Multiple Micronutrients, Lutein, and Docosahexaenoic Acid Supplementation during Lactation: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
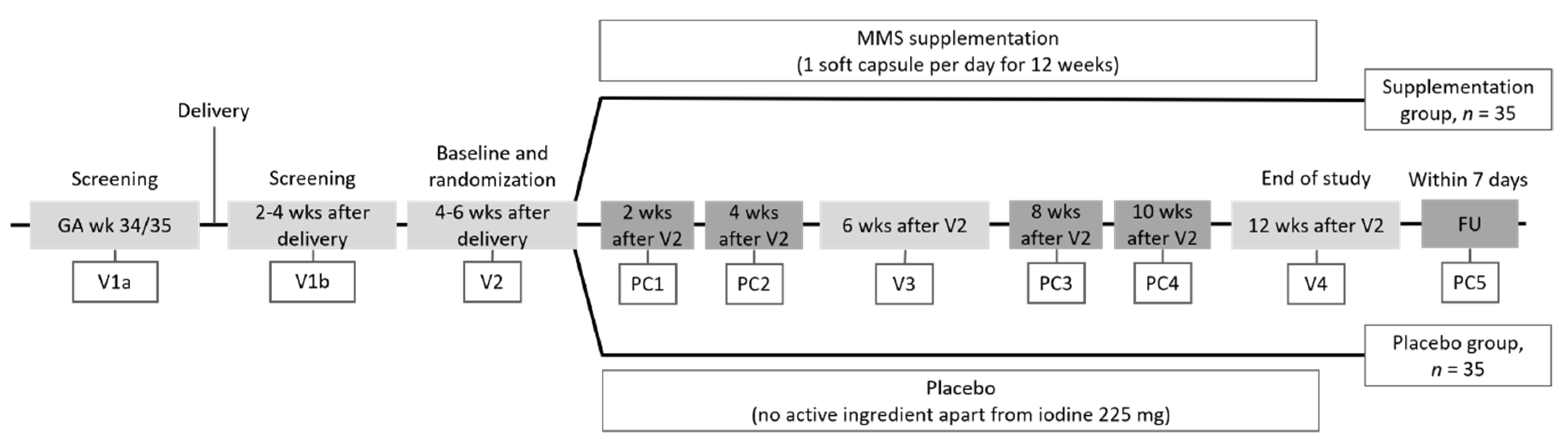
2.1. Trial Design
2.2. Study Population
2.3. Randomization to Study Product
2.4. Parameters Assessed
Biochemical Analyses
2.5. Power Calculation and Statistical Analyses
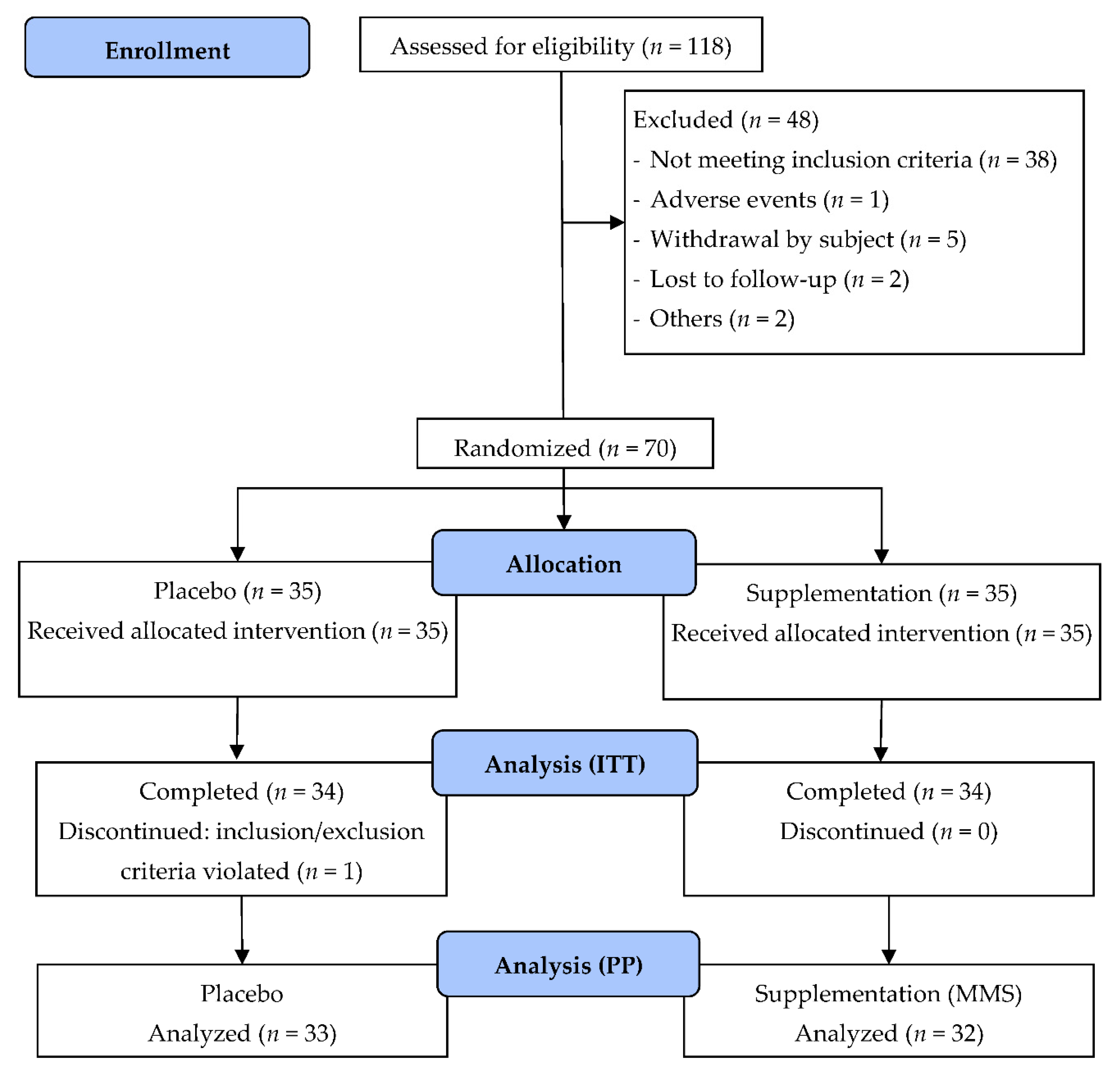
3. Results
3.1. Subject Characteristics
3.2. Efficacy Endpoints
3.2.1. Primary Endpoint
3.2.2. Secondary Maternal Endpoints
3.2.3. Exploratory Infant Endpoints
3.2.4. Safety Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ESPGHAN-Committee-on-Nutrition; Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; et al. Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. 2018, 9, 278s–294s. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Nutrition During Lactation; The National Academies Press: Washington, DC, USA, 1991. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune function and micronutrient requirements change over the life course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Calder, P.; Prescott, S.; Caplan, M. Scientific Review: The Role of Nutrients in Immune Function of Infants and Young Children. Emerging Evidence for Long-Chain Polyunsaturated Fatty Acids; Mead Johnson & Company: Glenview, IL, USA, 2007. [Google Scholar]
- Prentice, S. They are what you eat: Can nutritional factors during gestation and early infancy modulate the neonatal immune response? Front. Immunol. 2017, 8, 1641. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Colombo, J. Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Adv. Pediatr 2016, 63, 453–471. [Google Scholar] [CrossRef]
- Innis, S.M. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev. Neurosci. 2000, 22, 474–480. [Google Scholar] [CrossRef]
- Rees, A.; Sirois, S.; Wearden, A. Prenatal maternal docosahexaenoic acid intake and infant information processing at 4.5mo and 9mo: A longitudinal study. PLoS ONE 2019, 14, e0210984. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Catalina Hernandez-Rodas, M.; Valenzuela, A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot Essent Fatty Acids 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Bergmann, K.; Brenna, J.T.; Calder, P.C.; Campoy, C.; Clandinin, M.T.; Colombo, J.; Daly, M.; Decsi, T.; Demmelmair, H.; et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 2020, 111, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Cetin, I.; Agostoni, C.; Desoye, G.; Devlieger, R.; Emmett, P.M.; Ensenauer, R.; Hauner, H.; Herrera, E.; Hoesli, I.; et al. Pregnancy and Infants’ Outcome: Nutritional and Metabolic Implications. Crit. Rev. Food Sci. Nutr. 2016, 56, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Fidler, N.; Sauerwald, T.; Pohl, A.; Demmelmair, H.; Koletzko, B. Docosahexaenoic acid transfer into human milk after dietary supplementation: A randomized clinical trial. J. Lipid Res. 2000, 41, 1376–1383. [Google Scholar] [PubMed]
- Demmelmair, H.; Koletzko, B. Lipids in human milk. Best Pract Res. Clin. Endocrinol Metab 2018, 32, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple micronutrient needs in pregnancy in industrialized countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Stringham, J.M.; Johnson, E.J.; Hammond, B.R. Lutein across the Lifespan: From Childhood Cognitive Performance to the Aging Eye and Brain. Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Canfield, L.M.; Clandinin, M.T.; Davies, D.P.; Fernandez, M.C.; Jackson, J.; Hawkes, J.; Goldman, W.J.; Pramuk, K.; Reyes, H.; Sablan, B.; et al. Multinational study of major breast milk carotenoids of healthy mothers. Eur. J. Nutr. 2003, 42, 133–141. [Google Scholar] [CrossRef]
- Allen, L.H. Multiple micronutrients in pregnancy and lactation: An overview. Am. J. Clin. Nutr. 2005, 81, 1206S–1212S. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.K.; Balogun, O.O.; Ota, E.; Takahashi, K.; Mori, R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst. Rev. 2016, 2, CD010647. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.; West, K.J.; Black, R. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.T. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef]
- Fairbrother, N.; Hutton, E.K.; Stoll, K.; Hall, W.; Kluka, S. Psychometric evaluation of the Multidimensional Assessment of Fatigue scale for use with pregnant and postpartum women. Psychol. Assess. 2008, 20, 150–158. [Google Scholar] [CrossRef]
- Wambach, K.A. Maternal fatigue in breastfeeding primiparae during the first nine weeks postpartum. J. Hum. Lact 1998, 14, 219–229. [Google Scholar] [CrossRef]
- Federal Joint Committee. Maternity Guidelines: Guidelines for Medical Care During Pregnancy and After Childbirth; 24 November 2020. Available online: https://www.g-ba.de/richtlinien/19/ (accessed on 15 December 2020).
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-For-Age, Weight-For-Age, Weight-For-Length, Weight-For-Height and Body Mass Index-For-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; Available online: https://www.who.int/childgrowth/standards/technical_report/en/ (accessed on 6 November 2020).
- Glaser, C.; Demmelmair, H.; Koletzko, B. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J. Lipid Res. 2010, 51, 216–221. [Google Scholar] [CrossRef]
- Stimming, M.; Mesch, C.M.; Kersting, M.; Kalhoff, H.; Demmelmair, H.; Koletzko, B.; Schmidt, A.; Böhm, V.; Libuda, L. Vitamin E content and estimated need in German infant and follow-on formulas with and without long-chain polyunsaturated fatty acids (LC-PUFA) enrichment. J. Agric. Food Chem. 2014, 62, 10153–10161. [Google Scholar] [CrossRef]
- Hellmuth, C.; Koletzko, B.; Peissner, W. Aqueous normal phase chromatography improves quantification and qualification of homocysteine, cysteine and methionine by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2011, 879, 83–89. [Google Scholar] [CrossRef]
- Jensen, C.L.; Voigt, R.G.; Prager, T.C.; Zou, Y.L.; Fraley, J.K.; Rozelle, J.C.; Turcich, M.R.; Llorente, A.M.; Anderson, R.E.; Heird, W.C. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am. J. Clin. Nutr. 2005, 82, 125–132. [Google Scholar] [CrossRef]
- Salkind, N.J. Encyclopedia of Research Design; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010; Volumes 1–10. [Google Scholar]
- Juber, B.A.; Jackson, K.H.; Johnson, K.B.; Harris, W.S.; Baack, M.L. Breast milk DHA levels may increase after informing women: A community-based cohort study from South Dakota USA. Int. Breastfeed. J. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E. Docosahexaenoic acid supplementation in pregnancy and lactation. Am. J. Clin. Nutr. 2009, 89, 678s–684s. [Google Scholar] [CrossRef] [PubMed]
- Massari, M.; Novielli, C.; Mandò, C.; Di Francesco, S.; Della Porta, M.; Cazzola, R.; Panteghini, M.; Savasi, V.; Maggini, S.; Schaefer, E.; et al. Multiple micronutrients and docosahexaenoic acid supplementation during pregnancy: A randomized controlled study. Nutrients 2020, 12, 2432. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Grunewald, M.; Hellmuth, C.; Kirchberg, F.F.; Mearin, M.L.; Auricchio, R.; Castillejo, G.; Korponay-Szabo, I.R.; Polanco, I.; Roca, M.; Vriezinga, S.L.; et al. Variation and Interdependencies of Human Milk Macronutrients, Fatty Acids, Adiponectin, Insulin, and IGF-II in the European PreventCD Cohort. Nutrients 2019, 11, 2034. [Google Scholar] [CrossRef]
- Yuhas, R.; Pramuk, K.; Lien, E.L. Human milk fatty acid composition from nine countries varies most in DHA. Lipids 2006, 41, 851–858. [Google Scholar] [CrossRef]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef]
- Genzel-Boroviczény, O.; Wahle, J.; Koletzko, B. Fatty acid composition of human milk during the 1st month after term and preterm delivery. Eur. J. Pediatr. 1997, 156, 142–147. [Google Scholar] [CrossRef]
- Sauerwald, T.U.; Demmelmair, H.; Fidler, N.; Koletzko, B. Polyunsaturated fatty acid supply with human milk. Physiological aspects and in vivo studies of metabolism. Adv. Exp. Med. Biol. 2000, 478, 261–270. [Google Scholar]
- Demmelmair, H.; Ahmed, T.B.; Koletzko, B. Content, variability, and regulation of fatty acids in human milk. In Human Milk Sampling and Measurement of Energy-Yielding Nutrients and Other Macromolecules; McGuire, M.K., O’Connor, D.L., Eds.; Academic Press-Elsevier: London, UK, 2020; pp. 103–143. [Google Scholar]
- Reider, C.A.; Chung, R.Y.; Devarshi, P.P.; Grant, R.W.; Hazels Mitmesser, S. Inadequacy of Immune Health Nutrients: Intakes in US Adults, the 2005–2016 NHANES. Nutrients 2020, 12, 1735. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E. Micronutrient deficiency in women living in industrialized countries during the reproductive years: Is there a basis for supplementation with multiple micronutrients? J. Nutr. Disorders Ther. 2016, 6, 199. [Google Scholar] [CrossRef]
- Dawodu, A.; Tsang, R. Maternal vitamin D status: Effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv. Nutr. 2012, 3, 353–361. [Google Scholar] [CrossRef]
- Micronutrient Information Center. Immunity in depth. Linus Pauling Institute. 2016. Available online: http://lpi.oregonstate.edu/mic/health-disease/immunity (accessed on 10 May 2019).
- European Food Safety Authority. Update of the Tolerable Upper Intake Level for Vitamin D for Infants. 2018. Available online: https://www.efsa.europa.eu/de/efsajournal/pub/5365 (accessed on 6 November 2020).
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic & Laboratory Test. Reference, 14th ed.; Elsevier: St. Louis, MO, USA, 2019. [Google Scholar]
- Oberhelman, S.S.; Meekins, M.E.; Fischer, P.R.; Lee, B.R.; Singh, R.J.; Cha, S.S.; Gardner, B.M.; Pettifor, J.M.; Croghan, I.T.; Thacher, T.D. Maternal Vitamin D Supplementation to Improve the Vitamin D Status of Breast-fed Infants: A Randomized Controlled Trial. Mayo Clin. Proc. 2013, 88, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Drugs and Lactation Database (LactMed); National Library of Medicine (US): Bethesda, MD, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500914/ (accessed on 15 December 2020).
- Koletzko, B.; Bauer, C.P.; Cierpka, M.; Cremer, M.; Flothkötter, M.; Graf, C.; Heindl, I.; Hellmers, C.; Kersting, M.; Krawinkel, M.; et al. Ernährung und Bewegung von Säuglingen und stillenden Frauen. Mon. Kinderheilkd. 2016, 164, 771–798. [Google Scholar] [CrossRef]
- Bührer, C.; Genzel-Boroviczény, O.; Jochum, F.; Kauth, T.; Kersting, M.; Koletzko, B.; Mihatsch, W.; Przyrembel, H.; Reinehr, T.; Zimmer, P.; et al. Ernährung gesunder Säuglinge. Mon. Kinderheilkd. 2014, 162, 527–538. [Google Scholar] [CrossRef]
- Sherry, C.L.; Oliver, J.S.; Renzi, L.M.; Marriage, B.J. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J. Nutr. 2014, 144, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Ramlau-Hansen, C.H.; Møller, U.K.; Møller, J.; Thulstrup, A.M. Lactation—A risk factor for elevated plasma homocysteine? Ugeskr. Laeger 2003, 165, 2819–2823. [Google Scholar]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Canfield, L.M.; Taren, D.L.; Kaminsky, R.G.; Mahal, Z. Short-term beta-carotene supplementation of lactating mothers consuming diets low in vitamin A. J. Nutr. Biochem. 1999, 10, 532–538. [Google Scholar] [CrossRef]
- Cabezuelo, M.T.; Zaragozá, R.; Barber, T.; Viña, J.R. Role of Vitamin A in Mammary Gland Development and Lactation. Nutrients 2019, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Klemm, R.; Shamim, A.A.; Ali, H.; Rashid, M.; Shaikh, S.; Wu, L.; Mehra, S.; Labrique, A.; Katz, J.; et al. Effects of vitamin A and β-carotene supplementation on birth size and length of gestation in rural Bangladesh: A cluster-randomized trial. Am. J. Clin. Nutr. 2013, 97, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed]
- Low, F.M.; Gluckman, P.D.; Hanson, M.A. Developmental plasticity and epigenetic mechanisms underpinning metabolic and cardiovascular diseases. Epigenomics 2011, 3, 279–294. [Google Scholar] [CrossRef]
| Characteristics | Placebo (n = 33) | MMS (n = 32) | Total (n = 65) |
|---|---|---|---|
| Age, years | 31.7 ± 4.0 (21–38) | 31.7 ± 3.5 (25–39) | 31.7 ± 3.8 (21–39) |
| Race: | |||
| Caucasian | 33 (100.0) | 28 (87.5) | 61 (93.8) |
| Black/African American | 0 | 1 (3.1) | 1 (1.5) |
| Asian | 0 | 2 (6.3) | 2 (3.1) |
| Other | 0 | 1 (3.1) | 1 (1.5) |
| Weight, kg | 70.8 ± 10.29 (52.0–110.0) | 69.3 ± 13.30 (52.2–107.0) | 70.1 ± 11.80 (52.0–110.0) |
| Height, cm | 1.67 ± 0.08 (1.48–1.90) | 1.68 ± 0.06 (1.53–1.83) | 1.67 ± 0.07 (1.48–1.90) |
| Body mass index, kg/m2 | 25.29 ± 3.62 (19.8–38.1) | 24.6 ± 3.97 (19.3–32.4) | 25.0 ± 3.78 (19.3–38.1) |
| Gestational age, weeks: 1 | |||
| Early term | 6 (18.2) | 5 (15.6) | 11 (16.9) |
| Full term | 24 (72.7) | 21 (65.6) | 45 (69.2) |
| Late term | 3 (9.1) | 6 (18.8) | 9 (13.8) |
| Delivery type: | |||
| Vaginal | 27 (81.8) | 24 (75.0) | 51 (78.5) |
| Caesarean | 6 (18.2) | 8 (25.0) | 14 (21.5) |
| Delivery complications, Yes | 1 (3.0) | 5 (15.6) | 6 (9.2) |
| Infant birth weight, g | 3400.6 ± 355.6 (2860–4130) | 3556.9 ± 347.5 (2856–4375) | 3477.6 ± 357.7 (2856–4375) |
| Parameters | Placebo (n = 33) | MMS (n = 32) | LS Mean Difference (95% CI) 1 | p Value * |
|---|---|---|---|---|
| Mean ± SD (Range) | ||||
| Milk parameters: | ||||
| DHA (wt % TFA) (primary) | −0.05 ± 0.11 (−0.32 to 0.22) | 0.11 ± 0.12 (−0.23 to 0.32) | 0.15 (0.11–0.19) | <0.0001 |
| EPA, % | −0.01 ± 0.04 (−0.11 to 0.06) | 0.01 ± 0.03 (−0.06 to 0.06) | 0.0110 (0.0006–0.0214) | 0.038 |
| Mead acid, % | 0.019 ± 0.054 (−0.03 to 0.19) | −0.004 ± 0.015 (−0.07 to 0.01) | −0.022 (−0.040 to −0.003) | 0.024 |
| Beta carotene, ng/mL | −5.1 ± 17.2 (−48.0 to 28.0) | 22.7 ± 34.5 (−20.0 to 142.0) | 28.4 (15.0–41.9) | <0.0001 |
| Blood parameters: | ||||
| DHA, mg/L | −9.76 ± 8.98 (−37.0 to 6.9) | 7.14 ± 11.40 (−29.0 to 28.1) | 15.66 (11.96–19.36) | <0.0001 |
| DHA/TFA | −0.004 ± 0.007 (−0.008 to −0.001) | 0.009 ± 0.009 (−0.02 to 0.03) | 0.013 (0.010–0.016) | <0.0001 |
| Docosatetraenoic acid, mg/L | −0.44 ± 1.08 (−3.9 to 1.9) | −0.70 ± 0.87 (−2.2 to 1.8) | −0.46 (−0.86 to −0.05) | 0.0270 |
| EPA, mg/L | −2.84 ± 4.27 (−11.2 to 4.6) | 0.59 ± 4.48 (−10.6 to 13.5) | 2.21 (0.44–3.98) | 0.0155 |
| 25-OH-vitamin D, ng/mL | −1.02 ± 8.18 (−7.5 to 5.50) | 5.88 ± 9.98 (−8.4 to 28.9) | 7.82 (4.36–11.28) | <0.0001 |
| Folic acid, ng/mL | −1.73 ± 4.73 (−11.6 to 16.5) | 17.82 ± 9.51 (11.70−23.80) | 21.20 (17.84–24.56) | <0.0001 |
| Homocysteine, μM | 0.56 ± 1.47 (−3.51 to 3.72) | −1.08 ± 1.24 (−4.24 to 1.71) | −1.63 (−2.27 to −0.99) | <0.0001 |
| Vitamin B12, pg/mL | −17.9 ± 97.0 (−313.0 to 342.0) | 69.0 ± 135.7 (−190.0 to 510.0) | 89.89 (31.45–148.3) | 0.0031 |
| Beta carotene, ng/mL | −49.3 ± 160.3 (−443.0 to 222.0) | 223.3 ± 334.2 (−480.0 to 1182.0) | 296.35 (183.06–409.64) | <0.0001 |
| Lutein, ng/mL | −16.8 ± 33.1 (−91.0 to 40.0) | 5.8 ± 52.2 (−182.0 to 90.0) | 21.13 (4.15–38.31) | 0.0157 |
| Parameters | Placebo (n = 35) | MMS (n = 35) |
|---|---|---|
| Mothers with at least one AE, n | 19 | 18 |
| Mothers with at least one AE: 1 | ||
| Mild | 11 (57.9) | 10 (55.6) |
| Moderate | 7 (36.8) | 8 (44.4) |
| Severe | 1 (5.3) | 0 |
| Mothers with at least one SAE | 0 | 3 (16.7) |
| Mothers with at least one treatment-related AE 2 | 1 (5.3) | 1 (5.6) |
| Number of infant AEs, n | 9 | 7 |
| Infants with at least one AE: 1 | ||
| Mild | 2 (28.6) | 6 (66.7) |
| Moderate | 4 (57.1) | 3 (33.3) |
| Severe | 1 (14.3) | 0 |
| Infants with at least one SAE | 4 (57.1) | 2 (22.2) |
| Infants with at least one treatment-related AE | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaefer, E.; Demmelmair, H.; Horak, J.; Holdt, L.; Grote, V.; Maar, K.; Neuhofer, C.; Teupser, D.; Thiel, N.; Goeckeler-Leopold, E.; et al. Multiple Micronutrients, Lutein, and Docosahexaenoic Acid Supplementation during Lactation: A Randomized Controlled Trial. Nutrients 2020, 12, 3849. https://doi.org/10.3390/nu12123849
Schaefer E, Demmelmair H, Horak J, Holdt L, Grote V, Maar K, Neuhofer C, Teupser D, Thiel N, Goeckeler-Leopold E, et al. Multiple Micronutrients, Lutein, and Docosahexaenoic Acid Supplementation during Lactation: A Randomized Controlled Trial. Nutrients. 2020; 12(12):3849. https://doi.org/10.3390/nu12123849
Chicago/Turabian StyleSchaefer, Ella, Hans Demmelmair, Jeannie Horak, Lesca Holdt, Veit Grote, Karoline Maar, Christoph Neuhofer, Daniel Teupser, Nadja Thiel, Erwin Goeckeler-Leopold, and et al. 2020. "Multiple Micronutrients, Lutein, and Docosahexaenoic Acid Supplementation during Lactation: A Randomized Controlled Trial" Nutrients 12, no. 12: 3849. https://doi.org/10.3390/nu12123849
APA StyleSchaefer, E., Demmelmair, H., Horak, J., Holdt, L., Grote, V., Maar, K., Neuhofer, C., Teupser, D., Thiel, N., Goeckeler-Leopold, E., Maggini, S., & Koletzko, B. (2020). Multiple Micronutrients, Lutein, and Docosahexaenoic Acid Supplementation during Lactation: A Randomized Controlled Trial. Nutrients, 12(12), 3849. https://doi.org/10.3390/nu12123849





