The Protective Effects of Flavonoids in Cataract Formation through the Activation of Nrf2 and the Inhibition of MMP-9
Abstract
:1. Introduction
2. Types and Causes of Cataract Development
3. Pathophysiology of Cataracts
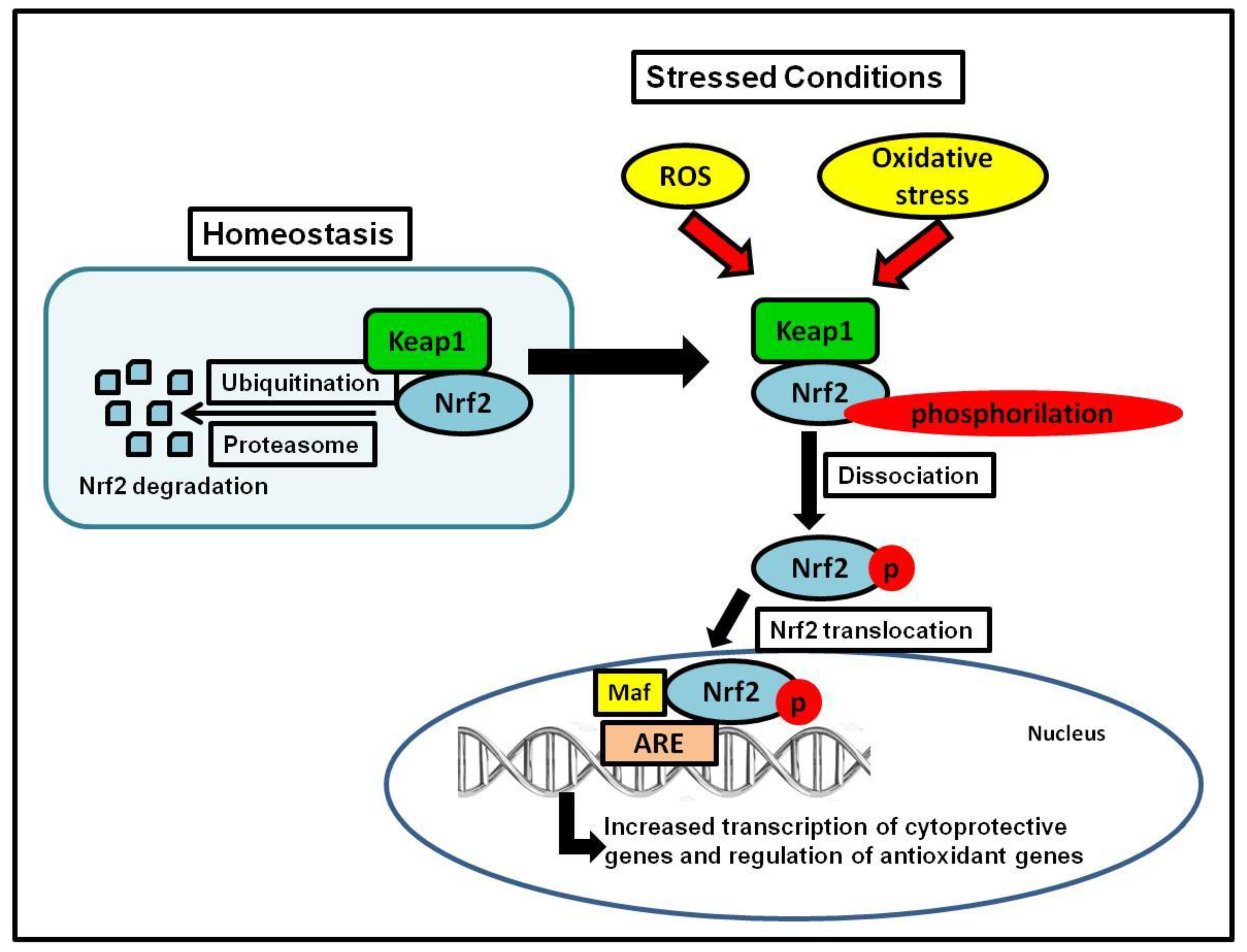
4. Nrf2 Reduces Oxidative Stress and Inflammation Levels
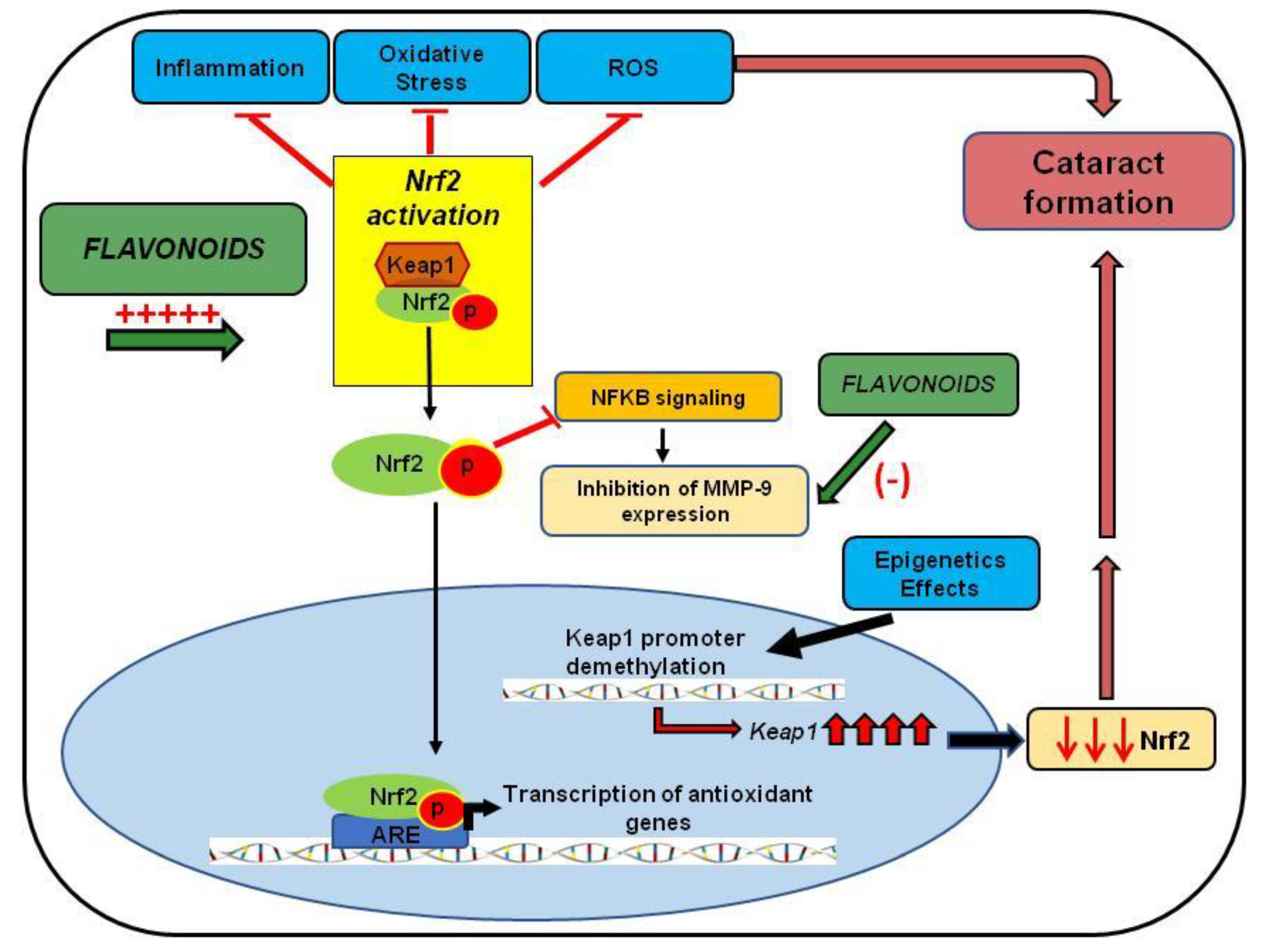
5. Nrf2 Activation and Cataracts
6. Epigenetics Modulation of Nrf2 Expression
7. Matrix Metalloproteinases Overexpression Induces Cataract Formation
8. Association between Nrf2 Expression and MMP-9 Activity
9. Polyphenols and Nrf2 Activation
10. Polyphenols and MMP-9 Inhibition
11. Polyphenols and Cataract Formation
11.1. Resveratrol
11.2. Curcumin
11.3. Quercetin
11.4. Epigallocatechin Gallate
11.5. Nigella sativa and Thymoquinone
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Blindness, and Vision Impairment Prevention. Available online: https://www.who.int/blindness/causes/priority/en/index1.html (accessed on 6 October 2020).
- Harding, J. Biochemistry epidemiology, and pharmacology. Cataract 1991, 195–217. [Google Scholar]
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species, and the Aging Eye: Specific Role of Metabolically Active Mitochondria in Maintaining Lens Function and in the Initiation of the Oxidation-Induced Maturity Onset Cataract—A Novel Platform of Mitochondria-Targeted Antioxidants with Broad Therapeutic Potential for Redox Regulation and Detoxification of Oxidants in Eye Diseases. Am. J. Ther. 2016, 23, e98–e117. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Gupta, V.; Christopher, A.F.; Malik, M.A.; Bansal, P. Nutraceuticals in the prevention of cataract—An evidence-based approach. Saudi J. Ophthalmol. 2017, 31, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Busbee, B.G.; Brown, M.M.; Brown, G.C.; Sharma, S. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology 2002, 109, 606–612; discussion 603–612. [Google Scholar] [CrossRef]
- Powe, N.R.; Schein, O.D.; Gieser, S.C.; Tielsch, J.M.; Luthra, R.; Javitt, J.; Steinberg, E.P. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch. Ophthalmol. 1994, 112, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Schein, O.D.; Steinberg, E.P.; Javitt, J.C.; Cassard, S.D.; Tielsch, J.M.; Steinwachs, D.M.; Legro, M.W.; Diener-West, M.; Sommer, A. Variation in cataract surgery practice and clinical outcomes. Ophthalmology 1994, 101, 1142–1152. [Google Scholar] [CrossRef]
- Javitt, J.C.; Street, D.A.; Tielsch, J.M.; Wang, Q.; Kolb, M.M.; Schien, O.; Sommer, A.; Bergner, M.; Steinberg, E.P. National outcomes of cataract extraction. Retinal detachment and endophthalmitis after outpatient cataract surgery. Cataract Patient Outcomes Research Team. Ophthalmology 1994, 101, 100–105; discussion 106. [Google Scholar] [CrossRef]
- Javitt, J.C.; Tielsch, J.M.; Canner, J.K.; Kolb, M.M.; Sommer, A.; Steinberg, E.P. National outcomes of cataract extraction. Increased risk of retinal complications associated with Nd: YAG laser capsulotomy. The Cataract Patient Outcomes Research Team. Ophthalmology 1992, 99, 1487–1497; discussion 1488–1497. [Google Scholar] [CrossRef]
- Cekić, S.; Zlatanović, G.; Cvetković, T.; Petrović, B. Oxidative stress in cataractogenesis. Bosn. J. Basic Med. Sci. 2010, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Mohan, M.; Sperduto, R.D.; Angra, S.K.; Milton, R.C.; Mathur, R.L.; Underwood, B.A.; Jaffery, N.; Pandya, C.B.; Chhabra, V.K.; Vajpayee, R.B. India-US case-control study of age-related cataracts. Arch. Ophthalmol. 1989, 107, 670–676. [Google Scholar] [CrossRef]
- Kaur, J.; Kukreja, S.; Kaur, A.; Malhotra, N.; Kaur, R. The oxidative stress in cataract patients. J. Clin. Diagn. Res. 2012, 6, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Farvardin-Jahromi, M.; Saadat, H. Null genotype of glutathione S-transferase M1 is associated with senile cataract susceptibility in non-smoker females. Biochem. Biophys. Res. Commun. 2004, 319, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Bjornstad, K.A.; Rosen, C.J.; Lin, S.; Blakely, E.A. Particle radiation alters expression of matrix metalloproteases resulting in ECM remodeling in human lens cells. Radiat. Environ. Biophys. 2007, 46, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, S.; Wormstone, I.M.; Marcantonio, J.M.; Gavrilovic, J.; Duncan, G. Induction of matrix metalloproteinases 2 and 9 following stress to the lens. Exp. Eye Res. 2000, 71, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Wormstone, I.M.; Tamiya, S.; Anderson, I.; Duncan, G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2301–2308. [Google Scholar]
- West-Mays, J.A.; Pino, G. Matrix Metalloproteinases as Mediators of Primary and Secondary Cataracts. Expert Rev. Ophthalmol. 2007, 2, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, D.J.; Pino, G.; Banh, A.; Nathu, Z.; Howchin, D.; Margetts, P.; Sivak, J.G.; West-Mays, J.A. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am. J. Pathol. 2006, 168, 69–79. [Google Scholar] [CrossRef]
- Vinson, J.A. Oxidative stress in cataracts. Pathophysiology 2006, 13, 151–162. [Google Scholar] [CrossRef]
- Gupta, V.B.; Rajagopala, M.; Ravishankar, B. Etiopathogenesis of cataract: An appraisal. Indian J. Ophthalmol. 2014, 62, 103–110. [Google Scholar] [CrossRef]
- Simon, H.; Zieve, D. New York: Time Health Guide; Last Updated and Reviewed on 23 June 2010; Cataract-Risk Factors (Internet). Available online: http://health.NYTimes.com/health/guides/disease/cataract/risk-factors (accessed on 6 October 2020).
- Hejtmancik, J.; Kaiser-Kupfer, M.; Piatigorsky, J. The Metabolic and Molecular Basis of Inherited Disease; McGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Khurana, K. Diseases of the Lens, Comprehensive Ophthalmology; New Age International (P) Ltd.: New Delhi, India, 2007; pp. 167–204. [Google Scholar]
- Bensch, K.G.; Fleming, J.E.; Lohmann, W. The role of ascorbic acid in senile cataract. Proc. Natl. Acad. Sci. USA 1985, 82, 7193–7196. [Google Scholar] [CrossRef] [Green Version]
- West, S.; Munoz, B.; Vitale, S.; Schein, O.; Maguire, M.; Bressler, N. Watermen Study-Ii-Smoking and Nuclear Opacities. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1097. [Google Scholar]
- Brian, G.; Taylor, H. Cataract blindness: Challenges for the 21st century. Bull. World Health Organ. 2001, 79, 249–256. [Google Scholar] [PubMed]
- Delcourt, C.; Carrière, I.; Ponton-Sanchez, A.; Lacroux, A.; Covacho, M.-J.; Papoz, L. Light exposure and the risk of cortical, nuclear, and posterior subcapsular cataracts: The Pathologies Oculaires Liees a l’Age (POLA) study. Arch. Ophthalmol. 2000, 118, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Derham, B.K.; Harding, J.J. α-Crystallin as a molecular chaperone. Prog. Retin. Eye Res. 1999, 18, 463–509. [Google Scholar] [CrossRef]
- Kador, P.F.; Robison, W.G., Jr.; Kinoshita, J.H. Inhibitors. Ann. Rey. Pharmacol. Toxicol 1985, 25, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kennedy, A. Therapeutic potential of antioxidants and diabetic retinopathy. Expert Opin. Investig. Drugs 2001, 10, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Jobling, A.I.; Augusteyn, R.C. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin. Exp. Optom. 2002, 85, 61–75. [Google Scholar] [CrossRef]
- Alexander, L.J.; Bowerman, L.; Thompson, L. The prevalence of the ocular side effects of chlorpromazine in the Tuscaloosa Veterans Administration patient population. J. Am. Optom. Assoc. 1985, 56, 872–876. [Google Scholar]
- Harding, J.J.; van Heyningen, R. Beer, cigarettes, and military work as risk factors for cataract. In Risk Factors for Cataract Development; Karger Publishers: Basel, Switzerland, 1989; Volume 17, pp. 13–16. [Google Scholar]
- Jacques, P.F.; Chylack, L.T.; McGandy, R.B.; Hartz, S.C. Antioxidant status in persons with and without senile cataract. Arch. Ophthalmol. 1988, 106, 337–340. [Google Scholar] [CrossRef]
- Liu, X.-F.; Hao, J.-L.; Xie, T.; Malik, T.H.; Lu, C.-B.; Liu, C.; Shu, C.; Lu, C.-W.; Zhou, D.-D. Nrf2 as a target for the prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell 2017, 16, 934–942. [Google Scholar] [CrossRef]
- Periyasamy, P.; Shinohara, T. Age-related cataracts: Role of unfolded protein response, Ca (2+) mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog. Retin. Eye Res. 2017, 60, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; Grattagliano, I.; Vendemiale, G.; Micelli-Ferrari, T.; Altomare, E. Protein Oxidation and Lens Opacity in Humans. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2461–2465. [Google Scholar]
- Ghahramani, M.; Yousefi, R.; Khoshaman, K.; Alavianmehr, M.-M. The impact of calcium ion on structure and aggregation propensity of peroxynitrite-modified lens crystallins: New insights into the pathogenesis of cataract disorders. Colloids Surfaces B Biointerfaces 2015, 125, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Duker, J.S.; Yoshida, Y.; Niki, E.; Rasmussen, H.; Russell, R.M.; Yeum, K.J. Oxidative stress and antioxidant status in older adults with early cataract. Eye 2009, 23, 1464–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truscott, R.J. Age-related nuclear cataract: A lens transport problem. Ophthalmic Res. 2000, 32, 185–194. [Google Scholar] [CrossRef]
- Wang, Z.; Su, D.; Sun, Z.; Liu, S.; Sun, L.; Li, Q.; Guan, L.; Liu, Y.; Ma, X.; Hu, S. MDM2 phosphorylation mediates H2O2-induced lens epithelial cells apoptosis and age-related cataract. Biochem. Biophys. Res. Commun. 2020, 528, 112–119. [Google Scholar] [CrossRef]
- Elanchezhian, R.; Palsamy, P.; Madson, C.J.; Mulhern, M.L.; Lynch, D.W.; Troia, A.M.; Usukura, J.; Shinohara, T. Low glucose under hypoxic conditions induces unfolded protein response and produces reactive oxygen species in lens epithelial cells. Cell Death Dis. 2012, 3, e301. [Google Scholar] [CrossRef] [Green Version]
- David, L.L.; Shearer, T.R. Role of proteolysis in lenses: A review. Lens Eye Toxic. Res. 1989, 6, 725–747. [Google Scholar]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar]
- Yu, S.; Khor, T.O.; Cheung, K.-L.; Li, W.; Wu, T.-Y.; Huang, Y.; Foster, B.A.; Kan, Y.W.; Kong, A.-N. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS ONE 2010, 5, e8579. [Google Scholar] [CrossRef] [Green Version]
- Palsamy, P.; Bidasee, K.R.; Shinohara, T. Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Exp. Eye Res. 2014, 121, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, F.; Sekhar, K.R.; Freeman, M.L.; Liebler, D.C. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J. Biol. Chem. 2005, 280, 31768–31775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, Y.; Iida, K.; Kang, M.I.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by the proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, Y. Nrf2 Is an Attractive Therapeutic Target for Retinal Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7469326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesic, M.J.; Simmons, S.O.; Bauer, R.; Jaspers, I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic. Biol. Med. 2011, 51, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Schumann, C.; Chan, S.; Khalimonchuk, O.; Khal, S.; Moskal, V.; Shah, V.; Alani, A.W.G.; Taratula, O.; Taratula, O. Mechanistic Nanotherapeutic Approach Based on siRNA-Mediated DJ-1 Protein Suppression for Platinum-Resistant Ovarian Cancer. Mol. Pharm. 2016, 13, 2070–2083. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.J.; Lan, D.H.; He, S.Z.; Ye, Z.; Li, P.; Zhai, W.; Chen, W.Q.; Huang, Y.; Fu, Y.; Sun, A.; et al. Nrf2 protects human lens epithelial cells against H(2)O(2)-induced oxidative and ER stress: The ATF4 may be involved. Exp. Eye Res. 2018, 169, 28–37. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, Y.; Huang, T. Human age-related cataracts: Epigenetic suppression of the nuclear factor erythroid 2-related factor 2-mediated antioxidant system. Mol. Med. Rep. 2015, 11, 1442–1447. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.; Hanselmann, C.; Gassmann, M.G.; Auf dem Keller, U.; Born-Berclaz, C.; Chan, K.; Kan, Y.W.; Werner, S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell. Biol. 2002, 22, 5492–5505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arisawa, T.; Tahara, T.; Shibata, T.; Nagasaka, M.; Nakamura, M.; Kamiya, Y.; Fujita, H.; Hasegawa, S.; Takagi, T.; Wang, F.Y.; et al. The relationship between Helicobacter pylori infection and promoter polymorphism of the Nrf2 gene in chronic gastritis. Int. J. Mol. Med. 2007, 19, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babizhayev, M.A. Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: Disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract disease. Cell Biochem. Funct. 2011, 29, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H. Involvement of Nrf2 in Ocular Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef]
- Elanchezhian, R.; Ramesh, E.; Sakthivel, M.; Isai, M.; Geraldine, P.; Rajamohan, M.; Jesudasan, C.N.; Thomas, P.A. Acetyl-L-carnitine prevents selenite-induced cataractogenesis in an experimental animal model. Curr. Eye Res. 2007, 32, 961–971. [Google Scholar] [CrossRef]
- Park, J.Y.; Kang, K.A.; Kim, K.C.; Cha, J.W.; Kim, E.H.; Hyun, J.W. Morin Induces Heme Oxygenase-1 via ERK-Nrf2 Signaling Pathway. J. Cancer Prev. 2013, 18, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Smith, A.J.; Lott, M.C.; Bao, Y.; Bowater, R.P.; Reddan, J.R.; Wormstone, I.M. Sulforaphane can protect lens cells against oxidative stress: Implications for cataract prevention. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5236–5248. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Ma, J.; Han, F.; Guo, X.; Meng, L.; Sun, Y.; Jin, C.; Duan, H.; Li, H.; Peng, Y. DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci. Rep. 2016, 6, 19396. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Luo, W.; Luo, X.; Yong, Z.; Zhong, X. Effects of Rosa laevigata Michx. extract on reactive oxygen species production and mitochondrial membrane potential in lens epithelial cells cultured under high glucose. Int. J. Clin. Exp. Med. 2015, 8, 15759–15765. [Google Scholar]
- Crabbe, M.J. Cataract as a conformational disease--the Maillard reaction, alpha-crystallin, and chemotherapy. Cell. Mol. Biol. (Noisy-le-grand) 1998, 44, 1047–1050. [Google Scholar]
- Harding, J.J. Viewing molecular mechanisms of ageing through a lens. Ageing Res. Rev. 2002, 1, 465–479. [Google Scholar] [CrossRef]
- Feige, M.J.; Hendershot, L.M. Disulfide bonds in ER protein folding and homeostasis. Curr. Opin. Cell Biol. 2011, 23, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhardt, F.; Lewin, J.; Cortese, R.; Rakyan, V.K.; Attwood, J.; Burger, M.; Burton, J.; Cox, T.V.; Davies, R.; Down, T.A.; et al. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat. Genet. 2006, 38, 1378–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razin, A.; Riggs, A.D. DNA methylation, and gene function. Science 1980, 210, 604–610. [Google Scholar] [CrossRef]
- Palsamy, P.; Ayaki, M.; Elanchezhian, R.; Shinohara, T. Promoter demethylation of Keap1 gene in human diabetic cataractous lenses. Biochem. Biophys. Res. Commun. 2012, 423, 542–548. [Google Scholar] [CrossRef]
- Elanchezhian, R.; Palsamy, P.; Madson, C.J.; Lynch, D.W.; Shinohara, T. Age-related cataracts: Homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem. Biol. Interact. 2012, 200, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Palsamy, P.; Bidasee, K.R.; Ayaki, M.; Augusteyn, R.C.; Chan, J.Y.; Shinohara, T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free Radic. Biol. Med. 2014, 72, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Palsamy, P.; Bidasee, K.R.; Shinohara, T. Selenite cataracts: Activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection. Biochim. Biophys. Acta 2014, 1842, 1794–1805. [Google Scholar] [CrossRef] [Green Version]
- Takamura, Y.; Sugimoto, Y.; Kubo, E.; Takahashi, Y.; Akagi, Y. Immunohistochemical study of apoptosis of lens epithelial cells in human and diabetic rat cataracts. Jpn. J. Ophthalmol. 2001, 45, 559–563. [Google Scholar] [CrossRef] [Green Version]
- de Iongh, R.U.; Wederell, E.; Lovicu, F.J.; McAvoy, J.W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: A model for cataract formation. Cells Tissues Organs 2005, 179, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-W.; Choi, J.A.; Jee, D. Matrix Metalloproteinase-1 and Matrix Metalloproteinase-9 in the Aqueous Humor of Diabetic Macular Edema Patients. PLoS ONE 2016, 11, e0159720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivak, J.M.; Fini, M.E. MMPs in the eye: Emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin. Eye Res. 2002, 21, 1–14. [Google Scholar] [CrossRef]
- Korol, A.; Pino, G.; Dwivedi, D.; Robertson, J.V.; Deschamps, P.A.; West-Mays, J.A. Matrix metalloproteinase-9-null mice are resistant to TGF-β-induced anterior subcapsular cataract formation. Am. J. Pathol. 2014, 184, 2001–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallas, S.L.; Rosser, J.L.; Mundy, G.R.; Bonewald, L.F. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for the release of TGF-beta from the bone matrix. J. Biol. Chem. 2002, 277, 21352–21360. [Google Scholar] [CrossRef] [Green Version]
- Imhof, B.A.; Vollmers, H.P.; Goodman, S.L.; Birchmeier, W. Cell-cell interaction and polarity of epithelial cells: Specific perturbation using a monoclonal antibody. Cell 1983, 35, 667–675. [Google Scholar] [CrossRef]
- Alapure, B.V.; Praveen, M.R.; Gajjar, D.; Vasavada, A.R.; Rajkumar, S.; Johar, K. Matrix metalloproteinase-9 activity in human lens epithelial cells of cortical, posterior subcapsular, and nuclear cataracts. J. Cataract Refract. Surg. 2008, 34, 2063–2067. [Google Scholar] [CrossRef]
- Trivedi, V.D.; Raman, B.; Ramakrishna, T.; Rao, C.M. Detection and assay of proteases using calf lens β-crystallin aggregate as substrate. J. Biochem. Biophys. Methods 1999, 40, 49–55. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Mao, L.; Wang, H.; Qiao, L.; Wang, X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediat. Inflamm. 2010, 2010, 238321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Sohn, D.H.; Jin, X.Y.; Kim, S.W.; Choi, S.C.; Seo, G.S. 2’,4’,6’-tris(methoxymethoxy) chalcone protects against trinitrobenzene sulfonic acid-induced colitis and blocks tumor necrosis factor-alpha-induced intestinal epithelial inflammation via heme oxygenase 1-dependent and independent pathways. Biochem. Pharmacol. 2007, 74, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Jeon, W.K.; Hong, H.Y.; Jeon, K.B.; Hahn, J.H.; Kim, Y.M.; Numazawa, S.; Yosida, T.; Park, E.H.; Lim, C.J. The anti-inflammatory activity of Phellinus linteus (Berk. & M.A. Curt.) is mediated through the PKCdelta/Nrf2/ARE signaling to up-regulation of heme oxygenase-1. J. Ethnopharmacol. 2007, 113, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Yang, A.Y.; Huang, M.T.; Liu, Y.; Lee, J.H.; Khor, T.O.; Su, Z.Y.; Shu, L.; Lu, Y.; Conney, A.H.; et al. Nrf2 null enhances UVB-induced skin inflammation and extracellular matrix damages. Cell Biosci. 2014, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Gan, F.F.; Ling, H.; Ang, X.; Reddy, S.A.; Lee, S.S.; Yang, H.; Tan, S.H.; Hayes, J.D.; Chui, W.K.; Chew, E.H. A novel shogaol analog suppresses cancer cell invasion and inflammation and displays cytoprotective effects through modulation of NF-κB and Nrf2-Keap1 signaling pathways. Toxicol. Appl. Pharmacol. 2013, 272, 852–862. [Google Scholar] [CrossRef]
- Hanneken, A.; Lin, F.F.; Johnson, J.; Maher, P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3164–3177. [Google Scholar] [CrossRef]
- Sampath, C.; Zhu, Y.; Sang, S.; Ahmedna, M. Bioactive compounds isolated from apple, tea, and ginger protect against dicarbonyl induced stress in cultured human retinal epithelial cells. Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 200–213. [Google Scholar] [CrossRef]
- Hu, X.; Liang, Y.; Zhao, B.; Wang, Y. Thymoquinone protects human retinal pigment epithelial cells against hydrogen peroxide-induced oxidative stress and apoptosis. J. Cell. Biochem. 2019, 120, 4514–4522. [Google Scholar] [CrossRef]
- Chen, F.; Lu, H.T.; Jiang, Y. Purification of paeoniflorin from Paeonia lactiflora Pall. by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1040, 205–208. [Google Scholar] [CrossRef]
- Lu, Y.S.; Jiang, Y.; Yuan, J.P.; Jiang, S.B.; Yang, Y.; Zhu, P.Y.; Sun, Y.Z.; Qi, R.Q.; Liu, T.; Wang, H.X.; et al. UVA Induced Oxidative Stress Was Inhibited by Paeoniflorin/Nrf2 Signaling or PLIN2. Front. Pharmacol. 2020, 11, 736. [Google Scholar] [CrossRef]
- Wankun, X.; Wenzhen, Y.; Min, Z.; Weiyan, Z.; Huan, C.; Wei, D.; Lvzhen, H.; Xu, Y.; Xiaoxin, L. Protective effect of paeoniflorin against oxidative stress in human retinal pigment epithelium in vitro. Mol. Vis. 2011, 17, 3512–3522. [Google Scholar]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.K.; Yang, W.Z.; Liang, R.S.; Shi, S.S.; Chen, J.P.; Chen, C.M.; Wang, C.H.; Xie, H.S.; Chen, Y.; Ouyang, L.Q. Effect of baicalin on matrix metalloproteinase-9 expression and blood-brain barrier permeability following focal cerebral ischemia in rats. Neurochem. Res. 2011, 36, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-B.; Jin, Y.-L.; Jia, Q.; Zhang, Y.; Li, L.-Y.; Liu, P.; Liu, Y.-T. Baicalin attenuates brain edema in a rat model of intracerebral hemorrhage. Inflammation 2014, 37, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.F.; Zhou, Q.M.; Du, J.; Zhang, H.; Lu, Y.Y.; Su, S.B. Baicalin suppresses migration, invasion, and metastasis of breast cancer via p38MAPK signaling pathway. Anticancer. Agents Med. Chem. 2013, 13, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chu, Q.; Ye, J. Determination of trans-Resveratrol in wines, herbs, and health food by capillary electrophoresis with electrochemical detection. Food Chem. 2002, 78, 255–260. [Google Scholar] [CrossRef]
- Wei, H.; Wang, S.; Zhen, L.; Yang, Q.; Wu, Z.; Lei, X.; Lv, J.; Xiong, L.; Xue, R. Resveratrol attenuates the blood-brain barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after cerebral ischemia-reperfusion in rats. J. Mol. Neurosci. 2015, 55, 872–879. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, X.; Jiang, X.; Peng, Y.; Huang, W.; Cheng, G.; Song, L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006, 78, 2564–2570. [Google Scholar] [CrossRef]
- Pandey, A.K.; Bhattacharya, P.; Shukla, S.C.; Paul, S.; Patnaik, R. Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: A molecular docking study exploring possible neuroprotection. Neural Regen. Res. 2015, 10, 568–575. [Google Scholar] [CrossRef]
- Gao, D.; Huang, T.; Jiang, X.; Hu, S.; Zhang, L.; Fei, Z. Resveratrol protects primary cortical neuron cultures from transient oxygen-glucose deprivation by inhibiting MMP-9. Mol. Med. Rep. 2014, 9, 2197–2204. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Zhang, X.; Gao, D.; Jiang, X.; Dong, W. Resveratrol inhibits MMP-9 expression by upregulating PPAR alpha expression in an oxygen glucose deprivation-exposed neuron model. Neurosci. Lett. 2009, 451, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jin, W.; Zhu, T.; Wang, J.; Yuan, B.; Jiang, J.; Liang, W.; Ma, Z. Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. J. Spinal Cord Med. 2015, 38, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.Y.; Cheng, B.H.; Li, J. Effect of Curcumin on Cerebral Ischemia-reperfusion Injury in Rats. Zhong Yao Cai 2015, 38, 344–349. [Google Scholar] [PubMed]
- Yeh, C.H.; Lin, Y.M.; Wu, Y.C.; Lin, P.J. Inhibition of NF-kappa B activation can attenuate ischemia/reperfusion-induced contractility impairment via decreasing cardiomyocytic proinflammatory gene up-regulation and matrix metalloproteinase expression. J. Cardiovasc. Pharmacol. 2005, 45, 301–309. [Google Scholar] [CrossRef]
- ’t Hart, B.A.; Copray, S.; Philippens, I. Apocynin, a Low Molecular Oral Treatment for Neurodegenerative Diseases. BioMed. Res. Int. 2014, 2014, 298020. [Google Scholar] [CrossRef]
- Tang, X.; Zhong, W.; Tu, Q.; Ding, B. NADPH oxidase mediates the expression of MMP-9 in cerebral tissue after ischemia-reperfusion damage. Neurol. Res. 2014, 36, 118–125. [Google Scholar] [CrossRef]
- Shao, B.; Bayraktutan, U. Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-β. Diabetes Obes. Metab. 2013, 15, 993–999. [Google Scholar] [CrossRef]
- Di Paola, R.; Menegazzi, M.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Protective effects of glycyrrhizin in a gut hypoxia (ischemia)-reoxygenation (reperfusion) model. Intensive Care Med. 2009, 35, 687–697. [Google Scholar] [CrossRef]
- Menegazzi, M.; Di Paola, R.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol. Res. 2008, 58, 22–31. [Google Scholar] [CrossRef]
- Genovese, T.; Menegazzi, M.; Mazzon, E.; Crisafulli, C.; Di Paola, R.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin reduces secondary inflammatory process after spinal cord compression injury in mice. Shock 2009, 31, 367–375. [Google Scholar] [CrossRef]
- Liang, B.; Guo, X.-L.; Jin, J.; Ma, Y.-C.; Feng, Z.-Q. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J. Gastroenterol. 2015, 21, 5271–5280. [Google Scholar] [CrossRef]
- Abe, K.; Ikeda, T.; Wake, K.; Sato, T.; Sato, T.; Inoue, H. Glycyrrhizin prevents lipopolysaccharide/D-galactosamine-induced liver injury through down-regulation of matrix metalloproteinase-9 in mice. J. Pharm. Pharmacol. 2008, 60, 91–97. [Google Scholar] [CrossRef]
- Park, W.H.; Kim, S.H.; Kim, C.H. A new matrix metalloproteinase-9 inhibitor 3,4-dihydroxycinnamic acid (caffeic acid) from methanol extract of Euonymus alatus: Isolation and structure determination. Toxicology 2005, 207, 383–390. [Google Scholar] [CrossRef]
- Mendonca, P.; Taka, E.; Bauer, D.; Cobourne-Duval, M.; Soliman, K.F. The attenuating effects of 1,2,3,4,6 penta-O-galloyl-β-d-glucose on inflammatory cytokines release from activated BV-2 microglial cells. J. Neuroimmunol. 2017, 305, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, Y.; Tatsuzaki, J.; Yang, J.; Kosano, H. Potential Therapeutic Agents, Polymethoxylated Flavones Isolated from Kaempferia parviflora for Cataract Prevention through Inhibition of Matrix Metalloproteinase-9 in Lens Epithelial Cells. Biol. Pharm. Bull. 2019, 42, 1658–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, M.N. Chlorogenic acids and other cinnamates--nature, occurrence, and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Bola, C.; Bartlett, H.; Eperjesi, F. Resveratrol, and the eye: Activity and molecular mechanisms. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, J.; Kadekaru, T.; Ogawa, K.; Hitoe, S.; Shimoda, H.; Hara, H. Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. Food Chem. 2013, 139, 129–137. [Google Scholar] [CrossRef]
- Higashi, Y.; Higashi, K.; Mori, A.; Sakamoto, K.; Ishii, K.; Nakahara, T. Anti-cataract Effect of Resveratrol in High-Glucose-Treated Streptozotocin-Induced Diabetic Rats. Biol. Pharm. Bull. 2018, 41, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Kyselová, Z.; Garcia, S.J.; Gajdosíková, A.; Gajdosík, A.; Stefek, M. Temporal relationship between lens protein oxidation and cataract development in streptozotocin-induced diabetic rats. Physiol. Res. 2005, 54, 49–56. [Google Scholar] [PubMed]
- Kawakubo, K.; Mori, A.; Sakamoto, K.; Nakahara, T.; Ishii, K. GP-1447, an inhibitor of aldose reductase, prevents the progression of diabetic cataract in rats. Biol. Pharm. Bull. 2012, 35, 866–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilak, J.C.; Banerjee, M.; Mohan, H.; Devasagayam, T.P. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother. Res. 2004, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, M.; Schaffer, P.M.; Zidan, J.; Bar Sela, G. Curcuma as a functional food in the control of cancer and inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin, and curcumin-like molecules: From spice to drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Fatma, N.; Bhargavan, B.; Kubo, E.; Kumar, A.; Singh, D.P. Specificity protein, Sp1-mediated increased expression of Prdx6 as a curcumin-induced antioxidant defense in lens epithelial cells against oxidative stress. Cell Death Dis. 2011, 2, e234. [Google Scholar] [CrossRef]
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Chindhu, S.; Mariammal, K.; Sudhandiran, G.; Arumugam, M. Anti-cataractogenic effect of curcumin and aminoguanidine against selenium-induced oxidative stress in the eye lens of Wistar rat pups: An in vitro study using an isolated lens. Chem. Biol. Interact. 2009, 181, 202–209. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Saraswat, M.; Mrudula, T.; Krishna, T.P.; Krishnaswamy, K.; Reddy, G.B. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2092–2099. [Google Scholar] [CrossRef]
- Cao, J.; Wang, T.; Wang, M. Investigation of the anti-cataractogenic mechanisms of curcumin through in vivo and in vitro studies. BMC Ophthalmol. 2018, 18, 48. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, J.; McLauchlan, W.R.; Williamson, G. Quercetin inhibits hydrogen peroxide-induced oxidation of the rat lens. Free Radic. Biol. Med. 1999, 26, 639–645. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, X.; Piao, M.J.; Oh, M.C.; Fernando, P.M.; Kang, K.A.; Ryu, Y.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Hyperoside Induces Endogenous Antioxidant System to Alleviate Oxidative Stress. J Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, S.; Ghosh, I.; Saha, G.; Dasgupta, S. EGCG prevents tryptophan oxidation of cataractous ocular lens human γ-crystallin in the presence of H2O2. Int. J. Biol. Macromol. 2015, 77, 287–292. [Google Scholar] [CrossRef]
- McNulty, R.; Wang, H.; Mathias, R.T.; Ortwerth, B.J.; Truscott, R.J.W.; Bassnett, S. Regulation of tissue oxygen levels in the mammalian lens. J. Physiol. 2004, 559, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Van Heyningen, R. Fluorescent Glucoside in the Human Lens. Nature 1971, 230, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Dutta, A.; Bag, S.; Biswas, P.; Das, A.K.; Dasgupta, S. Probing the inhibitory potency of epigallocatechin gallate against human γB-crystallin aggregation: Spectroscopic, microscopic and simulation studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 192, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Bag, S.; Bose, M.; Das, A.K.; Ghosh, A.K.; Dasgupta, S. Protection of human γB-crystallin from UV-induced damage by epigallocatechin gallate: Spectroscopic and docking studies. Mol. bioSyst. 2016, 12, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef]
- Demir, E.; Taysi, S.; Al, B.; Demir, T.; Okumus, S.; Saygili, O.; Saricicek, E.; Dirier, A.; Akan, M.; Tarakcioglu, M.; et al. The effects of Nigella sativa oil, thymoquinone, propolis, and caffeic acid phenethyl ester on radiation-induced cataract. Wien. Klin. Wochenschr. 2016, 128, 587–595. [Google Scholar] [CrossRef]
- Taysi, S.; Abdulrahman, Z.K.; Okumus, S.; Demir, E.; Demir, T.; Akan, M.; Saricicek, E.; Saricicek, V.; Aksoy, A.; Tarakcioglu, M. The radioprotective effect of Nigella sativa on nitrosative stress in lens tissue in radiation-induced cataract in the rat. Cutan. Ocul. Toxicol. 2015, 34, 101–106. [Google Scholar] [CrossRef]
- Shirazi, A.; Haddadi, G.H.; Asadi-Amoli, F.; Sakhaee, S.; Ghazi-Khansari, M.; Avand, A. Radioprotective effect of melatonin in reducing oxidative stress in rat lenses. Cell J. 2011, 13, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef]
- Ma, T.; Chen, T.; Li, P.; Ye, Z.; Zhai, W.; Jia, L.; Chen, W.; Sun, A.; Huang, Y.; Wei, S.; et al. Heme oxygenase-1 (HO-1) protects human lens epithelial cells (SRA01/04) against hydrogen peroxide (H2O2)-induced oxidative stress and apoptosis. Exp Eye Res. 2016, 146, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3832–3841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, G.; Kowluru, R.A. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J Cell Physiol. 2012, 227, 1052–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilliard, A.; Mendonca, P.; Russell, T.D.; Soliman, K.F.A. The Protective Effects of Flavonoids in Cataract Formation through the Activation of Nrf2 and the Inhibition of MMP-9. Nutrients 2020, 12, 3651. https://doi.org/10.3390/nu12123651
Hilliard A, Mendonca P, Russell TD, Soliman KFA. The Protective Effects of Flavonoids in Cataract Formation through the Activation of Nrf2 and the Inhibition of MMP-9. Nutrients. 2020; 12(12):3651. https://doi.org/10.3390/nu12123651
Chicago/Turabian StyleHilliard, Aaron, Patricia Mendonca, Tanya D. Russell, and Karam F. A. Soliman. 2020. "The Protective Effects of Flavonoids in Cataract Formation through the Activation of Nrf2 and the Inhibition of MMP-9" Nutrients 12, no. 12: 3651. https://doi.org/10.3390/nu12123651




