Sex-Specific Effects of Chronic Creatine Supplementation on Hippocampal-Mediated Spatial Cognition in the 3xTg Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Dietary Supplementation, and Experimental Design
2.2. Morris Water Maze (MWM)
2.3. Protein Extraction
2.4. Western Blots
2.5. Creatine Assay
2.6. Mitochondrial Oxygen Consumption Rates from Freshly Isolated Hippocampal Mitochondria
2.7. Statistical Analysis
3. Results
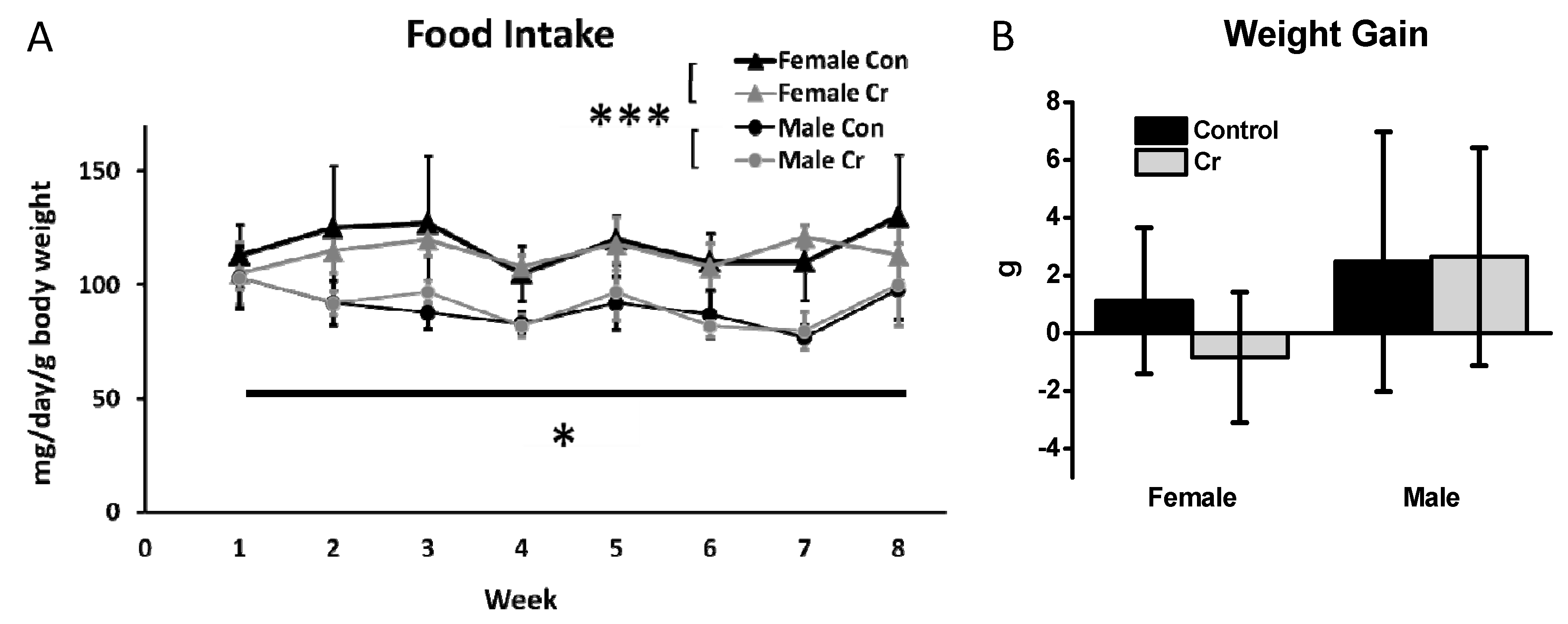
3.1. Food Intake and Weight Gain
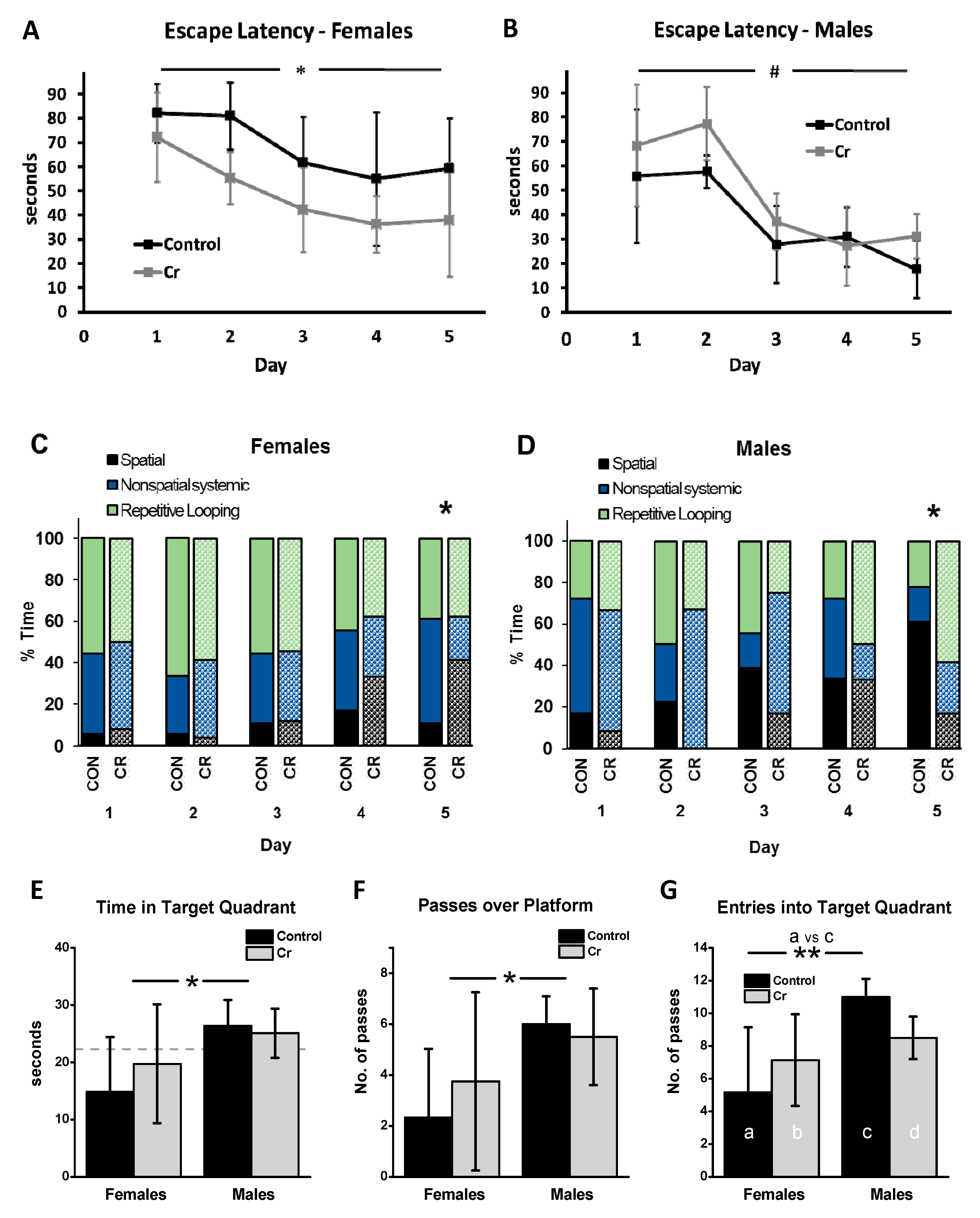
3.2. MWM Parameters: Acquisition Phase
3.2.1. Escape Latency
3.2.2. Search Strategy
3.2.3. Swim Speed
3.3. MWM Parameters: Retention Phase
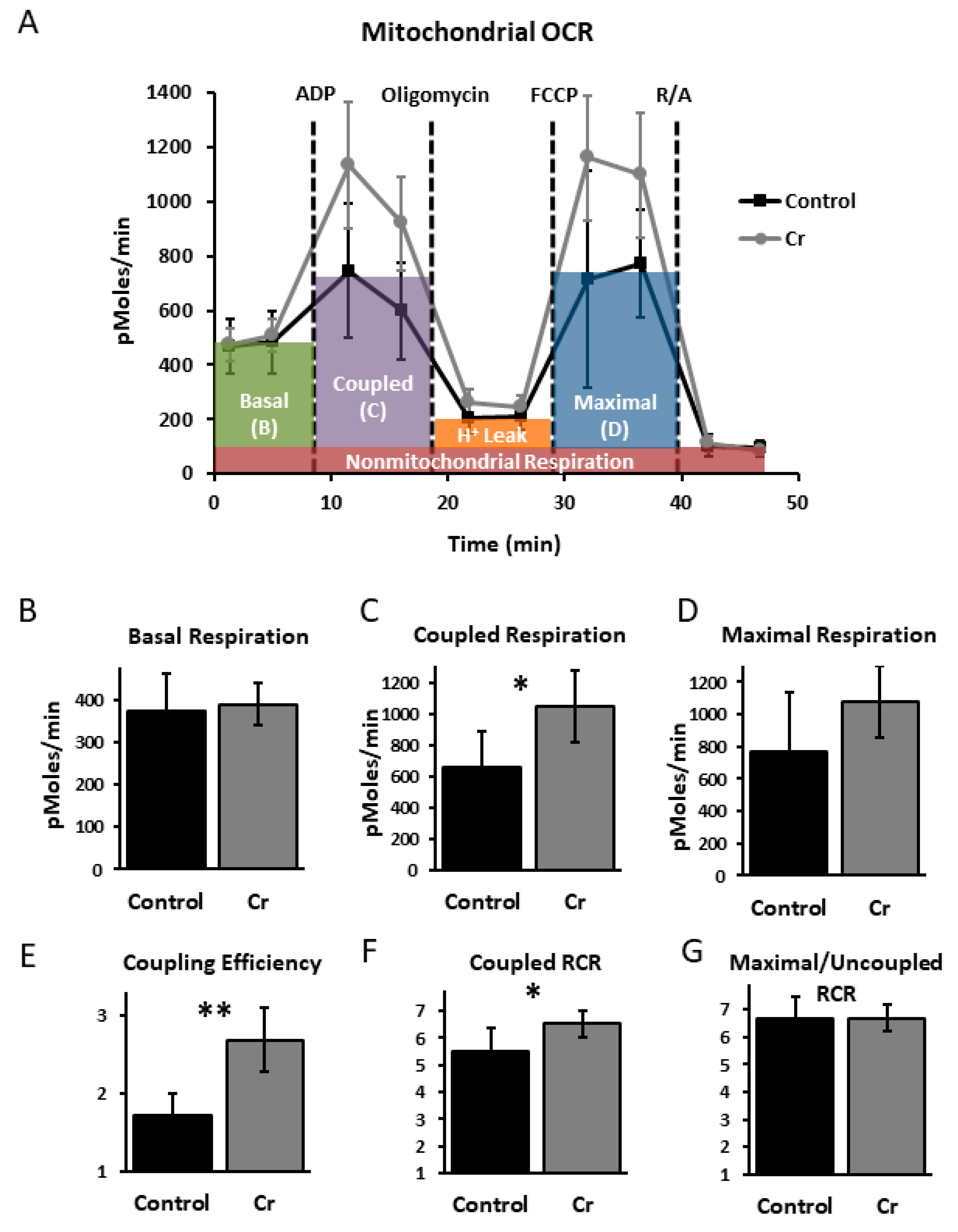
3.4. Hippocampal Mitochondrial Analysis
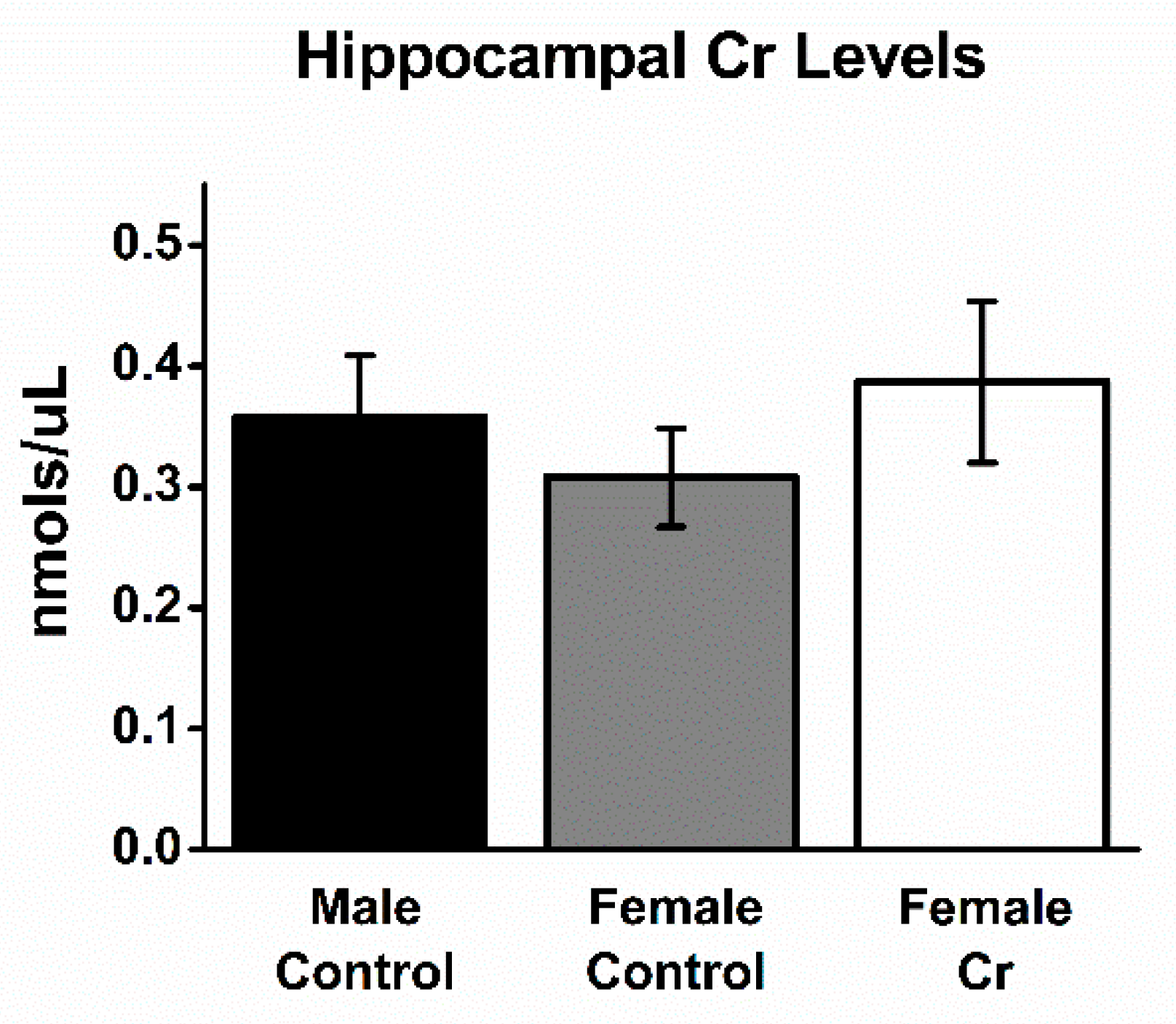
3.5. Hippocampal Cr Levels
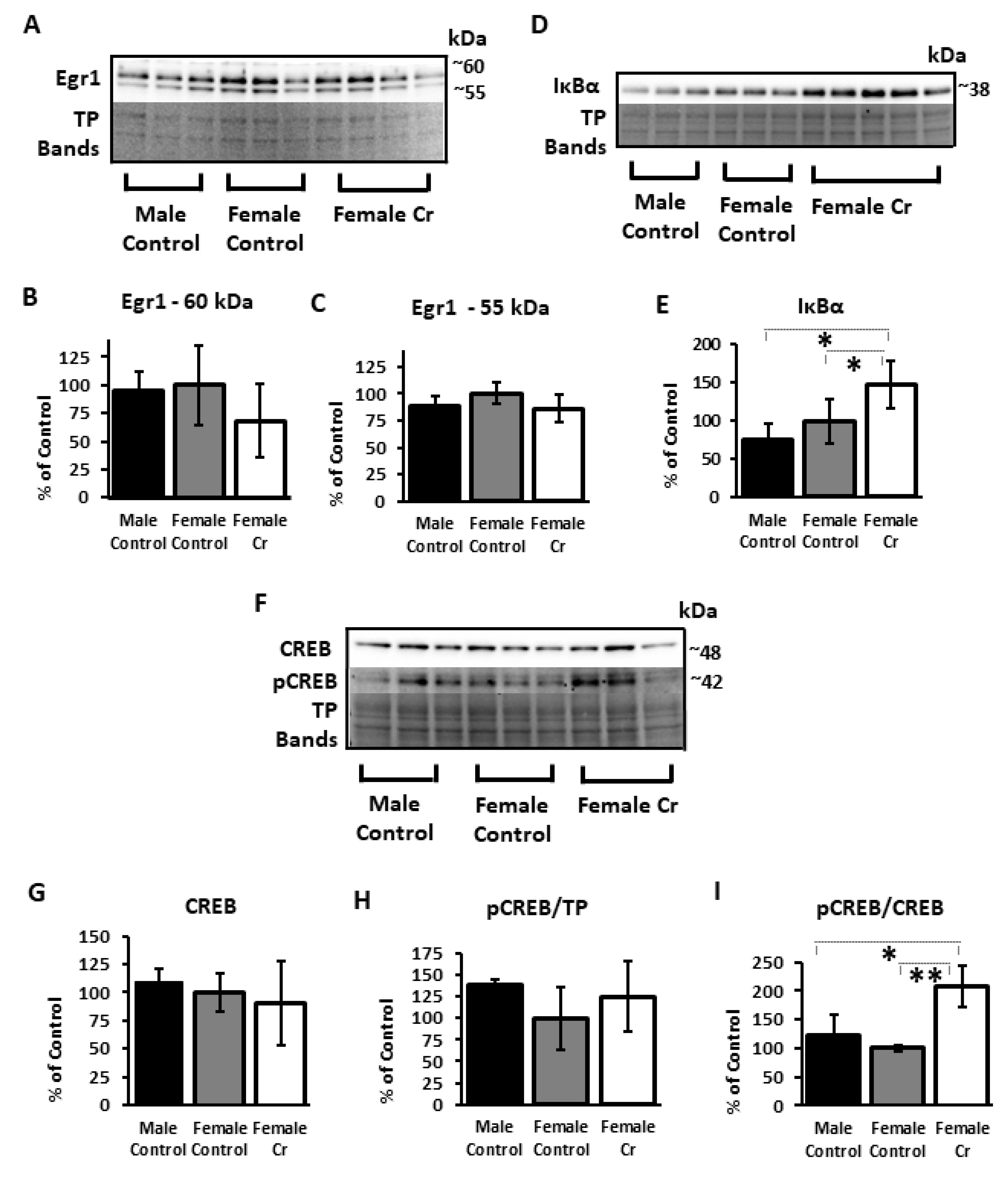
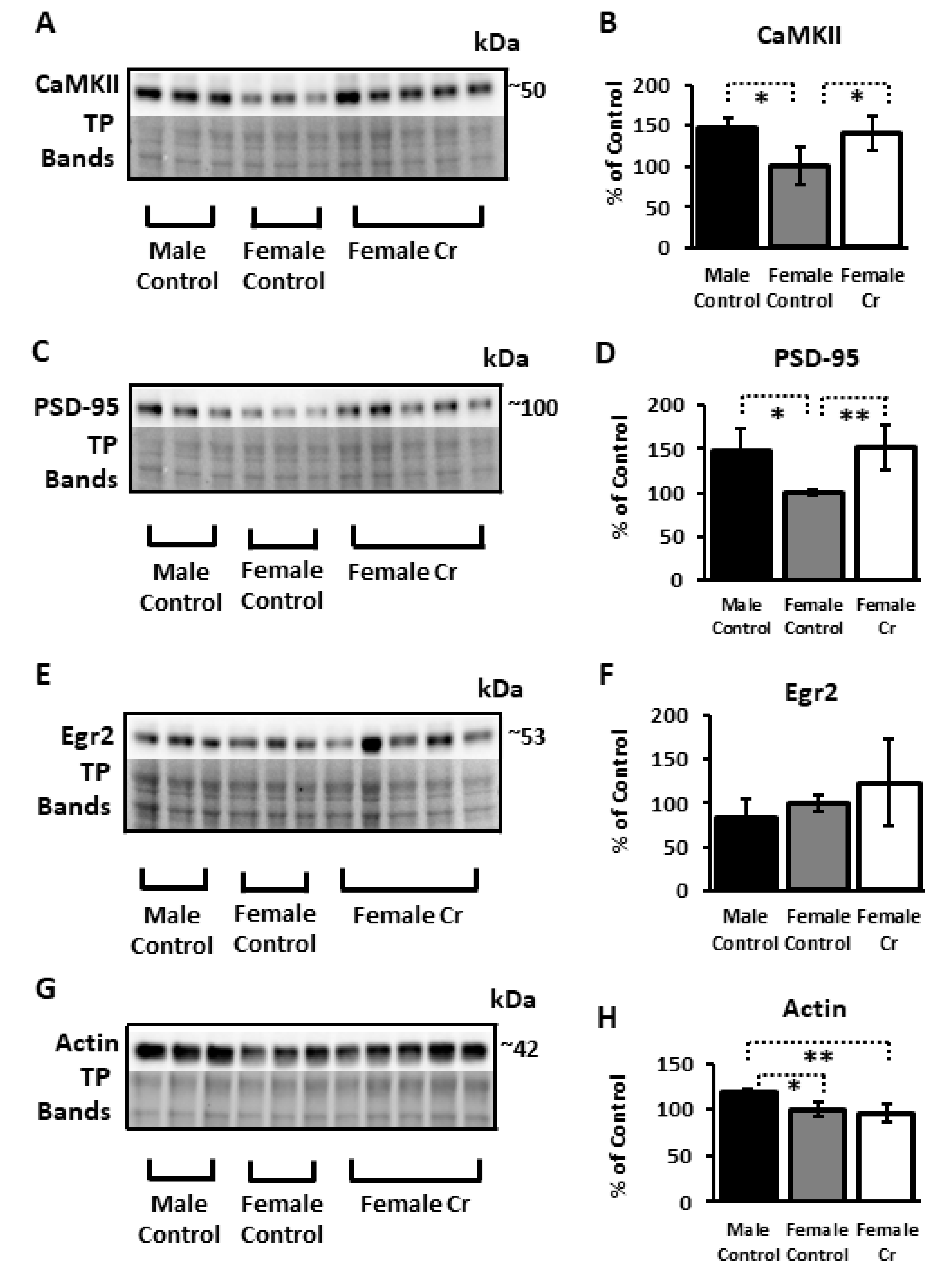
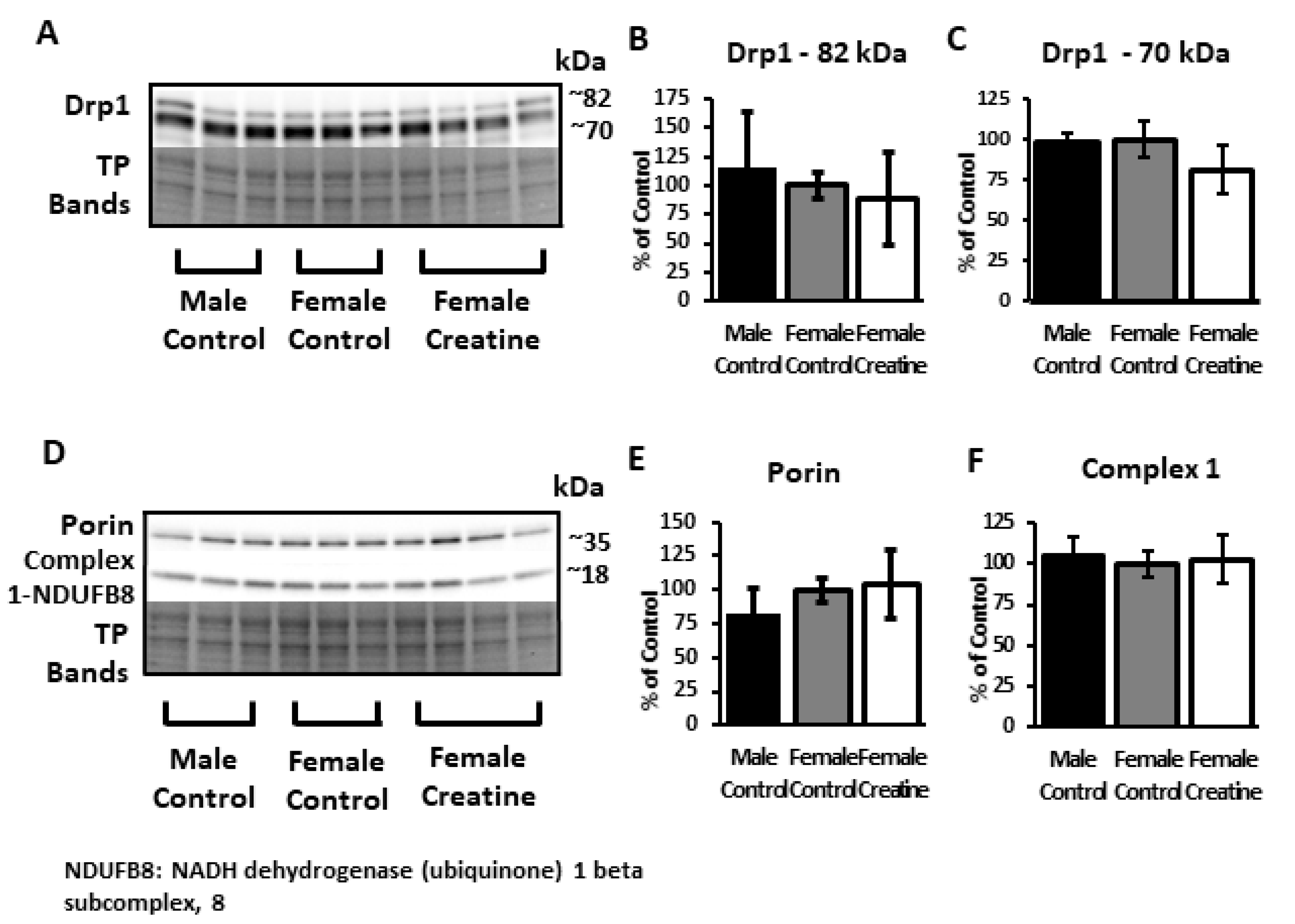
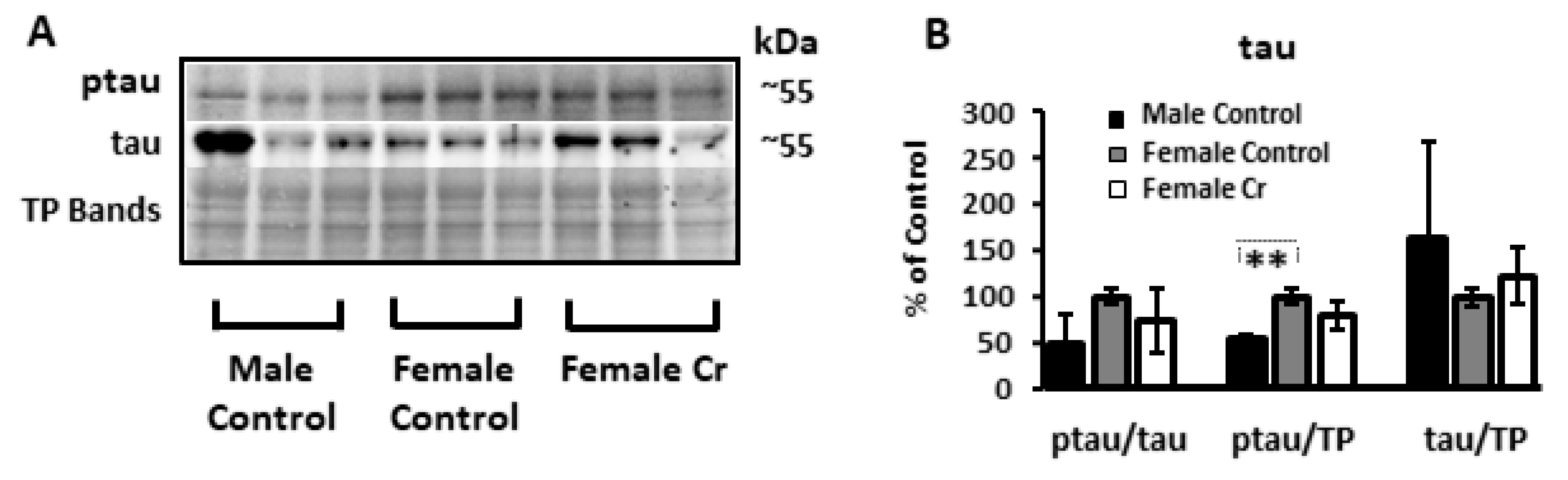
3.6. Western Blots
3.6.1. Transcription Factors and Related Proteins
3.6.2. Plasticity Proteins
3.6.3. Mitochondrial Proteins
3.6.4. AD-Related Proteins
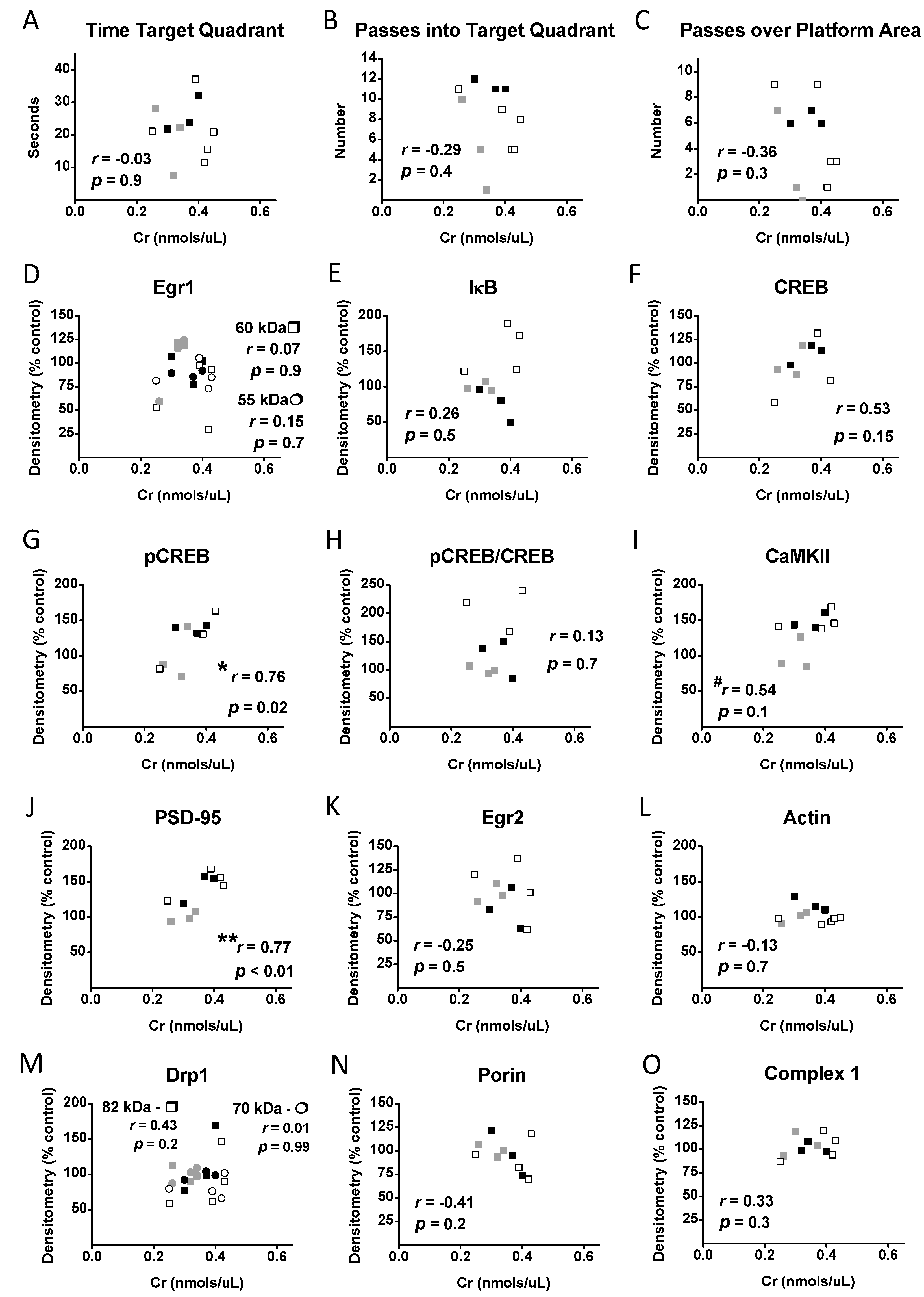
3.7. Correlational Analysis with Cr, Memory, and Protein Levels
4. Discussion
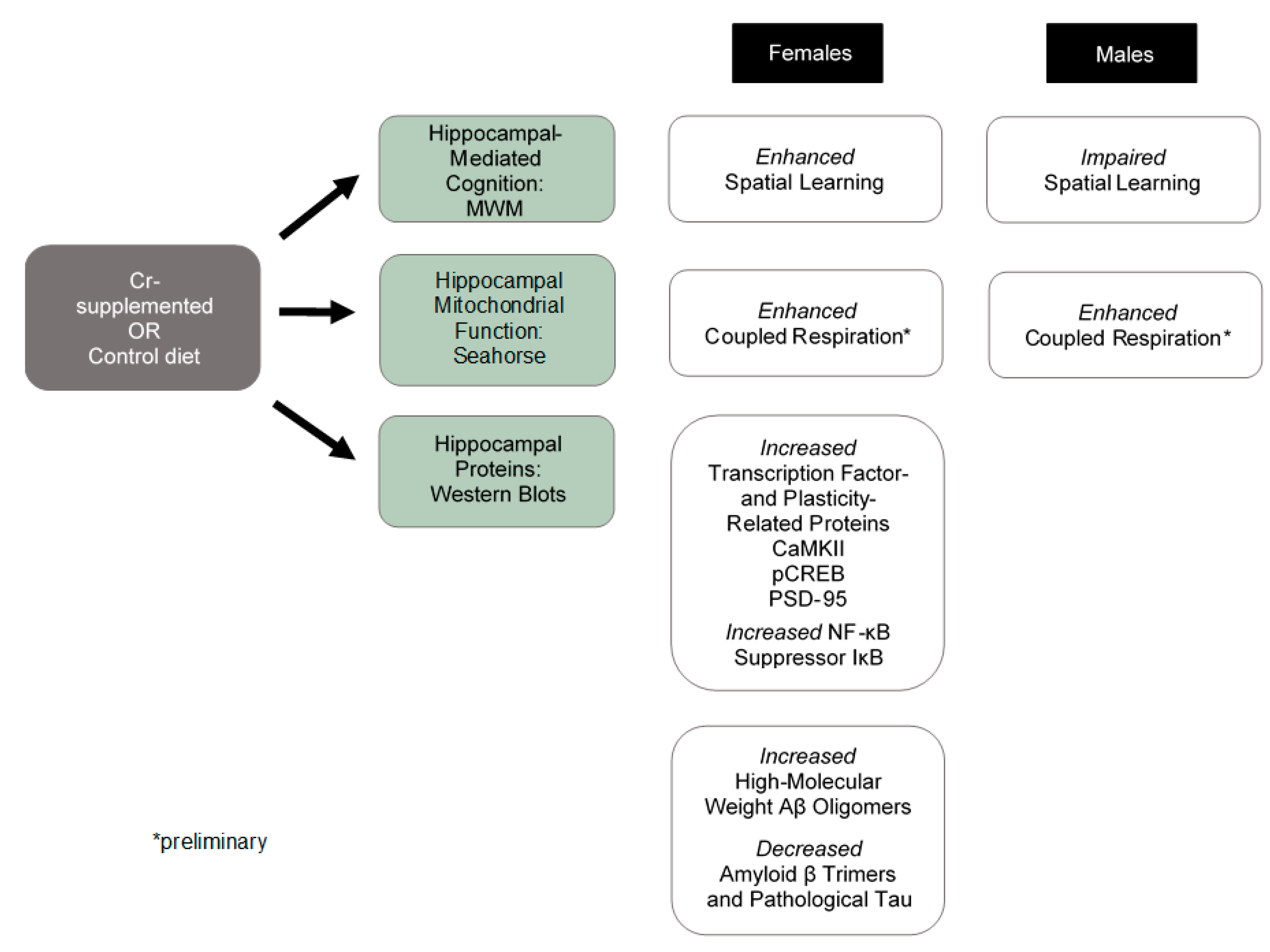
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [PubMed]
- Rae, C.D.; Broer, S. Creatine as a booster for human brain function. How might it work? Neurochem. Int. 2015, 89, 249–259. [Google Scholar] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [PubMed]
- Schlattner, U.; Tokaska-Schlattner, M.; Wallimann, T. Metabolite Channeling: Creatine Kinase Microcompartments. Encycl. Biol. Chem. 2013, 3, 80–85. [Google Scholar]
- Guimbal, C.; Kilimann, M.W. A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney. cDNA cloning and functional expression. J. Biol. Chem. 1993, 268, 8418–8421. [Google Scholar]
- Brewer, G.J.; Wallimann, T.W. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 2000, 74, 1968–1978. [Google Scholar]
- Mark, R.J.; Pang, Z.; Geddes, J.W.; Uchida, K.; Mattson, M.P. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: Involvement of membrane lipid peroxidation. J. Neurosci. 1997, 17, 1046–1054. [Google Scholar]
- Nicotera, P.; Leist, M.; Ferrando-May, E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol. Lett. 1998, 102–103, 139–142. [Google Scholar]
- Morris, J.C. Early-stage and preclinical Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2005, 19, 163–165. [Google Scholar]
- Snow, W.M.; Albensi, B.C. Neuronal Gene Targets of NF-kappaB and Their Dysregulation in Alzheimer’s Disease. Front. Mol. Neurosci. 2016, 9, 118. [Google Scholar]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar]
- Ferrer, I.; Marti, E.; Lopez, E.; Tortosa, A. NF-kB immunoreactivity is observed in association with beta A4 diffuse plaques in patients with Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 1998, 24, 271–277. [Google Scholar]
- Kitamura, Y.; Shimohama, S.; Ota, T.; Matsuoka, Y.; Nomura, Y.; Taniguchi, T. Alteration of transcription factors NF-κB and STAT1 in Alzheimer’s disease brains. Neurosci. Lett. 1997, 237, 17–20. [Google Scholar]
- Terai, K.; Matsuo, A.; McGeer, P.L. Enhancement of immunoreactivity for NF-kappa B in the hippocampal formation and cerebral cortex of Alzheimer’s disease. Brain Res. 1996, 735, 159–168. [Google Scholar] [PubMed]
- Kaltschmidt, B.; Uherek, M.; Wellmann, H.; Volk, B.; Kaltschmidt, C. Inhibition of NF-κB potentiates amyloid β-mediated neuronal apoptosis. Proc. Natl. Acad. Sci. USA 1999, 96, 9409–9414. [Google Scholar] [PubMed] [Green Version]
- Snow, W.M.; Stoesz, B.M.; Kelly, D.M.; Albensi, B.C. Roles for NF-kappaB and gene targets of NF-kappaB in synaptic plasticity, memory, and navigation. Mol. Neurobiol. 2014, 49, 757–770. [Google Scholar]
- Kaltschmidt, B.; Kaltschmidt, C. NF-B in the Nervous System. Cold Spring Harb. Perspect. Biol. 2009, 1, a001271. [Google Scholar]
- Juravleva, E.; Barbakadze, T.; Mikeladze, D.; Kekelidze, T. Creatine enhances survival of glutamate-treated neuronal/glial cells, modulates Ras/NF-kappaB signaling, and increases the generation of reactive oxygen species. J. Neurosci. Res. 2005, 79, 224–230. [Google Scholar] [PubMed]
- Snow, W.M.; Cadonic, C.; Cortes-Perez, C.; Roy Chowdhury, S.K.; Djordjevic, J.; Thomson, E.; Bernstein, M.J.; Suh, M.; Fernyhough, P.; Albensi, B.C. Chronic dietary creatine enhances hippocampal-dependent spatial memory, bioenergetics, and levels of plasticity-related proteins associated with. Learn. Mem. 2018, 25, 54–66. [Google Scholar] [PubMed]
- Frankland, P.W.; O’Brien, C.; Ohno, M.; Kirkwood, A.; Silva, A.J. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature 2001, 411, 309–313. [Google Scholar] [PubMed]
- Lilienbaum, A.; Israël, A. From Calcium to NF-κB Signaling Pathways in Neurons. Mol. Cell. Biol. 2003, 23, 2680–2698. [Google Scholar] [PubMed] [Green Version]
- Federman, N.; de la Fuente, V.; Zalcman, G.; Corbi, N.; Onori, A.; Passananti, C.; Romano, A. Nuclear factor kappaB-dependent histone acetylation is specifically involved in persistent forms of memory. J. Neurosci. 2013, 33, 7603–7614. [Google Scholar] [PubMed]
- Boersma, M.C.; Dresselhaus, E.C.; De Biase, L.M.; Mihalas, A.B.; Bergles, D.E.; Meffert, M.K. A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J. Neurosci. 2011, 31, 5414–5425. [Google Scholar]
- Rijpma, A.; van der Graaf, M.; Meulenbroek, O.; Olde Rikkert, M.G.M.; Heerschap, A. Altered brain high-energy phosphate metabolism in mild Alzheimer’s disease: A 3-dimensional 31P MR spectroscopic imaging study. Neuroimage Clin. 2018, 18, 254–261. [Google Scholar] [PubMed]
- Pettegrew, J.W.; Panchalingam, K.; Klunk, W.E.; McClure, R.J.; Muenz, L.R. Alterations of cerebral metabolism in probable Alzheimer’s disease: A preliminary study. Neurobiol. Aging 1994, 15, 117–132. [Google Scholar] [PubMed]
- Aksenov, M.Y.; Aksenova, M.V.; Payne, R.M.; Smith, C.D.; Markesbery, W.R.; Carney, J.M. The Expression of Creatine Kinase Isoenzymes in Neocortex of Patients with Neurodegenerative Disorders: Alzheimer’s and Pick’s Disease. Exp. Neurol. 1997, 146, 458–465. [Google Scholar] [PubMed]
- Laakso, M.P.; Hiltunen, Y.; Könönen, M.; Kivipelto, M.; Koivisto, A.; Hallikainen, M.; Soininen, H. Decreased brain creatine levels in elderly apolipoprotein E ε4 carriers. J. Neural Transm. 2003, 110, 267–275. [Google Scholar] [PubMed]
- AliMohammadi, M.; Eshraghian, M.; Zarindast, M.-R.; Aliaghaei, A.; Pishva, H. Effects of creatine supplementation on learning, memory retrieval, and apoptosis in an experimental animal model of Alzheimer disease. Med. J. Islam. Repub. Iran 2015, 29, 273. [Google Scholar]
- Klein, A.M.; Ferrante, R.J. The Neuroprotective Role of Creatine. In Creatine and Creatine Kinase in Health and Disease; Salomons, G.S., Wyss, M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 205–243. ISBN 978-1-4020-6486-9. [Google Scholar]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Clos, A.L.; Jackson, G.R.; Kayed, R. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 2011, 6, 39. [Google Scholar]
- David, D.C.; Hauptmann, S.; Scherping, I.; Schuessel, K.; Keil, U.; Rizzu, P.; Ravid, R.; Drose, S.; Brandt, U.; Muller, W.E.; et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 2005, 280, 23802–23814. [Google Scholar]
- Flannery, P.J.; Trushina, E. Mitochondrial dynamics and transport in Alzheimer’s disease. Mol. Cell. Neurosci. 2019, 98, 109–120. [Google Scholar] [PubMed]
- Parker, W.D.J.; Parks, J.; Filley, C.M.; Kleinschmidt-DeMasters, B.K. Electron transport chain defects in Alzheimer’s disease brain. Neurology 1994, 44, 1090–1096. [Google Scholar] [PubMed]
- Giachin, G.; Bouverot, R.; Acajjaoui, S.; Pantalone, S.; Soler-Lopez, M. Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3, 43. [Google Scholar] [PubMed] [Green Version]
- Billings, L.M.; Oddo, S.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 2005, 45, 675–688. [Google Scholar] [PubMed] [Green Version]
- Yang, J.-T.; Wang, Z.-J.; Cai, H.-Y.; Yuan, L.; Hu, M.-M.; Wu, M.-N.; Qi, J.-S. Sex Differences in Neuropathology and Cognitive Behavior in APP/PS1/tau Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2018, 34, 736–746. [Google Scholar]
- Allen, P.J.; D’Anci, K.E.; Kanarek, R.B.; Renshaw, P.F. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology 2010, 35, 534–546. [Google Scholar] [PubMed] [Green Version]
- Allen, P.J.; DeBold, J.F.; Rios, M.; Kanarek, R.B. Chronic high-dose creatine has opposing effects on depression-related gene expression and behavior in intact and sex hormone-treated gonadectomized male and female rats. Pharmacol. Biochem. Behav. 2015, 130, 22–33. [Google Scholar]
- Pike, C.J. Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 671–680. [Google Scholar]
- Snow, W.M.; Pahlavan, P.S.; Djordjevic, J.; McAllister, D.; Platt, E.E.; Alashmali, S.; Bernstein, M.J.; Suh, M.; Albensi, B.C. Morris Water Maze Training in Mice Elevates Hippocampal Levels of Transcription Factors Nuclear Factor (Erythroid-derived 2)-like 2 and Nuclear Factor Kappa B p65. Front. Mol. Neurosci. 2015, 8, 70. [Google Scholar]
- Guzowski, J.F.; McGaugh, J.L. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. USA 1997, 94, 2693–2698. [Google Scholar]
- Brody, D.L.; Holtzman, D.M. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 2006, 197, 330–340. [Google Scholar] [PubMed] [Green Version]
- Frezza, C.; Cipolat, S.; Scorrano, L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2007, 2, 287–295. [Google Scholar] [PubMed] [Green Version]
- Fonseca, T.B.; Sánchez-Guerrero, Á.; Milosevic, I.; Raimundo, N. Mitochondrial fission requires DRP1 but not dynamins. Nature 2019, 570, E34–E42. [Google Scholar] [PubMed]
- Hatami, A.; Albay, R.; Monjazeb, S.; Milton, S.; Glabe, C. Monoclonal Antibodies against Aβ42 Fibrils Distinguish Multiple Aggregation State Polymorphisms in vitro and in Alzheimer Disease Brain. J. Biol. Chem. 2014, 289, 32131–32143. [Google Scholar]
- Djordjevic, J.; Roy Chowdhury, S.; Snow, W.M.; Perez, C.; Cadonic, C.; Fernyhough, P.; Albensi, B.C. Early Onset of Sex-Dependent Mitochondrial Deficits in the Cortex of 3xTg Alzheimer’s Mice. Cells 2020, 9, 1541. [Google Scholar]
- Oddo, S.; Caccamo, A.; Kitazawa, M.; Tseng, B.P.; LaFerla, F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 2003, 24, 1063–1070. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar]
- Roddick, K.M.; Roberts, A.D.; Schellinck, H.M.; Brown, R.E. Sex and Genotype Differences in Odor Detection in the 3×Tg-AD and 5XFAD Mouse Models of Alzheimer’s Disease at 6 Months of Age. CHEMSE 2016, 41, 433–440. [Google Scholar]
- Colombo, D.; Caltagirone, C.; Padovani, A.; Sorbi, S.; Spalletta, G.; Simoni, L.; Ori, A.; Zagni, E. Gender Differences in Neuropsychiatric Symptoms in Mild to Moderate Alzheimer’s Disease Patients Undergoing Switch of Cholinesterase Inhibitors: A Post Hoc Analysis of the EVOLUTION Study. J. Womens Health 2018, 27, 1368–1377. [Google Scholar]
- Brownlow, M.L.; Joly-Amado, A.; Azam, S.; Elza, M.; Selenica, M.-L.; Pappas, C.; Small, B.; Engelman, R.; Gordon, M.N.; Morgan, D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res. 2014, 271, 79–88. [Google Scholar]
- Seney, M.L.; Huo, Z.; Cahill, K.; French, L.; Puralewski, R.; Zhang, J.; Logan, R.W.; Tseng, G.; Lewis, D.A.; Sibille, E. Opposite molecular signatures of depression in men and women. Biol. Psychiatry 2018, 84, 18–27. [Google Scholar] [PubMed]
- Bender, A.; Beckers, J.; Schneider, I.; Holter, S.M.; Haack, T.; Ruthsatz, T.; Vogt-Weisenhorn, D.M.; Becker, L.; Genius, J.; Rujescu, D.; et al. Creatine improves health and survival of mice. Neurobiol. Aging 2008, 29, 1404–1411. [Google Scholar] [PubMed]
- Kim, D.I.; Lee, K.H.; Gabr, A.A.; Choi, G.E.; Kim, J.S.; Ko, S.H.; Han, H.J. Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2820–2834. [Google Scholar]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer’s Disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar]
- O’Sullivan, N.C.; Croydon, L.; McGettigan, P.A.; Pickering, M.; Murphy, K.J. Hippocampal region-specific regulation of NF-kappaB may contribute to learning-associated synaptic reorganisation. Brain Res. Bull. 2010, 81, 385–390. [Google Scholar]
- Kaltschmidt, B.; Ndiaye, D.; Korte, M.; Pothion, S.; Arbibe, L.; Prullage, M.; Pfeiffer, J.; Lindecke, A.; Staiger, V.; Israel, A.; et al. NF-B Regulates Spatial Memory Formation and Synaptic Plasticity through Protein Kinase A/CREB Signaling. Mol. Cell. Biol. 2006, 26, 2936–2946. [Google Scholar]
- Walton, M.R.; Dragunow, M. Is CREB a key to neuronal survival? Trends Neurosci. 2000, 23, 48–53. [Google Scholar] [PubMed]
- Shenkar, R.; Yum, H.-K.; Arcaroli, J.; Kupfner, J.; Abraham, E. Interactions between CBP, NF-κB, and CREB in the lungs after hemorrhage and endotoxemia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L418–L426. [Google Scholar] [PubMed]
- Sun, P.; Enslen, H.; Myung, P.S.; Maurer, R.A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994, 8, 2527–2539. [Google Scholar] [PubMed] [Green Version]
- Souza, M.A.; Magni, D.V.; Guerra, G.P.; Oliveira, M.S.; Furian, A.F.; Pereira, L.; Marquez, S.V.; Ferreira, J.; Fighera, M.R.; Royes, L.F.F. Involvement of hippocampal CAMKII/CREB signaling in the spatial memory retention induced by creatine. Amino Acids 2012, 43, 2491–2503. [Google Scholar] [PubMed]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-κB Function in the Nervous System. Front. Immunol. 2019, 10, 1043. [Google Scholar] [PubMed]
- Nomura, A.; Zhang, M.; Sakamoto, T.; Ishii, Y.; Morishima, Y.; Mochizuki, M.; Kimura, T.; Uchida, Y.; Sekizawa, K. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br. J. Pharmacol. 2003, 139, 715–720. [Google Scholar] [PubMed] [Green Version]
- Jana, M.K.; Cappai, R.; Pham, C.L.L.; Ciccotosto, G.D. Membrane-bound tetramer and trimer Aβ oligomeric species correlate with toxicity towards cultured neurons. J. Neurochem. 2016, 136, 594–608. [Google Scholar] [PubMed] [Green Version]
- Townsend, M.; Shankar, G.M.; Mehta, T.; Walsh, D.M.; Selkoe, D.J. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: A potent role for trimers. J. Physiol. 2006, 572, 477–492. [Google Scholar]
- Crouch, P.J.; Hung, L.W.; Adlard, P.A.; Cortes, M.; Lal, V.; Filiz, G.; Perez, K.A.; Nurjono, M.; Caragounis, A.; Du, T.; et al. Increasing Cu bioavailability inhibits A oligomers and tau phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 381–386. [Google Scholar]
- Lesné, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar]
- Clark, J.K.; Furgerson, M.; Crystal, J.D.; Fechheimer, M.; Furukawa, R.; Wagner, J.J. Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice. Neurobiol. Learn. Mem. 2015, 125, 152–162. [Google Scholar]
- Park, S.-A.; Park, H.W.; Kim, N.-H.; Kim, Y.-H.; Kwak, M.-J.; Shin, J.-S.; Kim, C.-W. Effects of Tau on the activity of triose phosphate isomerase (TPI) in brain cells. Neurochem. Int. 2010, 56, 886–892. [Google Scholar]
- Carroll, J.C.; Rosario, E.R.; Kreimer, S.; Villamagna, A.; Gentzschein, E.; Stanczyk, F.Z.; Pike, C.J. Sex differences in β-amyloid accumulation in 3xTg-AD mice: Role of neonatal sex steroid hormone exposure. Brain Res. 2010, 1366, 233–245. [Google Scholar]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 289, 29–38. [Google Scholar]
- Li, Z.; Okamoto, K.; Hayashi, Y.; Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119, 873–887. [Google Scholar] [PubMed] [Green Version]
- Cane, M.; Maco, B.; Knott, G.; Holtmaat, A. The Relationship between PSD-95 Clustering and Spine Stability In Vivo. J. Neurosci. 2014, 34, 2075–2086. [Google Scholar] [PubMed]
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629. [Google Scholar] [PubMed] [Green Version]
- Ghosh, A.; Giese, K.P. Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol. Brain 2015, 8, 78. [Google Scholar] [PubMed] [Green Version]
- Pugazhenthi, S.; Wang, M.; Pham, S.; Sze, C.-I.; Eckman, C.B. Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol. Neurodegener. 2011, 6, 60. [Google Scholar]
- Savioz, A.; Leuba, G.; Vallet, P.G. A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease. Ageing Res. Rev. 2014, 18, 86–94. [Google Scholar]
- Teich, A.F.; Nicholls, R.E.; Puzzo, D.; Fiorito, J.; Purgatorio, R.; Fa’, M.; Arancio, O. Synaptic Therapy in Alzheimer’s Disease: A CREB-centric Approach. Neurotherapeutics 2015, 12, 29–41. [Google Scholar]




| Antibody | Description | Supplier (Catalogue No.) | Dilution |
|---|---|---|---|
| Egr1 | Rabbit polyclonal | Santa Cruz (sc-189) | 1:2000 |
| Egr2 | Rabbit monoclonal | Abcam (ab108399) | 1:7500 |
| PSD-95 | Rabbit monoclonal | Abcam (ab76115) | 1:2000 |
| IκBα | Rabbit monoclonal | Abcam (ab32518) | 1:1000 |
| CaMKII | Rabbit polyclonal | Santa Cruz (M-176) | 1:2000 |
| Drp1 | Mouse monoclonal | Abcam (ab156951) | 1:1000 |
| Actin | Rabbit polyclonal | Sigma (A5060) | 1:1000 |
| Total OXPHOS rodent WB antibody cocktail | Mouse monoclonal | Abcam (ab110413) | 1:1000 |
| Porin | Mouse monoclonal | Abcam (ab14734) | 1:1000 |
| pCREB (at Serine 133) | Rabbit monoclonal | Abcam (ab32096) | 1:1000 |
| CREB | Rabbit monoclonal | Abcam (ab32515) | 1:1000 |
| AT8 (ptau at Ser202 and Thr 205) | Mouse monoclonal | Thermo Fisher Scientific (MN1020) | 1:500 |
| Total tau | Rabbit polyclonal | Abcam (ab39524) | 1:1000 |
| mOC87 (amyloid fibril & APP) | Rabbit monoclonal | Abcam (ab201062) | 1:1000 |
| 4G8 (amyloid β 1–42) | Mouse monoclonal | Biolegend (800701) | 1:1000 |
| mOC64 (amyloid β 1–42) | Rabbit monoclonal | Abcam (ab201060) | 1:1000 |
| A11 (oligomeric amyloid β 1–42) | Rabbit polyclonal | Thermo Fisher Scientific (AHB0052) | 1:1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snow, W.M.; Cadonic, C.; Cortes-Perez, C.; Adlimoghaddam, A.; Roy Chowdhury, S.K.; Thomson, E.; Anozie, A.; Bernstein, M.J.; Gough, K.; Fernyhough, P.; et al. Sex-Specific Effects of Chronic Creatine Supplementation on Hippocampal-Mediated Spatial Cognition in the 3xTg Mouse Model of Alzheimer’s Disease. Nutrients 2020, 12, 3589. https://doi.org/10.3390/nu12113589
Snow WM, Cadonic C, Cortes-Perez C, Adlimoghaddam A, Roy Chowdhury SK, Thomson E, Anozie A, Bernstein MJ, Gough K, Fernyhough P, et al. Sex-Specific Effects of Chronic Creatine Supplementation on Hippocampal-Mediated Spatial Cognition in the 3xTg Mouse Model of Alzheimer’s Disease. Nutrients. 2020; 12(11):3589. https://doi.org/10.3390/nu12113589
Chicago/Turabian StyleSnow, Wanda M., Chris Cadonic, Claudia Cortes-Perez, Aida Adlimoghaddam, Subir K. Roy Chowdhury, Ella Thomson, Adama Anozie, Michael J. Bernstein, Kathleen Gough, Paul Fernyhough, and et al. 2020. "Sex-Specific Effects of Chronic Creatine Supplementation on Hippocampal-Mediated Spatial Cognition in the 3xTg Mouse Model of Alzheimer’s Disease" Nutrients 12, no. 11: 3589. https://doi.org/10.3390/nu12113589
APA StyleSnow, W. M., Cadonic, C., Cortes-Perez, C., Adlimoghaddam, A., Roy Chowdhury, S. K., Thomson, E., Anozie, A., Bernstein, M. J., Gough, K., Fernyhough, P., Suh, M., & Albensi, B. C. (2020). Sex-Specific Effects of Chronic Creatine Supplementation on Hippocampal-Mediated Spatial Cognition in the 3xTg Mouse Model of Alzheimer’s Disease. Nutrients, 12(11), 3589. https://doi.org/10.3390/nu12113589






