Meeting of Minds around Food Addiction: Insights from Addiction Medicine, Nutrition, Psychology, and Neurosciences
Abstract
:1. Introduction
1.1. From the Addiction Medicine Clinician Point of View: Towards a Definition of Food Addiction (FA)
1.2. From the Clinical Nutritionist’s Point of View: Food Addiction (FA) in the Context of Obesity Treatment
1.2.1. Prevalence of FA in the General and Obese Population
1.2.2. Association between Food Addiction and Obesity-Related Comorbidities
1.2.3. Rationale for a Systematic Screening of FA in Obese Patients
1.2.4. Proposed Therapy for Obese Patients with FA
1.3. From the Health Psychologist’s Point of View: Toward a More Comprehensive Psychological Approach to Food Addiction
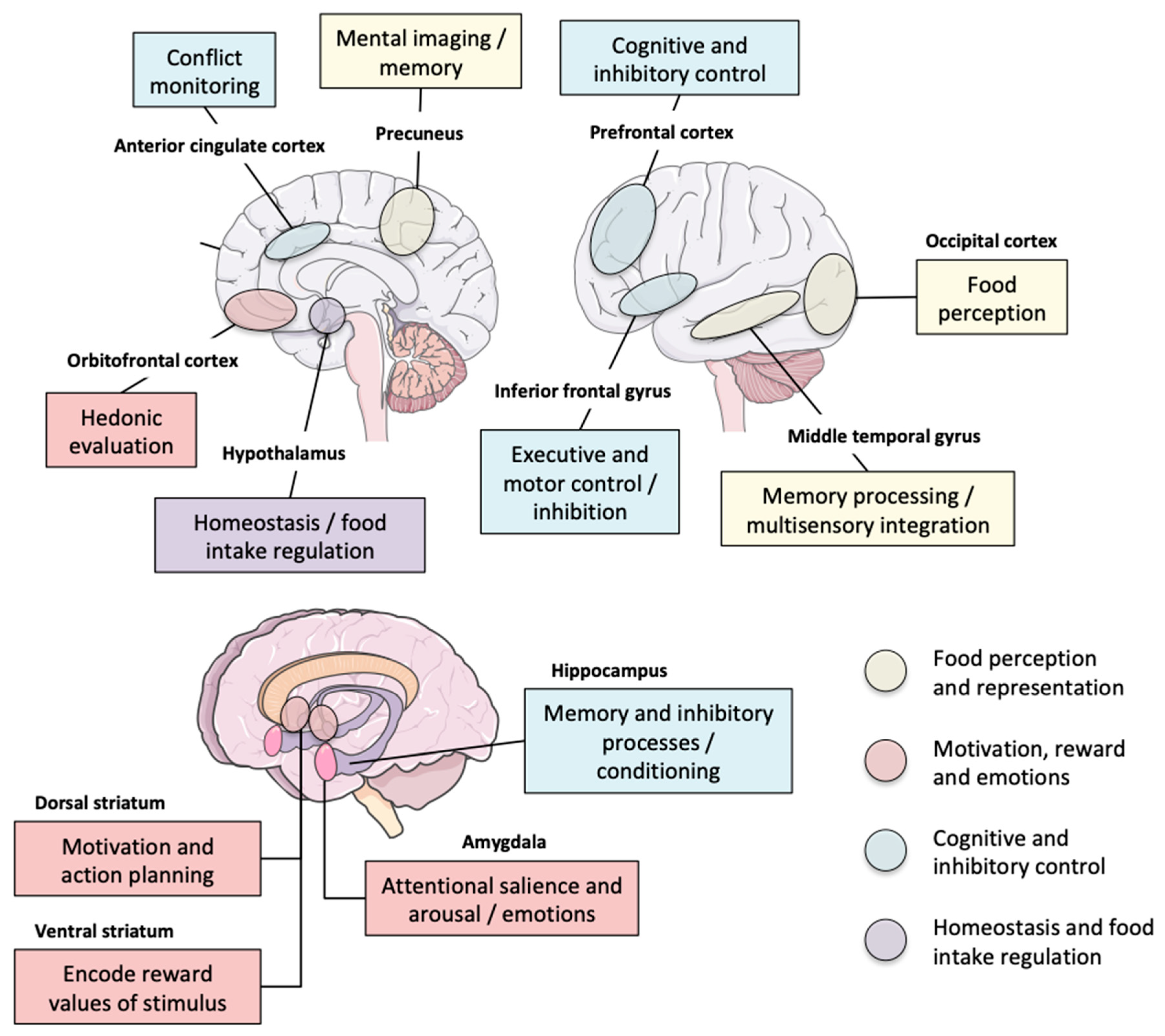
1.4. From the Behavioral Neuroscientist’s Point of View: Is There a “Food Addict” Brain?
2. General Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Randolph, T.G. The descriptive features of food addiction; addictive eating and drinking. Q. J. Stud. Alcohol 1956, 17, 198–224. [Google Scholar] [CrossRef]
- Markus, C.R.; Rogers, P.J.; Brouns, F.; Schepers, R. Eating dependence and weight gain; no human evidence for a ‘sugar-addiction’ model of overweight. Appetite 2017, 114, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Rogers, P.J.; Smit, H.J. Food craving and food “addiction”: A critical review of the evidence from a biopsychosocial perspective. Pharmacol. Biochem. Behav. 2000, 66, 3–14. [Google Scholar] [CrossRef]
- Gordon, E.L.; Ariel-Donges, A.H.; Bauman, V.; Merlo, L.J. What Is the Evidence for “Food Addiction?” A Systematic Review. Nutrients 2018, 10, 477. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef]
- Davis, C.; Carter, J.C. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite 2009, 53, 1–8. [Google Scholar] [CrossRef]
- Rogers, P.J. Food and drug addictions: Similarities and differences. Pharmacol. Biochem. Behav. 2017, 153, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.S.; Kotz, C.M.; Gosnell, B.A. Sugars: Hedonic aspects, neuroregulation, and energy balance. Am. J. Clin. Nutr. 2003, 78, 834S–842S. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 2012, 11, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Yokum, S.; Potenza, M.N.; Gearhardt, A.N. Neural systems implicated in obesity as an addictive disorder: From biological to behavioral mechanisms. Prog. Brain Res. 2016, 223, 329–346. [Google Scholar] [CrossRef]
- Carter, A.; Hendrikse, J.; Lee, N.; Yucel, M.; Verdejo-Garcia, A.; Andrews, Z.B.; Hall, W. The Neurobiology of “Food Addiction” and Its Implications for Obesity Treatment and Policy. Annu. Rev. Nutr. 2016, 36, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Guillem, K.; Vandaele, Y. Sugar addiction: Pushing the drug-sugar analogy to the limit. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Gearhardt, A.N. Food addiction in the light of DSM-5. Nutrients 2014, 6, 3653–3671. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Roberto, C.A.; Seamans, M.J.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale for children. Eat. Behav. 2013, 14, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pursey, K.M.; Stanwell, P.; Gearhardt, A.N.; Collins, C.E.; Burrows, T.L. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: A systematic review. Nutrients 2014, 6, 4552–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Society of Addition Medicine. Definition of Addiction. Available online: https://www.asam.org/quality-practice/definition-of-addiction (accessed on 15 September 2019).
- Hauck, C.; Cook, B.; Ellrott, T. Food addiction, eating addiction and eating disorders. Proc. Nutr. Soc. 2020, 79, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, P.C.; Kenny, P.J. Food addiction: A valid concept? Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 2506–2513. [Google Scholar] [CrossRef] [Green Version]
- Long, C.G.; Blundell, J.E.; Finlayson, G. A Systematic Review of the Application and Correlates of YFAS-Diagnosed ‘Food Addiction’ in Humans: Are Eating-Related ‘Addictions’ a Cause for Concern or Empty Concepts? Obes. Facts 2015, 8, 386–401. [Google Scholar] [CrossRef]
- Hebebrand, J.; Albayrak, Ö.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menzies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.C.; Sedgmond, J.; Maizey, L.; Chambers, C.D.; Lawrence, N.S. Food Addiction: Implications for the Diagnosis and Treatment of Overeating. Nutrients 2019, 11, 2086. [Google Scholar] [CrossRef] [Green Version]
- Bonder, R.; Davis, C.; Kuk, J.L.; Loxton, N.J. Compulsive “grazing” and addictive tendencies towards food. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2018, 26, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, S.T.; Wray, J.M. The clinical significance of drug craving. Ann. N. Y. Acad. Sci. 2012, 1248, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Boswell, R.G.; Kober, H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Rizk, M.T.; Treat, T.A. The association of food characteristics and individual differences with ratings of craving and liking. Appetite 2014, 79, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.A.; Gearhardt, A.N.; White, M.A. Food craving as a mediator between addictive-like eating and problematic eating outcomes. Eat. Behav. 2015, 19, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurt, R.T.; Edakkanambeth Varayil, J.; Mundi, M.S.; Martindale, R.G.; Ebbert, J.O. Designation of obesity as a disease: Lessons learned from alcohol and tobacco. Curr. Gastroenterol. Rep. 2014, 16, 415. [Google Scholar] [CrossRef]
- Spring, B.; Schneider, K.; Smith, M.; Kendzor, D.; Appelhans, B.; Hedeker, D.; Pagoto, S. Abuse potential of carbohydrates for overweight carbohydrate cravers. Psychopharmacology 2008, 197, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Saunders, J.B.; Degenhardt, L.; Reed, G.M.; Poznyak, V. Alcohol Use Disorders in ICD-11: Past, Present, and Future. Alcohol. Clin. Exp. Res. 2019, 43, 1617–1631. [Google Scholar] [CrossRef]
- Burrows, T.; Kay-Lambkin, F.; Pursey, K.; Skinner, J.; Dayas, C. Food addiction and associations with mental health symptoms: A systematic review with meta-analysis. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2018, 31, 544–572. [Google Scholar] [CrossRef]
- Ivezaj, V.; Wiedemann, A.A.; Grilo, C.M. Food addiction and bariatric surgery: A systematic review of the literature. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.L.; Gardiner, E.; Loxton, N.J. Investigating the relationship between reward sensitivity, impulsivity, and food addiction: A systematic review. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2020, 28, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Penzenstadler, L.; Soares, C.; Karila, L.; Khazaal, Y. Systematic Review of Food Addiction as Measured with the Yale Food Addiction Scale: Implications for the Food Addiction Construct. Curr. Neuropharmacol. 2019, 17, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Canan, F.; Karaca, S.; Sogucak, S.; Gecici, O.; Kuloglu, M. Eating disorders and food addiction in men with heroin use disorder: A controlled study. Eat. Weight Disord. EWD 2017, 22, 249–257. [Google Scholar] [CrossRef]
- Hauck, C.; Schipfer, M.; Ellrott, T.; Cook, B. The relationship between food addiction and patterns of disordered eating with exercise dependence: In amateur endurance athletes. Eat. Weight Disord. EWD 2019, 25, 1573–1582. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Q.; McGeary, J.; Cho, E.; Flint, A.; Wu, S.; Ascherio, A.; Rimm, E.; Field, A.; Qureshi, A. Indoor tanning bed use and risk of food addiction based on the modified Yale Food Addiction Scale. J. Biomed. Res. 2016, 31, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Mies, G.W.; Treur, J.L.; Larsen, J.K.; Halberstadt, J.; Pasman, J.A.; Vink, J.M. The prevalence of food addiction in a large sample of adolescents and its association with addictive substances. Appetite 2017, 118, 97–105. [Google Scholar] [CrossRef]
- Tinghino, B.; Lugoboni, F.; Amatulli, A.; Biasin, C.; Bramani Araldi, M.; Cantiero, D.; Cremaschini, M.; Galimberti, G.L.; Giusti, S.; Grosina, C.; et al. The FODRAT study (FOod addiction, DRugs, Alcohol and Tobacco): First data on food addiction prevalence among patients with addiction to drugs, tobacco and alcohol. Eat. Weight Disord. EWD 2020. [Google Scholar] [CrossRef]
- Lennerz, B.; Lennerz, J.K. Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef]
- Wiss, D.A.; Avena, N.; Rada, P. Sugar Addiction: From Evolution to Revolution. Front. Psychiatry 2018, 9, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westwater, M.L.; Fletcher, P.C.; Ziauddeen, H. Sugar addiction: The state of the science. Eur. J. Nutr. 2016, 55, 55–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Kochhar, K.P.; Khan, N.A. Fat Addiction: Psychological and Physiological Trajectory. Nutrients 2019, 11, 2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, E.M.; Smeal, J.K.; Gearhardt, A.N. Foods are differentially associated with subjective effect report questions of abuse liability. PLoS ONE 2017, 12, e0184220. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Sonneville, K.R.; Gearhardt, A.N. Subjective experiences of highly processed food consumption in individuals with food addiction. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 2019, 33, 144–153. [Google Scholar] [CrossRef]
- Junghanns, K.; Veltrup, C.; Wetterling, T. Craving shift in chronic alcoholics. Eur. Addict. Res. 2000, 6, 64–70. [Google Scholar] [CrossRef]
- King, W.C.; Chen, J.Y.; Mitchell, J.E.; Kalarchian, M.A.; Steffen, K.J.; Engel, S.G.; Courcoulas, A.P.; Pories, W.J.; Yanovski, S.Z. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA 2012, 307, 2516–2525. [Google Scholar] [CrossRef]
- Howard, M.; McMillen, C.; Nower, L.; Elze, D.; Edmond, T.; Bricout, J. Denial in addiction: Toward an integrated stage and process model—Qualitative findings. J. Psychoact. Drugs 2002, 34, 371–382. [Google Scholar] [CrossRef]
- Cassin, S.E.; Buchman, D.Z.; Leung, S.E.; Kantarovich, K.; Hawa, A.; Carter, A.; Sockalingam, S. Ethical, Stigma, and Policy Implications of Food Addiction: A Scoping Review. Nutrients 2019, 11, 710. [Google Scholar] [CrossRef] [Green Version]
- Meule, A. A Critical Examination of the Practical Implications Derived from the Food Addiction Concept. Curr. Obes. Rep. 2019, 8, 11–17. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.S.; Puhl, R.M.; Latner, J.D.; Lynott, D.; Reid, J.D.; Vakhitova, Z.; Hunter, J.A.; Scarf, D.; Jeanes, R.; Bouguettaya, A.; et al. The Effect of a Food Addiction Explanation Model for Weight Control and Obesity on Weight Stigma. Nutrients 2020, 12, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruddock, H.K.; Christiansen, P.; Jones, A.; Robinson, E.; Field, M.; Hardman, C.A. Believing in food addiction: Helpful or counterproductive for eating behavior? Obesity (Silver Spring Md.) 2016, 24, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Matta, J.; Zins, M.; Feral-Pierssens, A.L.; Carette, C.; Ozguler, A.; Goldberg, M.; Czernichow, S. Prévalence du surpoids, de l’obésité et des facteurs de risque cardio-métaboliques dans la cohorte Constances. Bull. Epidémiol. Hebd. 2016, 35–36, 640–646. [Google Scholar]
- The State of Obesity. Available online: http://healthyamericans.org/reports/stateofobesity2016/ (accessed on 20 September 2020).
- Malnick, S.D.; Knobler, H. The medical complications of obesity. QJM 2006, 99, 565–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Effertz, T.; Engel, S.; Verheyen, F.; Linder, R. The costs and consequences of obesity in Germany: A new approach from a prevalence and life-cycle perspective. Eur. J. Health Econ. 2016, 17, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Schwander, B.; Hiligsmann, M.; Nuijten, M.; Evers, S. Systematic review and overview of health economic evaluation models in obesity prevention and therapy. Expert Rev. Pharm. Outcomes Res. 2016, 16, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blouin, C.; Hamel, D.; Vandal, N.; Barry, A.D.; Lo, E.; Lacroix, G.; Laguë, J.; Langlois, M.-F.; Martel, S.; Michaud, P.-C.; et al. The economic consequences of obesity and overweight among adults in Quebec. Can. J. Public Health 2017, 107, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjostrom, L.; Narbro, K.; Sjostrom, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [Green Version]
- Eliasson, B.; Liakopoulos, V.; Franzen, S.; Naslund, I.; Svensson, A.M.; Ottosson, J.; Gudbjornsdottir, S. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: A nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol. 2015, 3, 847–854. [Google Scholar] [CrossRef]
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, A.; Bouchard, C.; Carlsson, B.; Karason, K.; Lonroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef] [Green Version]
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Sjostrom, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, A.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenoir, M.; Serre, F.; Cantin, L.; Ahmed, S.H. Intense sweetness surpasses cocaine reward. PLoS ONE 2007, 2, e698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, E.M.; Gearhardt, A.N. Associations of Food Addiction in a Sample Recruited to Be Nationally Representative of the United States. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2018, 26, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Som, M.; Val-Laillet, D.; Constant, A.; Moirand, R.; Thibault, R. Prevalence of food addiction (FA) diagnosed by the Yale Food Addiction Scale version 2.0 (YFAS 2.0): Prospective study in a referral centre for obesity surgery. Clin. Nutr. 2018, 37, S264. [Google Scholar] [CrossRef]
- Brunault, P.; Ducluzeau, P.H.; Bourbao-Tournois, C.; Delbachian, I.; Couet, C.; Reveillere, C.; Ballon, N. Food Addiction in Bariatric Surgery Candidates: Prevalence and Risk Factors. Obes. Surg. 2016, 26, 1650–1653. [Google Scholar] [CrossRef]
- Hauck, C.; Weiss, A.; Schulte, E.M.; Meule, A.; Ellrott, T. Prevalence of ‘Food Addiction’ as Measured with the Yale Food Addiction Scale 2.0 in a Representative German Sample and Its Association with Sex, Age and Weight Categories. Obes. Facts 2017, 10, 12–24. [Google Scholar] [CrossRef]
- Davis, C.; Curtis, C.; Levitan, R.D.; Carter, J.C.; Kaplan, A.S.; Kennedy, J.L. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 2011, 57, 711–717. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Stein, R.I.; Eagon, J.C.; Klein, S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity (Silver Spring Md.) 2014, 22, 1792–1798. [Google Scholar] [CrossRef] [Green Version]
- Davis, C. A commentary on the associations among ‘food addiction’, binge eating disorder, and obesity: Overlapping conditions with idiosyncratic clinical features. Appetite 2017, 115, 3–8. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Morgan, P.T.; Crosby, R.D.; Grilo, C.M. An examination of the food addiction construct in obese patients with binge eating disorder. Int. J. Eat. Disord. 2012, 45, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Grilo, C.M. An examination of food addiction in a racially diverse sample of obese patients with binge eating disorder in primary care settings. Compr. Psychiatry 2013, 54, 500–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, S.K.; Meule, A. Food Addiction and Bulimia Nervosa: New Data Based on the Yale Food Addiction Scale 2.0. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2016, 24, 518–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyici, S.; Koca, N.; Sigirli, D.; Aslan, B.B.; Guclu, M.; Kisakol, G. Food Addiction Correlates with Psychosocial Functioning More Than Metabolic Parameters in Patients with Obesity. Metab. Syndr. Relat. Disord. 2020, 18, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Segato, G.; De Luca, M.; De Marchi, F.; Foletto, M.; Vianello, M.; Valeri, M.; Favretti, F.; Enzi, G. Weight loss and postoperative complications in morbidly obese patients with binge eating disorder treated by laparoscopic adjustable gastric banding. Obes. Surg. 2005, 15, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wolnerhanssen, B.K.; Peters, T.; Kern, B.; Schotzau, A.; Ackermann, C.; von Flue, M.; Peterli, R. Predictors of outcome in treatment of morbid obesity by laparoscopic adjustable gastric banding: Results of a prospective study of 380 patients. Surg. Obes. Relat. Dis. 2008, 4, 500–506. [Google Scholar] [CrossRef]
- Steffen, K.J.; Engel, S.G.; Wonderlich, J.A.; Pollert, G.A.; Sondag, C. Alcohol and Other Addictive Disorders Following Bariatric Surgery: Prevalence, Risk Factors and Possible Etiologies. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2015, 23, 442–450. [Google Scholar] [CrossRef]
- Holgerson, A.A.; Clark, M.M.; Ames, G.E.; Collazo-Clavell, M.L.; Kellogg, T.A.; Graszer, K.M.; Kalsy, S.A.; Grothe, K. Association of Adverse Childhood Experiences and Food Addiction to Bariatric Surgery Completion and Weight Loss Outcome. Obes. Surg. 2018, 28, 3386–3392. [Google Scholar] [CrossRef]
- Lent, M.R.; Eichen, D.M.; Goldbacher, E.; Wadden, T.A.; Foster, G.D. Relationship of food addiction to weight loss and attrition during obesity treatment. Obesity (Silver Spring Md.) 2014, 22, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Burmeister, J.M.; Hinman, N.; Koball, A.; Hoffmann, D.A.; Carels, R.A. Food addiction in adults seeking weight loss treatment. Implications for psychosocial health and weight loss. Appetite 2013, 60, 103–110. [Google Scholar] [CrossRef]
- Sevincer, G.M.; Konuk, N.; Bozkurt, S.; Coskun, H. Food addiction and the outcome of bariatric surgery at 1-year: Prospective observational study. Psychiatry Res. 2016, 244, 159–164. [Google Scholar] [CrossRef]
- Conason, A.; Teixeira, J.; Hsu, C.H.; Puma, L.; Knafo, D.; Geliebter, A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013, 148, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, L.T. Substance use after bariatric surgery: A review. J. Psychiatr. Res. 2016, 76, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Reslan, S.; Saules, K.K.; Greenwald, M.K.; Schuh, L.M. Substance misuse following Roux-en-Y gastric bypass surgery. Subst. Use Misuse 2014, 49, 405–417. [Google Scholar] [CrossRef]
- David, L.A.; Sijercic, I.; Cassin, S.E. Preoperative and post-operative psychosocial interventions for bariatric surgery patients: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e12926. [Google Scholar] [CrossRef]
- Sharma, A.M.; Kushner, R.F. A proposed clinical staging system for obesity. Int. J. Obes. 2009, 33, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Padwal, R.S.; Pajewski, N.M.; Allison, D.B.; Sharma, A.M. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ 2011, 183, E1059–E1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, M.R.; Gleaves, D.H.; DiSimone-Weiss, R.T.; Furgueson, C.; Gayda, C.A.; Kolsky, P.A.; Neal-Walden, T.; Nelsen, L.A.; McKinney, S. Restraint, dieting, and the continuum model of bulimia nervosa. J. Abnorm. Psychol. 1996, 105, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Vogele, C. The psychology of eating. Front. Psychol. 2013, 4, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, P.; Smit, H.J.; Lightowler, H.J. The influence of restrained and external eating patterns on overeating. Appetite 2007, 49, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. The neurobiology of food intake in an obesogenic environment. Proc. Nutr. Soc. 2012, 71, 478–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reba-Harrelson, L.; Von Holle, A.; Hamer, R.M.; Swann, R.; Reyes, M.L.; Bulik, C.M. Patterns and prevalence of disordered eating and weight control behaviors in women ages 25-45. Eat. Weight Disord. EWD 2009, 14, e190–e198. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.L.; Abbott, M.J. Processes and pathways to binge eating: Development of an integrated cognitive and behavioural model of binge eating. J. Eat. Disord. 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchison, D.; Touyz, S.; Gonzalez-Chica, D.A.; Stocks, N.; Hay, P. How abnormal is binge eating? 18-Year time trends in population prevalence and burden. Acta Psychiatr. Scand. 2017, 136, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Koenders, P.G.; van Strien, T. Emotional eating, rather than lifestyle behavior, drives weight gain in a prospective study in 1562 employees. J. Occup. Environ. Med. 2011, 53, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Masheb, R.M.; Grilo, C.M. Emotional overeating and its associations with eating disorder psychopathology among overweight patients with binge eating disorder. Int. J. Eat. Disord. 2006, 39, 141–146. [Google Scholar] [CrossRef]
- De Young, K.P.; Zander, M.; Anderson, D.A. Beliefs about the emotional consequences of eating and binge eating frequency. Eat. Behav. 2014, 15, 31–36. [Google Scholar] [CrossRef]
- Pinaquy, S.; Chabrol, H.; Simon, C.; Louvet, J.P.; Barbe, P. Emotional eating, alexithymia, and binge-eating disorder in obese women. Obes. Res. 2003, 11, 195–201. [Google Scholar] [CrossRef]
- Van Strien, T.; Schippers, G.M.; Cox, W.M. On the relationship between emotional and external eating behavior. Addict. Behav. 1995, 20, 585–594. [Google Scholar] [CrossRef]
- Brunault, P.; Courtois, R.; Gearhardt, A.N.; Gaillard, P.; Journiac, K.; Cathelain, S.; Reveillere, C.; Ballon, N. Validation of the French Version of the DSM-5 Yale Food Addiction Scale in a Nonclinical Sample. Can. J. Psychiatry Rev. Can. Psychiatr. 2017, 62, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Constant, A.; Gautier, Y.; Coquery, N.; Thibault, R.; Moirand, R.; Val-Laillet, D. Emotional overeating is common and negatively associated with alcohol use in normal-weight female university students. Appetite 2018, 129, 186–191. [Google Scholar] [CrossRef]
- de Lauzon, B.; Romon, M.; Deschamps, V.; Lafay, L.; Borys, J.M.; Karlsson, J.; Ducimetiere, P.; Charles, M.A.; Fleurbaix Laventie Ville Sante Study, G. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J. Nutr. 2004, 134, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Pankevich, D.E.; Teegarden, S.L.; Hedin, A.D.; Jensen, C.L.; Bale, T.L. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J. Neurosci. 2010, 30, 16399–16407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogan, A.; Hevey, D. Eating styles in the morbidly obese: Restraint eating, but not emotional and external eating, predicts dietary behaviour. Psychol. Health 2013, 28, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.L.; White, K.S. The relation of anxiety, depression, and stress to binge eating behavior. J. Health Psychol. 2015, 20, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.Y.; Kim, K.H.; Woo, H.Y.; Shin, D.W.; Shin, Y.C.; Oh, K.S.; Shin, E.H.; Lim, S.W. Binge eating is associated with trait anxiety in Korean adolescent girls: A cross sectional study. BMC Womens Health 2017, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, D.L.; White, K.S. The Role of Anxiety in Binge Eating Behavior: A Critical Examination of Theory and Empirical Literature. Health Psychol. Res. 2013, 1, e19. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Murcia, S.; Aguera, Z.; Paslakis, G.; Munguia, L.; Granero, R.; Sanchez-Gonzalez, J.; Sanchez, I.; Riesco, N.; Gearhardt, A.N.; Dieguez, C.; et al. Food Addiction in Eating Disorders and Obesity: Analysis of Clusters and Implications for Treatment. Nutrients 2019, 11, 2633. [Google Scholar] [CrossRef] [Green Version]
- Elfhag, K.; Morey, L.C. Personality traits and eating behavior in the obese: Poor self-control in emotional and external eating but personality assets in restrained eating. Eat. Behav. 2008, 9, 285–293. [Google Scholar] [CrossRef]
- Lynam, D.R.; Smith, G.T.; Whiteside, S.P.; Cyders, M.A. Assessing Five Personality Pathways to Impulsive Behavior (Technical Report); Purdue University: West Lafayette, Indiana, 2006. [Google Scholar]
- Moynihan, A.B.; van Tilburg, W.A.; Igou, E.R.; Wisman, A.; Donnelly, A.E.; Mulcaire, J.B. Eaten up by boredom: Consuming food to escape awareness of the bored self. Front. Psychol. 2015, 6, 369. [Google Scholar] [CrossRef]
- Constant, A.; Val-Laillet, D.; Joubert, A.; Foret, K.; Thibault, R.; Moirand, R. Depressive symptoms are related to boredom proneness in patients receiving hospital care, regardless of alcohol status, lifestyle, or social support. J. Health Psychol. 2019. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Carr, D.; Granberg, E.M.; Major, B.; Robinson, E.; Sutin, A.R.; Brewis, A. How and why weight stigma drives the obesity ‘epidemic’ and harms health. BMC Med. 2018, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J. Obesity—Is food addiction to blame? Addiction 2011, 106, 1213–1214; discussion 1219–1220. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P. Structural equation modeling: Adjudging model fit. Personal. Individ. Differ. 2007, 42, 815–824. [Google Scholar] [CrossRef]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research, 2nd ed.; The Guilford Press: New York, NY, USA, 2015; p. xvii, 462. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Lindgren, E.; Gray, K.; Miller, G.; Tyler, R.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Food addiction: A common neurobiological mechanism with drug abuse. Front. Biosci. 2018, 23, 811–836. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. Review. The incentive sensitization theory of addiction: Some current issues. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 3137–3146. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci. 2005, 8, 1481–1489. [Google Scholar] [CrossRef]
- Volkow, N.D.; Baler, R.D. Addiction science: Uncovering neurobiological complexity. Neuropharmacology 2014, 76 Pt B, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Novelle, M.G.; Dieguez, C. Food Addiction and Binge Eating: Lessons Learned from Animal Models. Nutrients 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, N.; Vella, S.L.; Richardson, K. Is food addiction a valid phenomenon through the lens of the DSM-5? Aust. N. Z. J. Psychiatry 2014, 48, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Granero, R.; Hilker, I.; Aguera, Z.; Jimenez-Murcia, S.; Sauchelli, S.; Islam, M.A.; Fagundo, A.B.; Sanchez, I.; Riesco, N.; Dieguez, C.; et al. Food addiction in a Spanish sample of eating disorders: DSM-5 diagnostic subtype differentiation and validation data. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2014, 22, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Khine, M.T.; Ota, A.; Gearhardt, A.N.; Fujisawa, A.; Morita, M.; Minagawa, A.; Li, Y.; Naito, H.; Yatsuya, H. Validation of the Japanese Version of the Yale Food Addiction Scale 2.0 (J-YFAS 2.0). Nutrients 2019, 11, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardee, J.E.; Phaneuf, C.; Cope, L.; Zucker, R.; Gearhardt, A.; Heitzeg, M. Neural correlates of inhibitory control in youth with symptoms of food addiction. Appetite 2020, 148, 104578. [Google Scholar] [CrossRef] [PubMed]
- Beyer, F.; Garcia-Garcia, I.; Heinrich, M.; Schroeter, M.L.; Sacher, J.; Luck, T.; Riedel-Heller, S.G.; Stumvoll, M.; Villringer, A.; Witte, A.V. Neuroanatomical correlates of food addiction symptoms and body mass index in the general population. Hum. Brain Mapp. 2019, 40, 2747–2758. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Yokum, S.; Jahn, A.; Gearhardt, A.N. Food cue reactivity in food addiction: A functional magnetic resonance imaging study. Physiol. Behav. 2019, 208, 112574. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Garelli, S.; Agostini, A.; Filidei, E.; Fanelli, F.; Giorgetti, A.; Mezzullo, M.; Fucci, S.; Mazza, R.; Vicennati, V.; et al. Food addiction distinguishes an overweight phenotype that can be reversed by low calorie diet. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2018, 26, 657–670. [Google Scholar] [CrossRef]
- Osadchiy, V.; Labus, J.S.; Gupta, A.; Jacobs, J.; Ashe-McNalley, C.; Hsiao, E.Y.; Mayer, E.A. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE 2018, 13, e0201772. [Google Scholar] [CrossRef] [Green Version]
- Franken, I.H.A.; Nijs, I.M.T.; Toes, A.; van der Veen, F.M. Food addiction is associated with impaired performance monitoring. Biol. Psychol. 2018, 131, 49–53. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Yokum, S.; Orr, P.T.; Stice, E.; Corbin, W.R.; Brownell, K.D. Neural correlates of food addiction. Arch. Gen. Psychiatry 2011, 68, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Bohon, C.; Veldhuizen, M.G.; Small, D.M. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008, 117, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stice, E.; Spoor, S.; Ng, J.; Zald, D.H. Relation of obesity to consummatory and anticipatory food reward. Physiol. Behav. 2009, 97, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.S.; Pannacciulli, N.; Chen, K.; Salbe, A.D.; Del Parigi, A.; Hill, J.O.; Wing, R.R.; Reiman, E.M.; Krakoff, J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am. J. Clin. Nutr. 2007, 86, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, S.J.; Cedernaes, J.; Schioth, H.B. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: A meta-analysis of fMRI studies. PLoS ONE 2013, 8, e60393. [Google Scholar] [CrossRef]
- Ho, A.L.; Sussman, E.S.; Pendharkar, A.V.; Azagury, D.E.; Bohon, C.; Halpern, C.H. Deep brain stimulation for obesity: Rationale and approach to trial design. Neurosurg. Focus 2015, 38, E8. [Google Scholar] [CrossRef]
- Clark, S.M.; Martens, K.; Smith-Mason, C.E.; Hamann, A.; Miller-Matero, L.R. Validation of the Yale Food Addiction Scale 2.0 among a Bariatric Surgery Population. Obes. Surg. 2019, 29, 2923–2928. [Google Scholar] [CrossRef]
- Rodriguez-Martin, B.C.; Gallego-Arjiz, B. Overeaters Anonymous: A Mutual-Help Fellowship for Food Addiction Recovery. Front. Psychol. 2018, 9, 1491. [Google Scholar] [CrossRef] [Green Version]
- McKenna, R.A.; Rollo, M.E.; Skinner, J.A.; Burrows, T.L. Food Addiction Support: Website Content Analysis. JMIR Cardio 2018, 2, e10. [Google Scholar] [CrossRef] [Green Version]
- Hilker, I.; Sanchez, I.; Steward, T.; Jimenez-Murcia, S.; Granero, R.; Gearhardt, A.N.; Rodriguez-Munoz, R.C.; Dieguez, C.; Crujeiras, A.B.; Tolosa-Sola, I.; et al. Food Addiction in Bulimia Nervosa: Clinical Correlates and Association with Response to a Brief Psychoeducational Intervention. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2016, 24, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Schroder, R.; Sellman, D.; Elmslie, J. Addictive overeating: Lessons learned from medical students’ perceptions of Overeaters Anonymous. N. Z. Med. J. 2010, 123, 15–21. [Google Scholar] [PubMed]
- Cattivelli, R.; Pietrabissa, G.; Ceccarini, M.; Spatola, C.A.; Villa, V.; Caretti, A.; Gatti, A.; Manzoni, G.M.; Castelnuovo, G. ACTonFOOD: Opportunities of ACT to address food addiction. Front. Psychol. 2015, 6, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassin, S.; Leung, S.; Hawa, R.; Wnuk, S.; Jackson, T.; Sockalingam, S. Food Addiction Is Associated with Binge Eating and Psychiatric Distress among Post-Operative Bariatric Surgery Patients and May Improve in Response to Cognitive Behavioural Therapy. Nutrients 2020, 12, 2905. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, N.; Sokunbi, M.O.; Lawrence, A.D.; Lawrence, N.S.; Linden, D.E.J. Neurofeedback of visual food cue reactivity: A potential avenue to alter incentive sensitization and craving. Brain Imaging Behav. 2017, 11, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Kohl, S.H.; Veit, R.; Spetter, M.S.; Gunther, A.; Rina, A.; Luhrs, M.; Birbaumer, N.; Preissl, H.; Hallschmid, M. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: A randomized controlled trial in overweight and obese subjects. Neuroimage 2019, 191, 596–609. [Google Scholar] [CrossRef]
- Pretlow, R.A.; Stock, C.M.; Roeger, L.; Allison, S. Treatment of the sensory and motor components of urges to eat (eating addiction?): A mobile-health pilot study for obesity in young people. Eat. Weight Disord. EWD 2020. [Google Scholar] [CrossRef] [Green Version]

| Broader Categories | SUD Criteria |
|---|---|
| Impaired control | Substance often taken in larger amounts or over a longer period than was intended Craving, or a strong desire or urge to use the substance Persistent desire or repeated unsuccessful attempts to quit and/or control substance use Great deal of time is spent in activities necessary to obtain or use the substance or recover from its effects |
| Social impairment | Continued use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of the substance Recurrent substance use resulting in a failure to fulfill major role obligations at work, school, or home Important social, occupational, or recreational activities are given up or reduced because of substance use |
| Continued used despite risk | Recurrent substance use in situations in which it is physically hazardous Substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance |
| Pharmacological criteria | Tolerance: Need for markedly increased amounts of the substance to achieve intoxication or desired effect or Markedly diminished effect with continued use of the same amount of the substance Withdrawal: Withdrawal syndrome (differs by substance) or Substance is taken to relieve or avoid withdrawal symptoms |
| Articles Titles | Subjects | Exploration Methods | Main Results | References |
|---|---|---|---|---|
| Neural correlates of inhibitory control in youth with symptoms of FA | 76 young subjects (8.2–17.8 yo, 44 males) | Go/no-go task during BOLD fMRI | YFAS-positive subjects showed deactivation in three clusters: middle temporal gyrus/occipital gyrus, precuneus/calcarine sulcus, and inferior frontal gyrus | [130] |
| Neuroanatomical correlates of food addiction symptoms and body mass index in the general population | 625 subjects (Leipzig Research Centre for Civilization Diseases LIFE-Adult study), 20–59 yo, 45% women | BMI, personality questionnaires including YFAS and TFEQ, and brain structure via high-resolution 3T MRI | Small, additional contribution of YFAS symptom score to lower right lateral orbitofrontal cortex thickness over the effect of BMI | [131] |
| Food cue reactivity in FA: A functional magnetic resonance imaging study | 44 women with overweight or obesity, n = 20 with moderate-to-severe YFAS FA | YFAS, BOLD fMRI cue reactivity task | Subjects with FA exhibited modest, elevated responses in the sFG for highly processed food images and more robust, decreased activations for minimally processed food cues, whereas control subjects showed the opposite responses; Housefold items elicited greater activation than the food cues in regions associated with interoceptive awareness and visuospatial attention (e.g., INS, iFG, iPL) | [132] |
| FA distinguishes an overweight phenotype that can be reversed by low calorie diet | 36 overweight women | YFAS, 18 FDG-PET | Greater activation in thalamus, hypothalamus, midbrain, putamen, and occipital cortex (reward), but not in prefrontal and orbitofrontal cortices (control/reward receipt) in the high-YFAS versus low-YFAS group. In high-YFAS subjects, orbitofrontal responsiveness was inversely related to YFAS severity and hunger rating, and positive associations were observed between regional brain activation and lipid intake. A 3-month low-calorie diet abolished group differences in brain activation | [133] |
| Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects | 63 healthy subjects with and without elevated BMI (29 men and 34 women) | Fecal sampling, HAD anxiety and YFAS questionnaires, functional and anatomical connectivity of the amygdala, nucleus accumbens, and anterior insula | Direct positive association of indole metabolites with BMI and indirect positive association with YFAS through functional connectivity of the nucleus accumbens | [134] |
| FA is associated with impaired performance monitoring | 34 YFAS-positive and 34 control subjects | YFAS, Eriksen flanker task, and EEG measurement | YAFS-positive subjects have reduced ERN and Pe waves and demonstrate a higher number of errors on the flanker task, suggesting impaired performance monitoring | [135] |
| Neural correlates of FA | 49 healthy adolescent females ranging from lean to obese | YFAS, BOLD fMRI in response to receipt and anticipated receipt of palatable food (chocolate milkshake) | YFAS correlated with greater activation in the aCC, OFC, and amygdala in response to anticipated receipt of food. Participants with higher (n = 15) vs. lower (n = 11) YFAS showed greater activation in the DLPFC and CAU in response to anticipated receipt of food, but less activation in the lOFC in response to receipt of food | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constant, A.; Moirand, R.; Thibault, R.; Val-Laillet, D. Meeting of Minds around Food Addiction: Insights from Addiction Medicine, Nutrition, Psychology, and Neurosciences. Nutrients 2020, 12, 3564. https://doi.org/10.3390/nu12113564
Constant A, Moirand R, Thibault R, Val-Laillet D. Meeting of Minds around Food Addiction: Insights from Addiction Medicine, Nutrition, Psychology, and Neurosciences. Nutrients. 2020; 12(11):3564. https://doi.org/10.3390/nu12113564
Chicago/Turabian StyleConstant, Aymery, Romain Moirand, Ronan Thibault, and David Val-Laillet. 2020. "Meeting of Minds around Food Addiction: Insights from Addiction Medicine, Nutrition, Psychology, and Neurosciences" Nutrients 12, no. 11: 3564. https://doi.org/10.3390/nu12113564
APA StyleConstant, A., Moirand, R., Thibault, R., & Val-Laillet, D. (2020). Meeting of Minds around Food Addiction: Insights from Addiction Medicine, Nutrition, Psychology, and Neurosciences. Nutrients, 12(11), 3564. https://doi.org/10.3390/nu12113564





