environment and obesity among US adults: A geographical information system analysis. J. Epidemiol. Community Health 2018, 73, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.A.; Rutten, L.J.; Wilson, P.M.; Kumar, S.; Phelan, S.; Jacobson, R.M.; Fan, C.; Agunwamba, A. The impact of positive contextual factors on the association between adverse family experiences and obesity in a National Survey of Children. Prev. Med. 2018, 116, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Twaits, A.; Alwan, N.A. The association between area-based deprivation and change in body-mass index over time in primary school children: A population-based cohort study in Hampshire, UK. Int. J. Obes. 2019, 44, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Ford, D.E. The Community and Public Well-being Model: A New Framework and Graduate Curriculum for Addressing Adverse Childhood Experiences. Acad. Pediatrics 2017, 17, S9–S11. [Google Scholar] [CrossRef] [Green Version]
- Metzler, M.; Merrick, M.T.; Klevens, J.; Ports, K.A.; Ford, D.C. Adverse childhood experiences and life opportunities: Shifting the narrative. Child. Youth Serv. Rev. 2017, 72, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Kristenson, M.; Eriksen, H.R.; Sluiter, J.K.; Starke, D.; Ursin, H. Psychobiological mechanisms of socioeconomic differences in health. Soc. Sci. Med. 2004, 58, 1511–1522. [Google Scholar] [CrossRef]
- Gunstad, J.; Paul, R.H.; Spitznagel, M.; Cohen, R.A.; Williams, L.M.; Kohn, M.; Gordon, E. Exposure to early life trauma is associated with adult obesity. Psychiatry Res. 2006, 142, 31–37. [Google Scholar] [CrossRef]
- Friedman, E.M.; Montez, J.K.; Sheehan, C.M.; Guenewald, T.L.; Seeman, T.E. Childhood Adversities and Adult Cardiometabolic Health: Does the Quantity, Timing, and Type of Adversity Matter? J. Aging Health 2015, 27, 1311–1338. [Google Scholar] [CrossRef]
- Mhamdi, S.E.; Lemieux, A.; Abroug, H.; Salah, A.B.; Bouanene, I.; Salem, K.B.; al’Absi, M. Childhood exposure to violence is associated with risk for mental disorders and adult’s weight status: A community-based study in Tunisia. J. Public Health 2018, 41, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.; Burchenal, C.A.; Haber, L.; Batten, K.; Phillips, E. Understanding and effectively addressing disparities in obesity: A systematic review of the psychological determinants of emotional eating behaviours among Black women. Obes. Rev. 2020, 21, e13010. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A. Stress and Obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doom, J.R.; Lumeng, J.C.; Sturza, J.; Kaciroti, N.; Vazquez, D.M.; Miller, A.L. Longitudinal associations between overweight/obesity and stress biology in low-income children. Int. J. Obes. 2019, 44, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Barker, D. The fetal and infant origins of adult disease. Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Stellar, E. Stress and the Individual: Mechanisms Leading to Disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress, Adaption, and Disease. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Seeman, T.; Singer, B.; Rowe, J.; Horwitz, R.; McEwen, B. Price of adaptation—Allostatic load and its health consequences. MacArthur studies of successful aging. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef]
- Ottino-González, J.; Jurado, M.A.; García-García, I.; Caldú, X.; Prats-Soteras, X.; Tor, E.; Sender-Palacios, M.J.; Garolera, M. Allostatic load and executive functions in overweight adults. Psychoneuroendocrinology 2019, 106, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Nusslock, R.; Miller, G.E. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol. Psychiatry 2016, 80, 23–32. [Google Scholar] [CrossRef]
- Hostinar, C.E.; Nusslock, R.; Miller, G.E. Future Directions in the Study of Early-Life Stress and Physical and Emotional Health: Implications of the Neuroimmune Network Hypothesis. J. Clin. Child. Adolesc. Psychol. 2018, 47, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.; Krey, L.; McEwen, B. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr. Rev. 1986, 7, 284–301. [Google Scholar] [CrossRef] [PubMed]
- Kaess, M.; Whittle, S.; O’Brien-Simpson, L.; Allen, N.B.; Simmons, J.G. Childhood maltreatment, pituitary volume and adolescent hypothalamic-pituitary-adrenal axis—Evidence for a maltreatment-related attenuation. Psychoneuroendocrinology 2018, 98, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.W. Differential Susceptibility of the Developing Brain to Contextual Adversity and Stress. Neuropsychopharmacology 2015, 41, 142–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnell, J.E.; Wood, S.K. Putative Inflammatory Sensitive Mechanisms Underlying Risk or Resilience to Social Stress. Front. Behav. Neurosci. 2018, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Rapuano, K.M.; Laurent, J.S.; Hagler, D.J.; Hatton, S.N.; Thompson, W.K.; Jernigan, T.L.; Dale, A.M.; Casey, B.J.; Watts, R. Nucleus accumbens cytoarchitecture predicts weight gain in children. Proc. Natl. Acad. Sci. USA 2020, 117, 26977–26984. [Google Scholar] [CrossRef] [PubMed]
- Calem, M.; Bromis, K.; McGuire, P.; Morgan, C.; Kempton, M.J. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017, 14, 471–479. [Google Scholar] [CrossRef]
- Elsey, J.; Coates, A.; Lacadie, C.M.; McCrory, E.J.; Sinha, R.; Mayes, L.C.; Potenza, M.N. Childhood Trauma and Neural Responses to Personalized Stress, Favorite-Food and Neutral-Relaxing Cues in Adolescents. Neuropsychopharmacology 2015, 40, 1580–1589. [Google Scholar] [CrossRef] [Green Version]
- Berens, A.E.; Jensen, S.K.; Nelson, C.A. Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Med. 2017, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Wiss, D.A.; Criscitelli, K.; Gold, M.; Avena, N. Preclinical evidence for the addiction potential of highly palatable foods: Current developments related to maternal influence. Appetite 2017, 115, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Entringer, S.; Moog, N.K.; Toepfer, P.; Fair, D.A.; Simhan, H.N.; Heim, C.M.; Wadhwa, P.D. Intergenerational Transmission of Maternal Childhood Maltreatment Exposure: Implications for Fetal Brain Development. J. Am. Acad. Child. Adolesc. Psychiatry 2017, 56, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Nieto, S.J.; Kosten, T.A. Who’s your daddy? Behavioral and epigenetic consequences of paternal drug exposure. Int. J. Dev. Neurosci. 2019, 78, 109–121. [Google Scholar] [CrossRef]
- Costa, D.L.; Yetter, N.; DeSomer, H. Intergenerational transmission of paternal trauma among US Civil War ex-POWs. Proc. Natl. Acad. Sci. USA 2018, 115, 11215–11220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demetriou, C.A.; Veldhoven, K.; Relton, C.; Stringhini, S.; Kyriacou, K.; Vineis, P. Biological embedding of early-life exposures and disease risk in humans: A role for DNA methylation. Eur. J. Clin. Investig. 2015, 45, 303–332. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.-E.; Turecki, G. DNA methylation and childhood maltreatment: From animal models to human studies. Neuroscience 2014, 264, 142–156. [Google Scholar] [CrossRef] [Green Version]
- McGowan, P.O.; Szyf, M. The epigenetics of social adversity in early life: Implications for mental health outcomes. Neurobiol. Dis. 2010, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Szyf, M. DNA Methylation, Behavior and Early Life Adversity. J. Genet. Genom. 2013, 40, 331–338. [Google Scholar] [CrossRef]
- Palma-Gudiel, H.; Córdova-Palomera, A.; Eixarch, E.; Deuschle, M.; Fañanás, L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: A meta-analysis. Epigenetics 2015, 10, 893–902. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.; Schneper, L.M.; Notterman, D.A. DNA methylation, early life environment, and health outcomes. Pediatric Res. 2015, 79, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Bakusic, J.; Schaufeli, W.; Claes, S.; Godderis, L. Stress, burnout and depression: A systematic review on DNA methylation mechanisms. J. Psychosom. Res. 2017, 92, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-N. Epigenetic control of the expression of opioid receptor genes. Epigenetics 2008, 3, 119–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belzeaux, R.; Lalanne, L.; Kieffer, B.L.; Lutz, P.-E. Focusing on the Opioid System for Addiction Biomarker Discovery. Trends Mol. Med. 2018, 24, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, C.; Cottone, P. The role of the opioid system in binge eating disorder. CNS Spectr. 2015, 20, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, M.; Bonaventura, M.; Vezzoli, V.; Zaplatic, E.; Massimini, M.; Mai, S.; Sartorio, A.; Scacchi, M.; Persani, L.; Maccarrone, M.; et al. Preclinical and Clinical Evidence for a Distinct Regulation of Mu Opioid and Type 1 Cannabinoid Receptor Genes Expression in Obesity. Front. Genet. 2019, 10, 523. [Google Scholar] [CrossRef]
- Lutz, P.-E.; Gross, J.A.; Dhir, S.K.; Maussion, G.; Yang, J.; Bramoulle, A.; Meaney, M.J.; Turecki, G. Epigenetic Regulation of the Kappa Opioid Receptor by Child Abuse. Biol. Psychiatry 2018, 84, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.M.; Nestler, E.J. Neuroepigenetics and addiction. Sci. Direct 2018, 148, 747–765. [Google Scholar] [CrossRef]
- Denhardt, D.T. Effect of stress on human biology: Epigenetics, adaptation, inheritance, and social significance. J. Cell. Physiol. 2018, 233, 1975–1984. [Google Scholar] [CrossRef]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress. Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Kader, F.; Ghai, M.; Maharaj, L. The effects of DNA methylation on human psychology. Behav Brain Res. 2018, 346, 47–65. [Google Scholar] [CrossRef]
- Candler, T.; Kühnen, P.; Prentice, A.M.; Silver, M. Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease. Front. Neuroendocrinol. 2019, 54, 100773. [Google Scholar] [CrossRef] [PubMed]
- Groleau, P.; Joober, R.; Israel, M.; Zeramdini, N.; DeGuzman, R.; Steiger, H. Methylation of the dopamine D2 receptor (DRD2) gene promoter in women with a bulimia-spectrum disorder: Associations with borderline personality disorder and exposure to childhood abuse. J. Psychiatr. Res. 2014, 48, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, D.J.; Ashenhurst, J.R.; Cervantes, C.M.; Groman, S.M.; James, A.S.; Pennington, Z.T. Dissecting impulsivity and its relationships to drug addictions. Ann. N. Y. Acad. Sci. 2014, 1327, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Berg, A.; Kawasawa, Y.; Bixler, E.O.; Fernandez-Mendoza, J.; Whitsel, E.A.; Liao, D. Association between DNA methylation in obesity-related genes and body mass index percentile in adolescents. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, F.K.; Huyvaert, M.; Ortalli, A.; Canouil, M.; Lecoeur, C.; Verbanck, M.; Lobbens, S.; Khamis, A.; Marselli, L.; Marchetti, P.; et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int. J. Obes. 2020, 44, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, A.J.; Project, M. Dopamine gene methylation patterns are associated with obesity markers and carbohydrate intake. Brain Behav. 2018, 8, e01017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y. Epigenetic Mechanisms Link Maternal Diets and Gut Microbiome to Obesity in the Offspring. Front. Genet. 2018, 9, 342. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Sánchez-Alcoholado, L.; Cabrera-Mulero, A.; Lopez-Dominguez, R.; Carmona-Saez, P.; Garcia-Fuentes, E.; Moreno-Indias, I.; Tinahones, F.J. Gut Microbiota Composition Is Associated with the Global DNA Methylation Pattern in Obesity. Front. Genet. 2019, 10, 613. [Google Scholar] [CrossRef] [Green Version]
- Miro-Blanch, J.; Yanes, O. Epigenetic Regulation at the Interplay Between Gut Microbiota and Host Metabolism. Front. Genet. 2019, 10, 638. [Google Scholar] [CrossRef]
- Miller, A.L.; Lee, H.J.; Lumeng, J.C. Obesity-associated biomarkers and executive function in children. Pediatr Res. 2015, 77, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Favieri, F.; Forte, G.; Casagrande, M. The Executive Functions in Overweight and Obesity: A Systematic Review of Neuropsychological Cross-Sectional and Longitudinal Studies. Front. Psychol 2019, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, K.; Sbarra, D.A. Body mass and cognitive decline are indirectly associated via inflammation among aging adults. Brain Behav Immun 2017, 60, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.L.; Lumeng, J.C. Pathways of Association from Stress to Obesity in Early Childhood. Obesity 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michels, N. Biological underpinnings from psychosocial stress towards appetite and obesity during youth: Research implications towards metagenomics, epigenomics and metabolomics. Nutr. Res. Rev. 2019, 282–293. [Google Scholar] [CrossRef]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain–gut–microbiome interactions in obesity and food addiction. Nat. Rev. Gastroentero 2020, 1–18. [Google Scholar] [CrossRef]
- Wonderlich, J.A.; Breithaupt, L.; Thompson, J.C.; Crosby, R.D.; Engel, S.G.; Fischer, S. The impact of neural responses to food cues following stress on trajectories of negative and positive affect and binge eating in daily life. J. Psychiatr. Res. 2018, 102, 14–22. [Google Scholar] [CrossRef]
- Chami, R.; Monteleone, A.; Treasure, J.; Monteleone, P. Stress hormones and eating disorders. Mol. Cell. Endocrinol. 2019, 497, 110349. [Google Scholar] [CrossRef]
- Sinha, R. Role of addiction and stress neurobiology on food intake and obesity. Biol. Psychol. 2018, 131, 5–13. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Baker, D.A.; Funk, D.; Lê, A.D.; Shaham, Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacol 2016, 41, 335–356. [Google Scholar] [CrossRef]
- McCaul, M.E.; Wand, G.S.; Weerts, E.M.; Xu, X. A paradigm for examining stress effects on alcohol-motivated behaviors in participants with alcohol use disorder. Addict. Biol. 2017, 23, 836–845. [Google Scholar] [CrossRef] [Green Version]
- Blaine, S.K. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 2017, 122, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.C. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology 2018, 122. [Google Scholar] [CrossRef] [PubMed]
- George, O.; Moal, M.; Koob, G.F. Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiol. Behav. 2012, 106, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koob, G.F.; Schulkin, J. Addiction and Stress: An Allostatic View. Neurosci. Biobehav. Rev. 2019, 106, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Forster, G.L.; Anderson, E.M.; Scholl, J.L.; Lukkes, J.L.; Watt, M.J. Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity. Neurobiol. Stress 2018, 9, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Parkes, D.; Fitzgerald, L.; Underhill, D.; Garami, J.; Levy-Gigi, E.; Stramecki, F.; Valikhani, A.; Frydecka, D.; Misiak, B. The relationship between childhood trauma, early-life stress, and alcohol and drug use, abuse, and addiction: An integrative review. Curr. Psychol. 2018, 1–6. [Google Scholar] [CrossRef]
- Kreek, M.; Nielsen, D.A.; Butelman, E.R.; LaForge, S.K. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat. Neurosci. 2005, 8, 1450–1457. [Google Scholar] [CrossRef]
- Daughters, S.B.; Richards, J.M.; Gorka, S.M.; Sinha, R. HPA axis response to psychological stress and treatment retention in residential substance abuse treatment: A prospective study. Drug Alcohol Depend. 2009, 105, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Levran, O.; Peles, E.; Randesi, M.; da Rosa, J.; Shen, P.-H.; Rotrosen, J.; Adelson, M.; Kreek, M. Genetic variations in genes of the stress response pathway are associated with prolonged abstinence from heroin. Pharmacogenomics 2018, 19, 333–341. [Google Scholar] [CrossRef]
- Jacques, A.; Chaaya, N.; Beecher, K.; Ali, S.A.; Belmer, A.; Bartlett, S. The impact of sugar consumption on stress driven, emotional and addictive behaviors. Neurosci. Biobehav. Rev. 2019, 103, 178–199. [Google Scholar] [CrossRef]
- Evans, C.; Cahill, C. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Brady, K.T.; Dansky, B.S.; Sonne, S.C.; Saladin, M.E. Posttraumatic Stress Disorder and Cocaine Dependence: Order of Onset. Am. J. Addict. 1998, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Cottler, L.B.; Compton, W.M.; Mager, D.; Spitznagel, E.L.; Janca, A. Posttraumatic stress disorder among substance users from the general population. Am. J. Psychiatry 1992, 149, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.T.; Trott, J.M.; Rajbhandari, A.K.; Li, K.; Walwyn, W.M.; Evans, C.J.; Fanselow, M.S. Chronic opioid pretreatment potentiates the sensitization of fear learning by trauma. Neuropsychopharmacol 2019, 45, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Volkow, N.D.; Koob, G.F.; McLellan, T.A. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G. Brain disease model of addiction: Why is it so controversial? Lancet Psychiatry 2015, 2, 677–679. [Google Scholar] [CrossRef] [Green Version]
- Mukhara, D.; Banks, M.L.; Neigh, G.N. Stress as a Risk Factor for Substance Use Disorders: A Mini-Review of Molecular Mediators. Front. Behav. Neurosci. 2018, 12, 309. [Google Scholar] [CrossRef] [Green Version]
- Karkhanis, A.; Holleran, K.M.; Jones, S.R. International Review of Neurobiology. Int. Rev. Neurobiol. 2017, 136, 53–88. [Google Scholar] [CrossRef]
- Joutsa, J.; Karlsson, H.K.; Majuri, J.; Nuutila, P.; Helin, S.; Kaasinen, V.; Nummenmaa, L. Binge eating disorder and morbid obesity are associated with lowered mu-opioid receptor availability in the brain. Psychiatry Res. Neuroimaging 2018, 276, 41–45. [Google Scholar] [CrossRef]
- Novick, A.M.; Levandowski, M.L.; Laumann, L.; Philip, N.S.; Price, L.H.; Tyrka, A.R. The effects of early life stress on reward processing. J. Psychiatr. Res. 2018, 101, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Meule, A. Chapter 16 An Addiction Perspective on Eating Disorders and Obesity. Eat. Disord. Obes. Child. Adolesc. 2019, 99–104. [Google Scholar] [CrossRef]
- Monnier, L.; Schlienger, J.-L.; Colette, C.; Bonnet, F. The obesity treatment dilemma: Why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, K.; Anderson, D.A.; Anderson, L.M.; Reilly, E.E.; Gorrell, S. Dietary restraint: What’s the harm? A review of the relationship between dietary restraint, weight trajectory and the development of eating pathology. Clin. Obes. 2016, 6, 89–100. [Google Scholar] [CrossRef]
- Keys, A.; Brožek, J.; Henschel, A.; Mickelsen, O.; Taylor, H.L. The Biology of Human Starvation, Vols. 1 & 2; University of Minnesota Press: Minneapolis, MN, USA, 1950. [Google Scholar]
- Dunn, T.M.; Bratman, S. On orthorexia nervosa: A review of the literature and proposed diagnostic criteria. Eat. Behav. 2016, 21, 11–17. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Mann, T.; Vinas, D.; Hunger, J.M.; Dejager, J.; Taylor, S.E. Low calorie dieting increases cortisol. Psychosom Med. 2010, 72, 357. [Google Scholar] [CrossRef] [Green Version]
- Pietiläinen, K.H.; Saarni, S.E.; Kaprio, J.; Rissanen, A. Does dieting make you fat? A twin study. Int. J. Obes. 2011, 36, 456–464. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Bi, T.; Gong, G.; Jiang, Q.; Chen, H. Why Do Most Restrained Eaters Fail in Losing Weight?: Evidence from an fMRI Study. Psychol. Res. Behav Manag. 2019, 12, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Wiss, D.A.; Brewerton, T.D. Incorporating food addiction into disordered eating: The disordered eating food addiction nutrition guide (DEFANG). Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2017, 22, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Meule, A. A Critical Examination of the Practical Implications Derived from the Food Addiction Concept. Curr. Obes. Rep. 2019, 8, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, A.J. Weight stigma is stressful. A review of evidence for the Cyclic Obesity/Weight-Based Stigma model. Appetite 2014, 82, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiyama, A.J.; Epel, E.S.; McClatchey, T.M.; Poelke, G.; Kemeny, M.E.; McCoy, S.K.; Daubenmier, J. Associations of Weight Stigma with Cortisol and Oxidative Stress Independent of Adiposity. Health Psychol. 2014, 33, 862–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, M.; Robinson, E.; Sutin, A.R. Perceived overweight and suicidality among US adolescents from 1999 to 2017. Int. J. Obes. 2020, 44, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.; Robinson, E.; Daly, M.; Terracciano, A. Weight discrimination and unhealthy eating-related behaviors. Appetite 2016, 102, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Major, B.; Rathbone, J.A.; Blodorn, A.; Hunger, J.M. The Countervailing Effects of Weight Stigma on Weight-Loss Motivation and Perceived Capacity for Weight Control. Personal. Soc. Psychol. Bull. 2020, 146167220903184. [Google Scholar] [CrossRef]
- Ahorsu, D.K.; Lin, C.-Y.; Imani, V.; Griffiths, M.D.; Su, J.-A.; Latner, J.D.; Marshall, R.D.; Pakpour, A.H. A prospective study on the link between weight-related self-stigma and binge eating: Role of food addiction and psychological distress. Int. J. Eat. Disord. 2020, 53, 442–450. [Google Scholar] [CrossRef]
- Pudney, E.V.; Himmelstein, M.S.; Puhl, R.M.; Foster, G.D. Distressed or not distressed? A mixed methods examination of reactions to weight stigma and implications for emotional wellbeing and internalized weight bias. Soc. Sci. Med. 2020, 249, 112854. [Google Scholar] [CrossRef]
- Alberga, A.S.; Edache, I.Y.; Forhan, M.; Russell-Mayhew, S. Weight bias and health care utilization: A scoping review. Prim. Heal. Care Res. Dev. 2019, 20, e116. [Google Scholar] [CrossRef]
- Hunger, J.M.; Smith, J.P.; Tomiyama, A.J. An Evidence-Based Rationale for Adopting Weight-Inclusive Health Policy. Soc. Issues Policy Rev. 2020, 14, 73–107. [Google Scholar] [CrossRef] [Green Version]
- Latner, J.D.; Puhl, R.M.; Murakami, J.M.; O’Brien, K.S. Food addiction as a causal model of obesity. Effects on stigma, blame, and perceived psychopathology. Appetite 2014, 77, 79–84. [Google Scholar] [CrossRef]
- O’Brien, K.S.; Puhl, R.M.; Latner, J.D.; Lynott, D.; Reid, J.D.; Vakhitova, Z.; Hunter, J.A.; Scarf, D.; Jeanes, R.; Bouguettaya, A.; et al. The Effect of a Food Addiction Explanation Model for Weight Control and Obesity on Weight Stigma. Nutrients 2020, 12, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassin, S.E.; Buchman, D.Z.; Leung, S.E.; Kantarovich, K.; Hawa, A.; Carter, A.; Sockalingam, S. Ethical, Stigma, and Policy Implications of Food Addiction: A Scoping Review. Nutrients 2019, 11, 710. [Google Scholar] [CrossRef] [Green Version]
- Rubin, L.P. Maternal and Pediatric Health and Disease: Integrating Biopsychosocial Models and Epigenetics. Pediatric Res. 2015, 79, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, E.C.; Soare, T.W.; Zhu, Y.; Simpkin, A.J.; Suderman, M.J.; Klengel, T.; Smith, A.; Ressler, K.; Relton, C.L. Sensitive periods for the effect of childhood adversity on DNA methylation: Results from a prospective, longitudinal study. Biol. Psychiatry 2019, 85, 838–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabard-Durnam, L.J.; McLaughlin, K.A. Do Sensitive Periods Exist for Exposure to Adversity? Biol. Psychiatry 2019, 85, 789–791. [Google Scholar] [CrossRef]
- Matikainen-Ankney, B.A.; Kravitz, A.V. Persistent effects of obesity: A neuroplasticity hypothesis. Ann. N. Y. Acad. Sci. 2018, 1428, 221–239. [Google Scholar] [CrossRef]
- Theall, K.P.; Chaparro, P.M.; Denstel, K.; Bilfield, A.; Drury, S.S. Childhood obesity and the associated roles of neighborhood and biologic stress. Prev. Med. Rep. 2019, 14, 100849. [Google Scholar] [CrossRef]
- Rung, J.M.; Peck, S.; Hinnenkamp, J.E.; Preston, E.; Madden, G.J. Changing Delay Discounting and Impulsive Choice: Implications for Addictions, Prevention, and Human Health. Perspect. Behav Sci. 2019, 42, 397–417. [Google Scholar] [CrossRef]
- Bortz, W.M. Biological basis of determinants of health. Am. J. Public Health 2005, 95, 389–392. [Google Scholar] [CrossRef]
- Lei, M.-K.; Beach, S.R.; Simons, R.L. Biological embedding of neighborhood disadvantage and collective efficacy: Influences on chronic illness via accelerated cardiometabolic age. Dev. Psychopathol. 2018, 30, 1797–1815. [Google Scholar] [CrossRef]
- Dube, S.R. Continuing conversations about adverse childhood experiences (ACEs) screening: A public health perspective. Child. Abus. Neglect. 2018, 85, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.J.; Carr, D.; Granberg, E.M.; Major, B.; Robinson, E.; Sutin, A.R.; Brewis, A. How and why weight stigma drives the obesity ‘epidemic’ and harms health. BMC Med. 2018, 16, 123. [Google Scholar] [CrossRef]
- Yang, L.; Wong, L.Y.; Grivel, M.M.; Hasin, D.S. Stigma and substance use disorders. Curr. Opin. Psychiatry 2017, 30, 378. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Nergiz-Unal, R.; Dedebayraktar, D.; Akyol, A.; Pekcan, A.G.; Besler, H.T.; Buyuktuncer, Z. How does food addiction influence dietary intake profile? PLoS ONE 2018, 13, e0195541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juul, F.; Martinez-Steele, E.; Parekh, N.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 2018, 120, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Rico-Campà, A.; Martínez-González, M.A.; Alvarez-Alvarez, I.; de Mendonça, R.; de la Fuente-Arrillaga, C.; Gómez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef] [Green Version]
- Ruddock, H.K.; Hardman, C.A. Food Addiction Beliefs Amongst the Lay Public: What Are the Consequences for Eating Behaviour? Curr. Addict. Rep. 2017, 4, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Moran, A.; Musicus, A.; Soo, J.; Gearhardt, A.N.; Gollust, S.E.; Roberto, C.A. Believing that certain foods are addictive is associated with support for obesity-related public policies. Prev. Med. 2016, 90, 39–46. [Google Scholar] [CrossRef]
- Rodgers, R.F.; Sonneville, K. Research for leveraging food policy in universal eating disorder prevention. Int. J. Eat. Disord. 2018, 51, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Mallarino, C.; Gómez, L.F.; González-Zapata, L.; Cadena, Y.; Parra, D.C. Advertising of ultra-processed foods and beverages: Children as a vulnerable population. Rev. Saúde Pública 2013, 47, 1006–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, B.; Kelly, B.; Vandevijvere, S.; Baur, L. Young adults: Beloved by food and drink marketers and forgotten by public health? Health Promot. Int. 2016, 31, 954–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Story, M.; French, S. Food Advertising and Marketing Directed at Children and Adolescents in the US. Int. J. Behav. Nutr. Phys. 2004, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grier, S.A.; Kumanyika, S.K. The Context for Choice: Health Implications of Targeted Food and Beverage Marketing to African Americans. Am. J. Public Health 2008, 98, 1616–1629. [Google Scholar] [CrossRef]
- Grier, S.; Kumanyika, S.K. Targeting Interventions for Ethnic Minority and Low-Income Populations. Future Child. 2006, 16, 187–207. [Google Scholar] [CrossRef]
- Gearhardt, A.; Roberts, M.; Ashe, M. If Sugar is Addictive… What Does it Mean for the Law? J. Law Med. Ethics 2013, 41, 46–49. [Google Scholar] [CrossRef] [Green Version]
- Bragg, M.A.; Pageot, Y.K.; Amico, A.; Miller, A.N.; Gasbarre, A.; Rummo, P.E.; Elbel, B. Fast food, beverage, and snack brands on social media in the United States: An examination of marketing techniques utilized in 2000 brand posts. Pediatr. Obes. 2019, 15, e12606. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Yokum, S.; Harris, J.L.; Epstein, L.H.; Lumeng, J.C. Neural response to fast food commercials in adolescents predicts intake. Am. J. Clin. Nutr. 2020, 111, 493–502. [Google Scholar] [CrossRef]
- Thoits, P.A. Stress and Health: Major Findings and Policy Implications. J. Health Soc. Behav 2010, 51, S41–S53. [Google Scholar] [CrossRef] [Green Version]
- Wiss, D.A. A Biopsychosocial Overview of the Opioid Crisis: Considering Nutrition and Gastrointestinal Health. Front. Public Health 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiss, D.A.; Avena, N.; Gold, M. Food Addiction and Psychosocial Adversity: Biological Embedding, Contextual Factors, and Public Health Implications. Nutrients 2020, 12, 3521. https://doi.org/10.3390/nu12113521
Wiss DA, Avena N, Gold M. Food Addiction and Psychosocial Adversity: Biological Embedding, Contextual Factors, and Public Health Implications. Nutrients. 2020; 12(11):3521. https://doi.org/10.3390/nu12113521
Chicago/Turabian StyleWiss, David A., Nicole Avena, and Mark Gold. 2020. "Food Addiction and Psychosocial Adversity: Biological Embedding, Contextual Factors, and Public Health Implications" Nutrients 12, no. 11: 3521. https://doi.org/10.3390/nu12113521
APA StyleWiss, D. A., Avena, N., & Gold, M. (2020). Food Addiction and Psychosocial Adversity: Biological Embedding, Contextual Factors, and Public Health Implications. Nutrients, 12(11), 3521. https://doi.org/10.3390/nu12113521




