Dietary Habits and Risk of Early-Onset Dementia in an Italian Case-Control Study
Abstract
1. Introduction
2. Methods
3. Results
3.1. Characteristics of the Study Population
3.2. Assessment of Dietary Habits
3.3. Assessment of the Relation between Dietary Habits and Dementia Risk
3.4. Assessment of the Relation between Dietary Patterns and Dementia Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Dementia. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 15 November 2020).
- Wolters, F.J.; Ikram, M.A. Epidemiology of dementia: The burden on society, the challenges for research. Methods Mol. Biol. 2018, 1750, 3–14. [Google Scholar]
- Rossor, M.N.; Fox, N.C.; Mummery, C.J.; Schott, J.M.; Warren, J.D. The diagnosis of young-onset dementia. Lancet Neurol. 2010, 9, 793–806. [Google Scholar] [CrossRef]
- Vieira, R.T.; Caixeta, L.; Machado, S.; Silva, A.C.; Nardi, A.E.; Arias-Carrion, O.; Carta, M.G. Epidemiology of early-onset dementia: A review of the literature. Clin. Pract. Epidemiol. Ment. Health 2013, 9, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Okumura, Y. Job loss after diagnosis of early-onset dementia: A matched cohort study. J. Alzheimers Dis. 2017, 60, 1231–1235. [Google Scholar] [CrossRef]
- Sikes, P.; Hall, M. The impact of parental young onset dementia on children and young people’s educational careers. Br. Educ. Res. J. 2018, 44, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Chiari, A.; Vinceti, G.; Adani, G.; Tondelli, M.; Galli, C.; Fiondella, L.; Costa, M.; Molinari, M.A.; Filippini, T.; Zamboni, G.; et al. Epidemiology of early onset dementia and its clinical presentations in the province of Modena, Italy. Alzheimer’s Dement. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jarmolowicz, A.I.; Chen, H.Y.; Panegyres, P.K. The patterns of inheritance in early-onset dementia: Alzheimer’s disease and frontotemporal dementia. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Koedam, E.L.; Lauffer, V.; van der Vlies, A.E.; van der Flier, W.M.; Scheltens, P.; Pijnenburg, Y.A. Early-versus late-onset Alzheimer’s disease: More than age alone. J. Alzheimers Dis. 2010, 19, 1401–1408. [Google Scholar] [CrossRef]
- Cations, M.; Withall, A.; Low, L.F.; Draper, B. What is the role of modifiable environmental and lifestyle risk factors in young onset dementia? Eur. J. Epidemiol. 2016, 31, 107–124. [Google Scholar] [CrossRef]
- Cations, M.; Withall, A.; Draper, B. Modifiable risk factors for young onset dementia. Curr. Opin. Psychiatry 2019, 32, 138–143. [Google Scholar] [CrossRef]
- Angeloni, C.; Businaro, R.; Vauzour, D. The role of diet in preventing and reducing cognitive decline. Curr. Opin. Psychiatry 2020, 33, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Stamati, P.; Siokas, V.; Aloizou, A.M.; Karampinis, E.; Arseniou, S.; Rakitskii, V.N.; Tsatsakis, A.; Spandidos, D.A.; Gozes, I.; Mitsias, P.D.; et al. Does SCFD1 rs10139154 polymorphism decrease Alzheimer’s disease risk? J. Mol. Neurosci. 2019, 69, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Fish consumption and cognitive decline with age in a large community study. Arch. Neurol. 2005, 62, 1849–1853. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006, 67, 1370–1376. [Google Scholar] [CrossRef]
- Nooyens, A.C.; Bueno-de-Mesquita, H.B.; van Boxtel, M.P.; van Gelder, B.M.; Verhagen, H.; Verschuren, W.M. Fruit and vegetable intake and cognitive decline in middle-aged men and women: The Doetinchem Cohort Study. Br. J. Nutr. 2011, 106, 752–761. [Google Scholar] [CrossRef]
- Martinez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvado, J.; San Julian, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.A. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Babyak, M.A.; Craighead, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Strauman, T.A.; Sherwood, A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 2010, 55, 1331–1338. [Google Scholar] [CrossRef]
- Tanaka, T.; Talegawkar, S.A.; Jin, Y.; Colpo, M.; Ferrucci, L.; Bandinelli, S. Adherence to a mediterranean diet protects from cognitive decline in the invecchiare in Chianti study of aging. Nutrients 2018, 10, 2007. [Google Scholar] [CrossRef]
- Berti, V.; Murray, J.; Davies, M.; Spector, N.; Tsui, W.H.; Li, Y.; Williams, S.; Pirraglia, E.; Vallabhajosula, S.; McHugh, P.; et al. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J. Nutr. Health Aging 2015, 19, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Murray, J.; Davies, M.; Williams, S.; Pirraglia, E.; Spector, N.; Tsui, W.H.; Li, Y.; Butler, T.; Osorio, R.S.; et al. Nutrient intake and brain biomarkers of Alzheimer’s disease in at-risk cognitively normal individuals: A cross-sectional neuroimaging pilot study. BMJ Open 2014, 4, e004850. [Google Scholar] [CrossRef] [PubMed]
- Adani, G.; Filippini, T.; Garuti, C.; Malavolti, M.; Vinceti, G.; Zamboni, G.; Tondelli, M.; Galli, C.; Costa, M.; Vinceti, M.; et al. Environmental risk factors for early-onset Alzheimer’s dementia and frontotemporal dementia: A case-control study in northern Italy. Int. J. Environ. Res. Public Health 2020, 17, 7941. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Fiore, M.; Tesauro, M.; Malagoli, C.; Consonni, M.; Violi, F.; Arcolin, E.; Iacuzio, L.; Oliveri Conti, G.; Cristaldi, A.; et al. Clinical and lifestyle factors and risk of amyotrophic lateral sclerosis: A population-based case-control study. Int. J. Environ. Res. Public Health 2020, 17, 857. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori 2003, 89, 594–607. [Google Scholar] [CrossRef]
- Pasanisi, P.; Berrino, F.; Bellati, C.; Sieri, S.; Krogh, V. Validity of the Italian EPIC questionnaire to assess past diet. IARC Sci. Publ. 2002, 156, 41–44. [Google Scholar]
- Filippini, T.; Malagoli, C.; Wise, L.A.; Malavolti, M.; Pellacani, G.; Vinceti, M. Dietary cadmium intake and risk of cutaneous melanoma: An Italian population-based case-control study. J. Trace Elem. Med. Biol. 2019, 56, 100–106. [Google Scholar] [CrossRef]
- Malavolti, M.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Vinceti, M.; Filippini, T. Lead exposure in an Italian population: Food content, dietary intake and risk assessment. Food Res. Int. 2020, 137, 109370. [Google Scholar] [CrossRef]
- Sieri, S.; Krogh, V.; Saieva, C.; Grobbee, D.E.; Bergmann, M.; Rohrmann, S.; Tjonneland, A.; Ferrari, P.; Chloptsios, Y.; Dilis, V.; et al. Alcohol consumption patterns, diet and body weight in 10 European countries. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 4), S81–S100. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Malavolti, M.; Agnoli, C.; Crespi, C.M.; Fiorentini, C.; Farnetani, F.; Longo, C.; Ricci, C.; Albertini, G.; Lanzoni, A.; et al. Diet quality and risk of melanoma in an Italian population. J. Nutr. 2015, 145, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Agnoli, C.; Krogh, V.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Frasca, G.; et al. A priori-defined dietary patterns are associated with reduced risk of stroke in a large Italian cohort. J. Nutr. 2011, 141, 1552–1558. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef]
- Di Marco, L.Y.; Marzo, A.; Munoz-Ruiz, M.; Ikram, M.A.; Kivipelto, M.; Ruefenacht, D.; Venneri, A.; Soininen, H.; Wanke, I.; Ventikos, Y.A.; et al. Modifiable lifestyle factors in dementia: A systematic review of longitudinal observational cohort studies. J. Alzheimers Dis. 2014, 42, 119–135. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Zielinska, M.A.; Bialecka, A.; Pietruszka, B.; Hamulka, J. Vegetables and fruit, as a source of bioactive substances, and impact on memory and cognitive function of elderly. Postepy Hig. Med. Dosw. (Online) 2017, 71, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Alperovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Ascherio, A.; Grodstein, F. Fruit and vegetable consumption and cognitive decline in aging women. Ann. Neurol. 2005, 57, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.; Morris, M.C. Association of strawberries and anthocyanidin intake with Alzheimer’s dementia risk. Nutrients 2019, 11, 3060. [Google Scholar] [CrossRef]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.; Hofman, A.; Rosner, B.; Stampfer, M.J.; Witteman, J.C.; Breteler, M.M. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am. J. Clin. Nutr. 2009, 90, 170–176. [Google Scholar] [CrossRef]
- Larrieu, S.; Letenneur, L.; Helmer, C.; Dartigues, J.F.; Barberger-Gateau, P. Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J. Nutr. Health Aging 2004, 8, 150–154. [Google Scholar]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef]
- Samieri, C.; Morris, M.C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish intake, genetic predisposition to Alzheimer disease, and decline in global cognition and memory in 5 cohorts of older persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef]
- Pappalardo, A.M.; Copat, C.; Ferrito, V.; Grasso, A.; Ferrante, M. Heavy metal content and molecular species identification in canned tuna: Insights into human food safety. Mol. Med. Rep. 2017, 15, 3430–3437. [Google Scholar] [CrossRef]
- Filippini, T.; Cilloni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018, 50, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Lo Voi, A.; De Simone, A.; Serpe, F.P.; Anastasio, A.; Pepe, T.; Cacace, D.; Severino, L. Heavy metals in canned tuna from Italian markets. J. Food Prot. 2013, 76, 355–359. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G.; Cuttone, G.; Giungato, D.; Garofalo, R. Occurrence of toxic metals (Hg, Cd and Pb) in fresh and canned tuna: Public health implications. Food Chem. Toxicol. 2010, 48, 3167–3170. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Mandrioli, J.; Violi, F.; Bargellini, A.; Weuve, J.; Fini, N.; Grill, P.; Michalke, B. Lead, cadmium and mercury in cerebrospinal fluid and risk of amyotrophic lateral sclerosis: A case-control study. J. Trace Elem. Med. Biol. 2017, 43, 121–125. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Assmann, K.E.; Andreeva, V.A.; Ferry, M.; Hercberg, S.; Galan, P. Consumption of dairy products and cognitive functioning: Findings from the SU.VI.MAX 2 study. J. Nutr. Health Aging 2016, 20, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Kato, Y.; Nishita, Y.; Tange, C.; Nakamoto, M.; Tomida, M.; Imai, T.; Ando, F.; Shimokata, H. Cereal intake increases and dairy products decrease risk of cognitive decline among elderly female Japanese. J. Prev. Alzheimers Dis. 2014, 1, 160–167. [Google Scholar]
- Lee, J.; Fu, Z.; Chung, M.; Jang, D.J.; Lee, H.J. Role of milk and dairy intake in cognitive function in older adults: A systematic review and meta-analysis. Nutr. J. 2018, 17, 82. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The hisayama Study1–3. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef]
- Rahman, A.; Baker, P.S.; Allman, R.M.; Zamrini, E. Dietary factors and cognitive impairment in community-dwelling elderly. J. Nutr. Health Aging 2007, 11, 49–54. [Google Scholar]
- Ano, Y.; Nakayama, H. Preventive effects of dairy products on dementia and the underlying mechanisms. Int. J. Mol. Sci. 2018, 19, 1927. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: The cache county study on memory, health and aging. Am. J. Clin. Nutr. 2013, 98, 1263–1271. [Google Scholar] [CrossRef]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Singh-Manoux, A.; Marmot, M.G.; Brunner, E.J. Education attenuates the association between dietary patterns and cognition. Dement. Geriatr. Cogn. Disord. 2009, 27, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Desport, J.C.; Jesus, P.; Mbelesso, P.; Ndamba-Bandzouzi, B.; Dartigues, J.F.; Clement, J.P.; Preux, P.M.; Guerchet, M. Diet, Alcohol consumption and cognitive disorders in central Africa: A study from the EPIDEMCA program. J. Nutr. Health Aging 2015, 19, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C. Dietary fat composition and dementia risk. Neurobiol. Aging 2014, 35 (Suppl. 2), S59–S64. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Alkerwi, A. Chocolate intake is associated with better cognitive function: The maine-syracuse longitudinal study. Appetite 2016, 100, 126–132. [Google Scholar] [CrossRef]
- Moreira, A.; Diogenes, M.J.; de Mendonca, A.; Lunet, N.; Barros, H. Chocolate consumption is associated with a lower risk of cognitive decline. J. Alzheimers Dis. 2016, 53, 85–93. [Google Scholar] [CrossRef]
- Barrera-Reyes, P.K.; de Lara, J.C.; Gonzalez-Soto, M.; Tejero, M.E. Effects of cocoa-derived polyphenols on cognitive function in humans. Systematic review and analysis of methodological aspects. Plant. Foods Hum. Nutr. 2020, 75, 1–11. [Google Scholar] [CrossRef]
- Calabro, R.S.; De Cola, M.C.; Gervasi, G.; Portaro, S.; Naro, A.; Accorinti, M.; Manuli, A.; Marra, A.; De Luca, R.; Bramanti, P. The efficacy of cocoa polyphenols in the treatment of mild cognitive impairment: A retrospective study. Medicina 2019, 55, 156. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N. Coffee consumption and risk of dementia and Alzheimer’s disease: A dose-response meta-analysis of prospective studies. Nutrients 2018, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Cilloni, S.; Malavolti, M.; Violi, F.; Vescovi, L.; Bargellini, A.; Vinceti, M. Aluminum and tin: Food contamination and dietary intake in an Italian population. J. Trace Elem. Med. Biol. 2019, 52, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Malavolti, M.; Farnetani, F.; Longo, C.; Filippini, T.; Pellacani, G.; Vinceti, M. Food and beverage consumption and melanoma risk: A population-based case-control study in northern Italy. Nutrients 2019, 11, 2206. [Google Scholar] [CrossRef]
- Lao, Y.; Hou, L.; Li, J.; Hui, X.; Yan, P.; Yang, K. Association between alcohol intake, mild cognitive impairment and progression to dementia: A dose-response meta-analysis. Aging Clin. Exp. Res. 2020. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of wine consumption with Alzheimer’s disease. Nutrients 2020, 12, 206. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, D.H.; Li, J.; Wang, Y.J.; Gao, C.; Chen, M. A 2-year follow-up study of alcohol consumption and risk of dementia. Clin. Neurol. Neurosurg. 2006, 108, 378–383. [Google Scholar] [CrossRef]
- Orgogozo, J.M.; Dartigues, J.F.; Lafont, S.; Letenneur, L.; Commenges, D.; Salamon, R.; Renaud, S.; Breteler, M.B. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev. Neurol. 1997, 153, 185–192. [Google Scholar]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Berendsen, A.M.; Kang, J.H.; Feskens, E.J.M.; de Groot, C.; Grodstein, F.; van de Rest, O. Association of long-term adherence to the MIND diet with cognitive function and cognitive decline in American women. J. Nutr. Health Aging 2018, 22, 222–229. [Google Scholar] [CrossRef]
- Feart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garcia, M.I.; Toledo, E.; Razquin, C.; Dominguez, L.J.; Maragarone, D.; Martinez-Gonzalez, J.; Martinez-Gonzalez, M.A. “A priori” dietary patterns and cognitive function in the SUN project. Neuroepidemiology 2020, 54, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Tangney, C.; Hong, L.; Barnes, L.L.; Schneider, J.; Bennett, D.; Morris, M. O1–05–03: Accordance to Dietary Approaches to Stop Hypertension (DASH) is associated with slower cognitive decline. Alzheimers Dement. 2013, 9, P135. [Google Scholar] [CrossRef]
- Tangney, C.C.; Li, H.; Wang, Y.; Barnes, L.; Schneider, J.A.; Bennett, D.A.; Morris, M.C. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 2014, 83, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Hosking, D.E.; Eramudugolla, R.; Cherbuin, N.; Anstey, K.J. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019, 15, 581–589. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Barnes, L.L.; Bennett, D.; Aggarwal, N. MIND diet score more predictive than DASH or Mediterranean Diet scores. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014, 10, P166. [Google Scholar] [CrossRef]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. MIND diet associated with reduced incidence and delayed progression of parkinsonism in old age. J. Nutr Health Aging 2018, 22, 1211–1215. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Fakuda, K.; Leurgans, S.; Aggarwal, N.; Morris, M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J. Prev. Alzheimers Dis. 2019, 6, 267–273. [Google Scholar]
- Cherian, L.; Wang, Y.; Holland, T.; Agarwal, P.; Aggarwal, N.; Morris, M.C. DASH and Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diets are associated with fewer depressive symptoms over time. J. Gerontol. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Morris, M.C. MIND Diet Intervention and Cognitive Decline (MIND). 2018; Available online: https://mind-diet-trial.org/meet-the-researchers/; https://clinicaltrials.gov/ct2/show/NCT02817074; (accessed on 15 November 2020). [Google Scholar]
- Morris, M.C. Nutrition and risk of dementia: Overview and methodological issues. Ann. N. Y. Acad. Sci. 2016, 1367, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Biazus-Sehn, L.F.; Schuch, F.B.; Firth, J.; Stigger, F.S. Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 89, 104048. [Google Scholar] [CrossRef] [PubMed]
- Whitty, E.; Mansour, H.; Aguirre, E.; Palomo, M.; Charlesworth, G.; Ramjee, S.; Poppe, M.; Brodaty, H.; Kales, H.C.; Morgan-Trimmer, S.; et al. Efficacy of lifestyle and psychosocial interventions in reducing cognitive decline in older people: Systematic review. Ageing Res. Rev. 2020, 62, 101113. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, I.; Biggeri, L.; Laureti, T.; Secondi, L. Exploring the Italians’ food habits and tendency towards a sustainable diet: The Mediterranean eating pattern. Agric. Agric. Sci. Procedia 2016, 8, 433–440. [Google Scholar] [CrossRef]


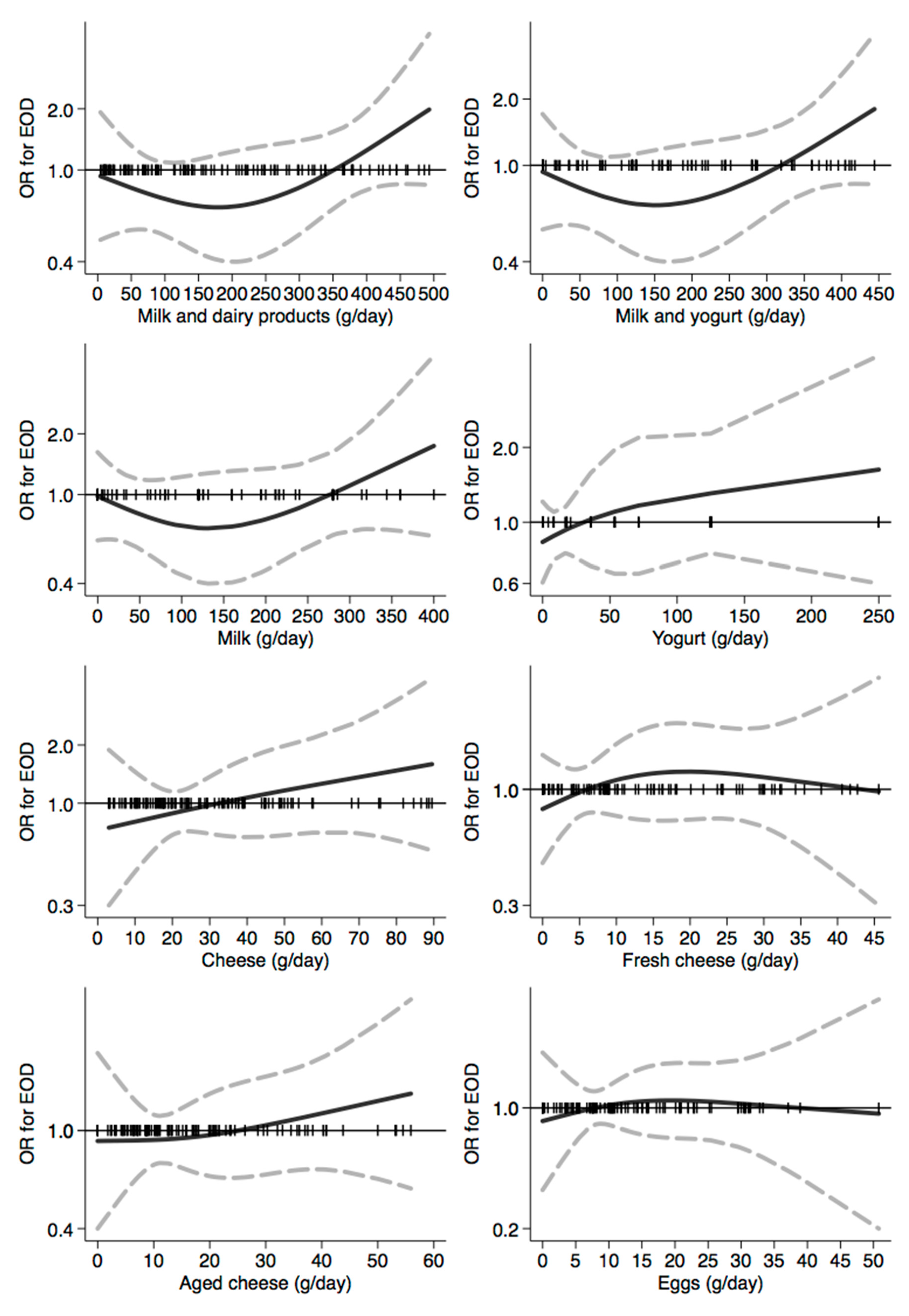
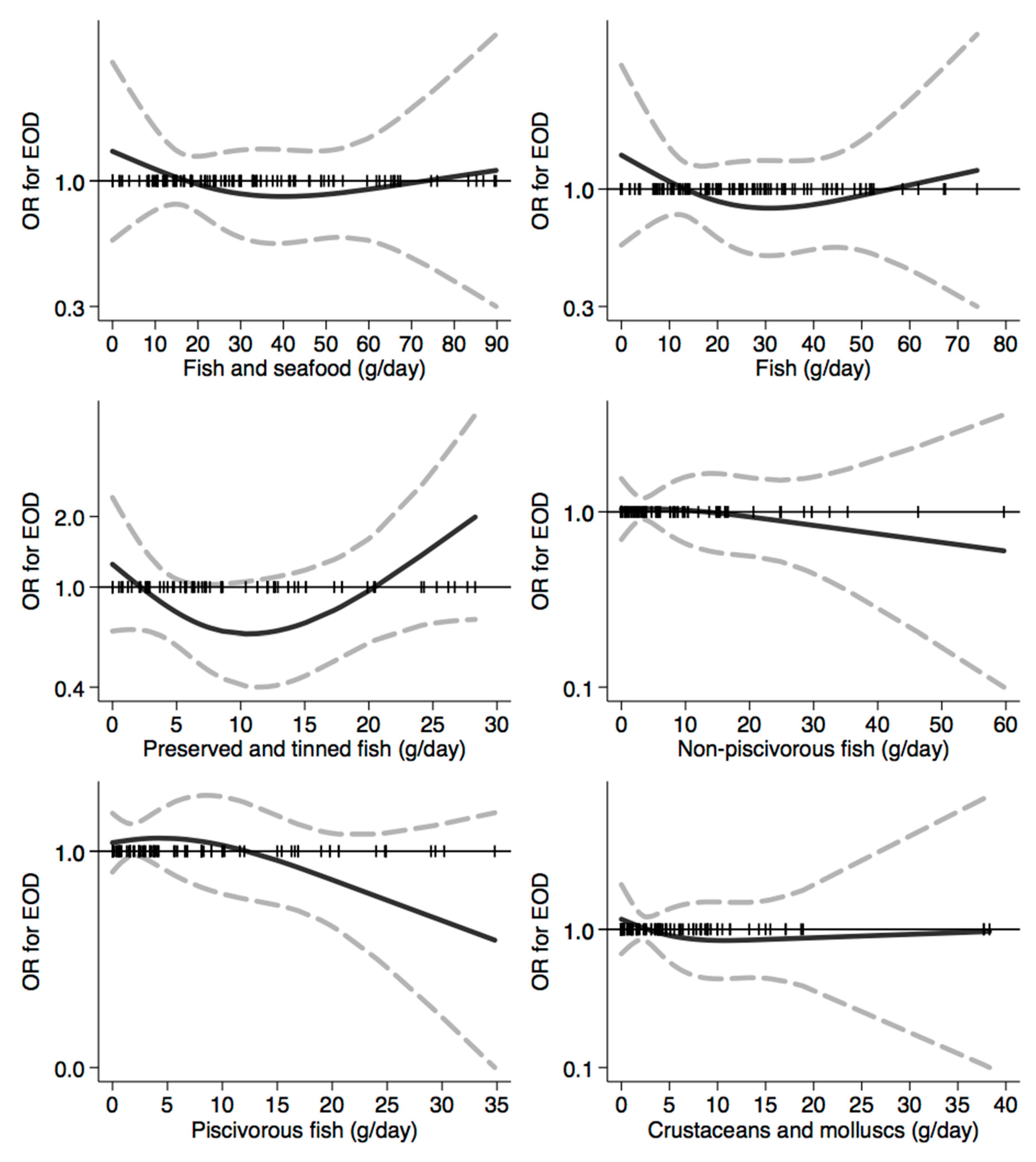







| Controls | All EOD Cases | EO-AD Cases | EO-FTD Cases | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| All subjects | 54 (100) | 54 (100) | 30 (100) | 18 (100) |
| Age at questionnaire filling | ||||
| Mean (standard deviation) | 63.8 (9.6) | 66.2 (4.6) | 65.9 (4.5) | 66.6 (4.7) |
| <65 years | 28 (51.8) | 19 (35.2) | 11 (36.7) | 5 (27.8) |
| ≥65 years | 26 (48.2) | 35 (64.8) | 19 (63.3) | 13 (72.2) |
| Sex | ||||
| Men | 23 (42.6) | 24 (44.4) | 11 (36.7) | 10 (55.6) |
| Women | 31 (57.4) | 30 (55.6) | 19 (63.3) | 8 (44.4) |
| Educational attainment | ||||
| Primary or less | 11 (20.4) | 13 (24.1) | 6 (20.0) | 5 (27.8) |
| Middle school | 11 (20.4) | 20 (37.0) | 10 (33.3) | 8 (44.4) |
| High school | 21 (38.9) | 18 (33.3) | 12 (40.0) | 4 (22.2) |
| College or more | 11 (20.4) | 3 (5.6) | 2 (6.7) | 1 (5.6) |
| Marital status | ||||
| Married/unmarried partner | 48 (88.9) | 45 (83.3) | 23 (76.7) | 16 (88.9) |
| Single | 3 (5.6) | 1 (1.9) | 1 (3.3) | 0 (0.0) |
| Separated/divorced | 2 (3.7) | 2 (3.7) | 0 (0.0) | 2 (11.1) |
| Widowed | 1 (1.9) | 6 (11.1) | 6 (20.0) | 0 (0.0) |
| Dietary Pattern | Controls | EOD Cases | EO-AD Cases | EO-FTD Cases |
|---|---|---|---|---|
| GM diet (range 0–9) | 4.4 (1.7) | 4.1 (1.5) | 4.1 (1.5) | 4.1 (1.5) |
| DASH diet (rang 8–40) | 23.7 (5.5) | 23.1 (5.0) | 23.5 (5.4) | 23.6 (4.4) |
| MIND diet (range 0–15) | 7.8 (1.3) | 7.1 (1.4) | 7.2 (1.4) | 7.2 (1.3) |
| All EOD | EO-AD | EO-FTD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Food Items | Median | Cases/ Controls | OR | (95% CI) | Cases/ Controls | OR | (95% CI) | Cases/ Controls | OR | (95% CI) |
| GM diet | ||||||||||
| 1st tertile (ref.) | 3 | 24/19 | 1.00 | - | 14/19 | 1.00 | - | 7/19 | 1.00 | - |
| 2nd tertile | 5 | 18/18 | 0.76 | (0.30–1.96) | 10/18 | 0.73 | (0.24–2.17) | 6/18 | 0.87 | (0.22–3.35) |
| 3nd tertile | 6 | 12/17 | 0.45 | (0.16–1.26) | 6/17 | 0.40 | (0.12–1.35) | 5/17 | 0.60 | (0.14–2.61) |
| Linear trend | 0.84 | (0.65-1.09) | 0.84 | (0.62–1.13) | 0.83 | (0.57–1.20) | ||||
| DASH diet | ||||||||||
| 1st tertile (ref.) | 18 | 21/19 | 1.00 | - | 10/19 | 1.00 | - | 7/19 | 1.00 | - |
| 2nd tertile | 25 | 22/19 | 0.87 | (0.35–2.15) | 13/19 | 1.08 | (0.37–3.17) | 7/19 | 0.80 | (0.22–3.00) |
| 3nd tertile | 29 | 11/16 | 0.60 | (0.21–1.72) | 7/16 | 0.79 | (0.23–2.74) | 4/16 | 0.71 | (0.14–3.52) |
| Linear trend | 0.98 | (0.90–1.06) | 0.99 | (0.91–1.08) | 1.01 | (0.89–1.14) | ||||
| MIND diet | ||||||||||
| 1st tertile (ref.) | 6.5 | 30/16 | 1.00 | - | 16/16 | 1.00 | - | 9/16 | 1.00 | - |
| 2nd tertile | 7.5 | 15/22 | 0.32 | (0.12–0.83) | 9/22 | 0.39 | (0.13–1.15) | 5/22 | 0.31 | (0.07–1.28) |
| 3nd tertile | 9.0 | 9/16 | 0.31 | (0.11–0.90) | 5/16 | 0.32 | (0.09–1.13) | 4/16 | 0.45 | (0.10–2.00) |
| Linear trend | 0.66 | (0.47–0.91) | 0.67 | (0.46–0.98) | 0.66 | (0.41–1.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, T.; Adani, G.; Malavolti, M.; Garuti, C.; Cilloni, S.; Vinceti, G.; Zamboni, G.; Tondelli, M.; Galli, C.; Costa, M.; et al. Dietary Habits and Risk of Early-Onset Dementia in an Italian Case-Control Study. Nutrients 2020, 12, 3682. https://doi.org/10.3390/nu12123682
Filippini T, Adani G, Malavolti M, Garuti C, Cilloni S, Vinceti G, Zamboni G, Tondelli M, Galli C, Costa M, et al. Dietary Habits and Risk of Early-Onset Dementia in an Italian Case-Control Study. Nutrients. 2020; 12(12):3682. https://doi.org/10.3390/nu12123682
Chicago/Turabian StyleFilippini, Tommaso, Giorgia Adani, Marcella Malavolti, Caterina Garuti, Silvia Cilloni, Giulia Vinceti, Giovanna Zamboni, Manuela Tondelli, Chiara Galli, Manuela Costa, and et al. 2020. "Dietary Habits and Risk of Early-Onset Dementia in an Italian Case-Control Study" Nutrients 12, no. 12: 3682. https://doi.org/10.3390/nu12123682
APA StyleFilippini, T., Adani, G., Malavolti, M., Garuti, C., Cilloni, S., Vinceti, G., Zamboni, G., Tondelli, M., Galli, C., Costa, M., Chiari, A., & Vinceti, M. (2020). Dietary Habits and Risk of Early-Onset Dementia in an Italian Case-Control Study. Nutrients, 12(12), 3682. https://doi.org/10.3390/nu12123682






