Menopause Delays the Typical Recovery of Pre-Exercise Hepcidin Levels after High-Intensity Interval Running Exercise in Endurance-Trained Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Interval Running Protocol
2.4. Blood Collection
2.5. Blood Analysis
2.6. Statistical Analysis
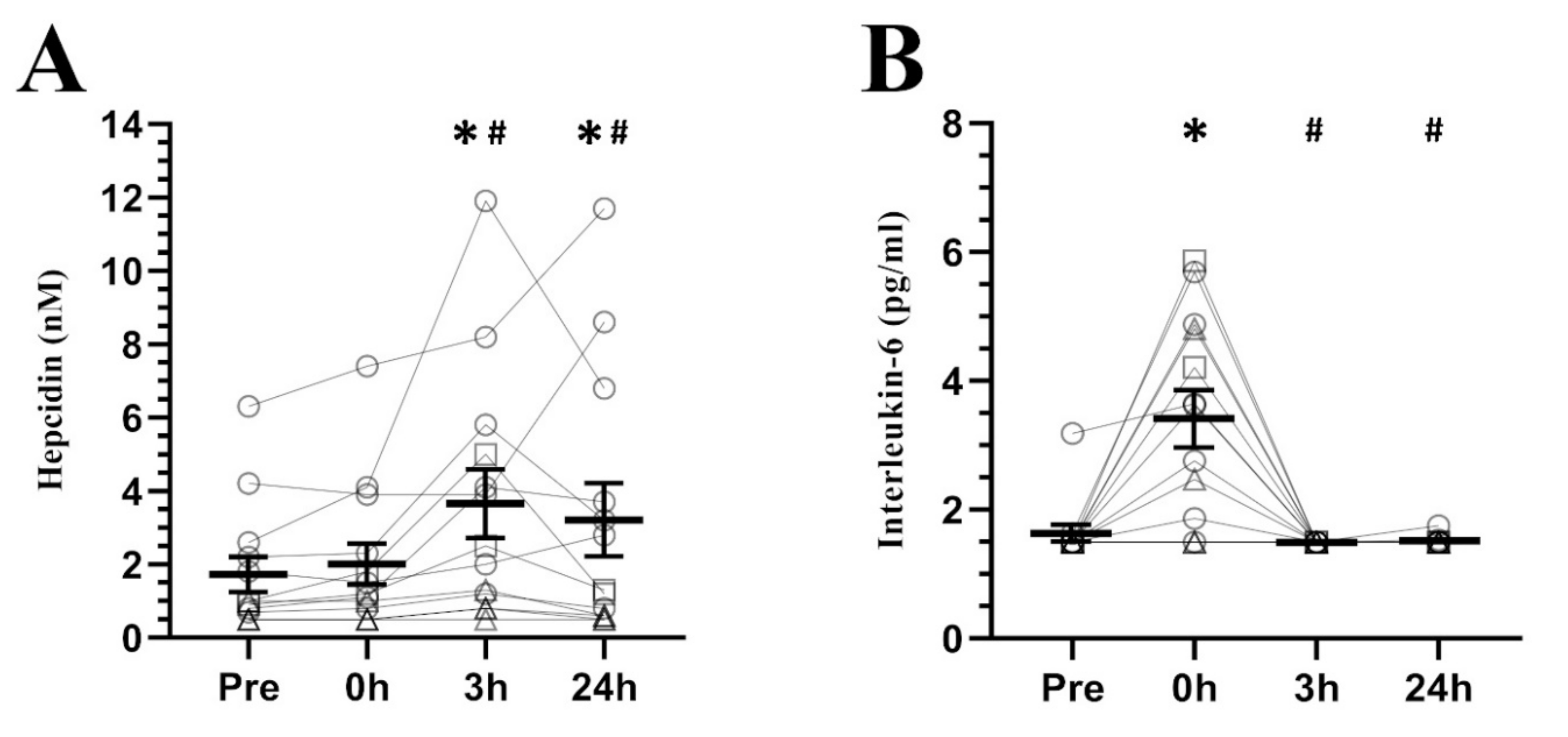
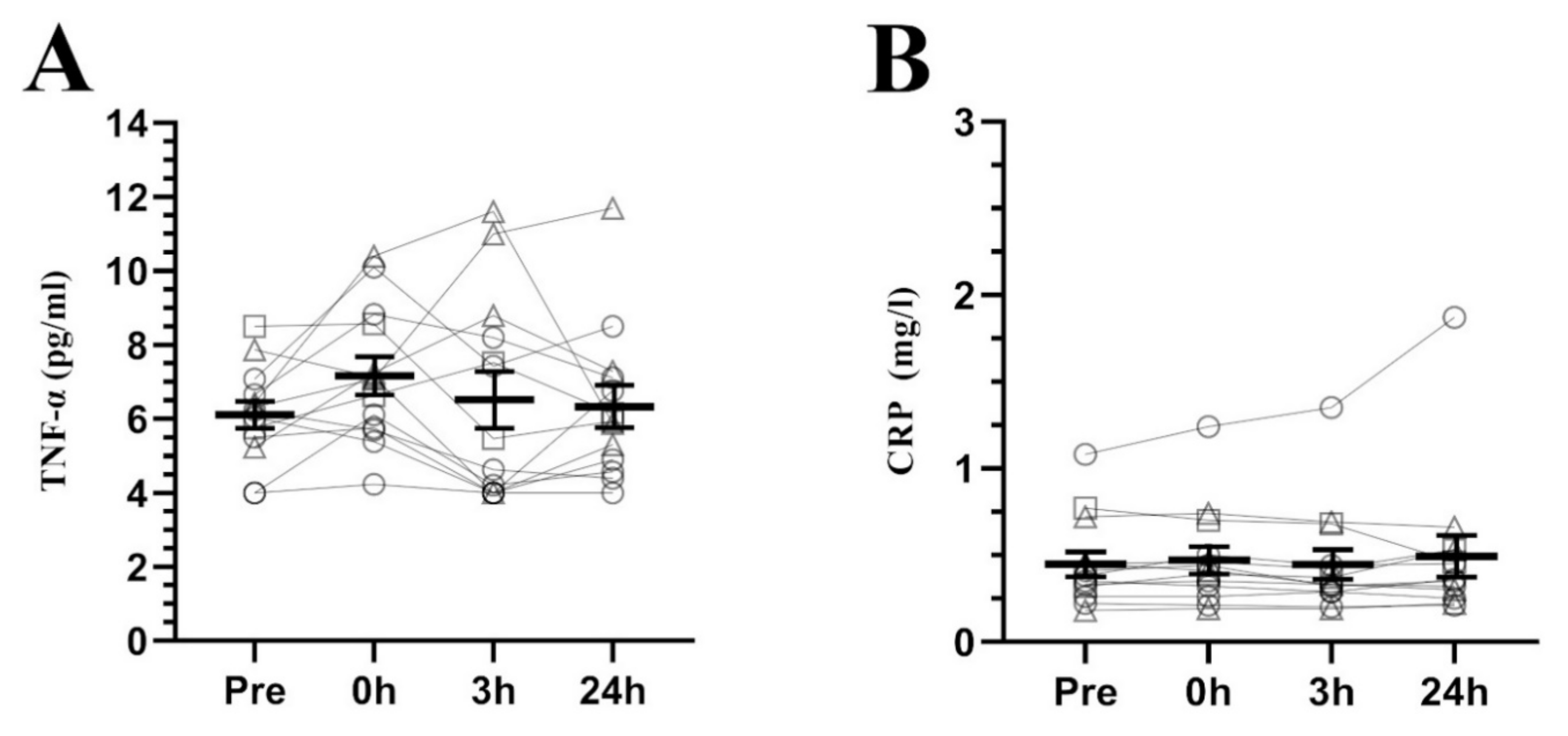
3. Results
4. Discussion
5. Practical Applications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Alfaro-Magallanes, V.M.; Babitt, J.L. Bone morphogenic proteins in iron homeostasis. Bone 2020, 138, 115495. [Google Scholar] [CrossRef]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A New Mouse Liver-specific Gene, Encoding a Protein Homologous to Human Antimicrobial Peptide Hepcidin, Is Overexpressed during Iron Overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, A.; Neitz, S.; Mägert, H.-J.; Schulz, A.; Forssmann, W.-G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Alex Ruwe, T.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-L.; Rouault, T.A. How does hepcidin hinder ferroportin activity? Blood 2018, 131, 840–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The Menopause Transition: Signs, Symptoms, and Management Options. J. Clin. Endocrinol. Metab. 2020. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Postmenopausal health interventions: Time to move on from the Women’s Health Initiative? Ageing Res. Rev. 2018, 48, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Pelle, E.; Huang, X. Iron and Menopause: Does Increased Iron Affect the Health of Postmenopausal Women? Antioxid. Redox Signal. 2009, 11, 2939–2943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; Van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; Den Heijer, M.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, 218–226. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, S.; Wang, L.; Li, J.; Qu, G.; He, J.; Rong, H.; Ji, H.; Liu, S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef]
- Lehtihet, M.; Bonde, Y.; Beckman, L.; Berinder, K.; Hoybye, C.; Rudling, M.; Sloan, J.H.; Konrad, R.J.; Angelin, B. Circulating Hepcidin-25 Is Reduced by Endogenous Estrogen in Humans. PLoS ONE 2016, 11, e0148802. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17β-Estradiol Inhibits Iron Hormone Hepcidin Through an Estrogen Responsive Element Half-Site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar] [CrossRef]
- Klip, I.T.; Voors, A.A.; Swinkels, D.W.; Bakker, S.J.L.; Kootstra-Ros, J.E.; Lam, C.S.; van der Harst, P.; van Veldhuisen, D.J.; van der Meer, P. Serum ferritin and risk for new-onset heart failure and cardiovascular events in the community. Eur. J. Heart Fail. 2017, 19, 348–356. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Chow, B.K.; Howes, P.S.; Shamayeva, G.; Baron, J.A.; Dalman, R.L.; Malenka, D.J.; Ozaki, C.K.; Lavori, P.W. Decreased Cancer Risk After Iron Reduction in Patients with Peripheral Arterial Disease: Results from a Randomized Trial. J. Natl. Cancer Inst. 2008, 100, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Zhang, X.; Jiang, J.; Zhao, G.; Wang, Y.; Xu, Y.; Xu, X.; Ma, H. Postmenopausal Iron Overload Exacerbated Bone Loss by Promoting the Degradation of Type I Collagen. BioMed Res. Int. 2017, 2017, 1345193. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Sánchez-Oliver, A.J.; Mata-Ordoñez, F.; Feria-Madueño, A.; Grimaldi-Puyana, M.; López-Samanes, Á.; Pérez-López, A. Effects of an acute exercise bout on serum hepcidin levels. Nutrients 2018, 10, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedlar, C.R.; Brugnara, C.; Bruinvels, G.; Burden, R. Iron balance and iron supplementation for the female athlete: A practical approach. Eur. J. Sport Sci. 2018, 18, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron status and the acute post-exercise hepcidin response in athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef]
- Ishibashi, A.; Maeda, N.; Sumi, D.; Goto, K. Elevated Serum Hepcidin Levels during an Intensified Training Period in Well-Trained Female Long-Distance Runners. Nutrients 2017, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Karl, J.P.; Lieberman, H.R.; Cable, S.J.; Williams, K.W.; Young, A.J.; McClung, J.P. Randomized, double-blind, placebo-controlled trial of an iron-fortified food product in female soldiers during military training: Relations between iron status, serum hepcidin, and inflammation. Am. J. Clin. Nutr. 2010, 92, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Moretti, D.; Mettler, S.; Zeder, C.; Lundby, C.; Geurts-Moetspot, A.; Monnard, A.; Swinkels, D.W.; Brittenham, G.M.; Zimmermann, M.B. An intensified training schedule in recreational male runners is associated with increases in erythropoiesis and inflammation and a net reduction in plasma hepcidin. Am. J. Clin. Nutr. 2018, 108, 1324–1333. [Google Scholar] [CrossRef]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, D.W.; Tjalsma, H.; Yeap, B.B.; Trinder, D.; Peeling, P. Oral contraception does not alter typical post-exercise interleukin-6 and hepcidin levels in females. J. Sci. Med. Sport 2015, 18, 8–12. [Google Scholar] [CrossRef]
- Newlin, M.K.; Williams, S.; McNamara, T.; Tjalsma, H.; Swinkels, D.W.; Haymes, E.M. The Effects of Acute Exercise Bouts on Hepcidin in Women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 79–88. [Google Scholar] [CrossRef]
- Roecker, L.; Meier-Buttermilch, R.; Brechtel, L.; Nemeth, E.; Ganz, T. Iron-regulatory protein hepcidin is increased in female athletes after a marathon. Eur. J. Appl. Physiol. 2005, 95, 569–571. [Google Scholar] [CrossRef]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Auersperger, I.; Knap, B.; Jerin, A.; Blagus, R.; Lainscak, M.; Skitek, M.; Skof, B. The Effects of 8 Weeks of Endurance Running on Hepcidin Concentrations, Inflammatory Parameters, and Iron Status in Female Runners. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Auersperger, I.; Škof, B.; Leskošek, B.; Knap, B.; Jerin, A.; Lainscak, M. Exercise-Induced Changes in Iron Status and Hepcidin Response in Female Runners. PLoS ONE 2013, 8, e58090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grindler, N.M.; Santoro, N.F. Menopause and exercise. Menopause 2015, 22, 1351–1358. [Google Scholar] [CrossRef]
- Kortas, J.; Ziemann, E.; Antosiewicz, J. Effect of HFE gene mutation on changes in iron metabolism induced by nordic walking in elderly women. Clin. Interv. Aging 2020, 15, 663–671. [Google Scholar] [CrossRef]
- Kortas, J.; Prusik, K.; Flis, D.; Prusik, K.; Ziemann, E.; Antosiewicz, J. Effect of nordic walking training on iron metabolism in elderly women. Clin. Interv. Aging 2015, 10, 1889–1896. [Google Scholar] [CrossRef] [Green Version]
- Kortas, J.; Kuchta, A.; Prusik, K.; Prusik, K.; Ziemann, E.; Labudda, S.; Ćwiklińska, A.; Wieczorek, E.; Jankowski, M.; Antosiewicz, J. Nordic walking training attenuation of oxidative stress in association with a drop in body iron stores in elderly women. Biogerontology 2017, 18, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Delamater, L.; Santoro, N. Management of the Perimenopause. Clin. Obstet. Gynecol. 2018, 61, 419–432. [Google Scholar] [CrossRef]
- Peeling, P.; Blee, T.; Goodman, C.; Dawson, B.; Claydon, G.; Beilby, J.; Prins, A. Effect of Iron Injections on Aerobic-Exercise Performance of Iron-Depleted Female Athletes. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Barba-Moreno, L.; Cupeiro, R.; Romero-Parra, N.; Janse de Jonge, X.A.K.; Peinado, A.B. Cardiorespiratory Responses to Endurance Exercise Over the Menstrual Cycle and with Oral Contraceptive Use. J. Strength Cond. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Onate, J.; Morrison, S. Differential effects of fatigue on movement variability. Gait Posture 2014, 39, 888–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troutt, J.S.; Rudling, M.; Persson, L.; Ståhle, L.; Angelin, B.; Butterfield, A.M.; Schade, A.E.; Cao, G.; Konrad, R.J. Circulating Human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin. Chem. 2012, 58, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.; Morimoto, Y.; Cooney, R.V.; Franke, A.A.; Shvetsov, Y.B.; Le Marchand, L.; Haiman, C.A.; Kolonel, L.N.; Goodman, M.T.; Maskarinec, G. Dietary Red and Processed Meat Intake and Markers of Adiposity and Inflammation: The Multiethnic Cohort Study. J. Am. Coll. Nutr. 2017, 36, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Holven, K.B.; Gil, A.; Rangel-Huerta, O.D. Milk and Dairy Product Consumption and Inflammatory Biomarkers: An Updated Systematic Review of Randomized Clinical Trials. Adv. Nutr. 2019, 10, S239–S250. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Margolis, L.M.; Murphy, N.E.; McClung, H.L.; Martini, S.; Gundersen, Y.; Castellani, J.W.; Karl, J.P.; Teien, H.K.; Madslien, E.H.; et al. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol. Rep. 2016, 4, e12820. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, D.W.; Tjalsma, H.; Trinder, D.; Peeling, P. Effect of Exercise Modality and Intensity on Postexercise Interleukin-6 and Hepcidin Levels. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 178–186. [Google Scholar] [CrossRef]
- Diepeveen, L.E.; Laarakkers, C.M.M.; Martos, G.; Pawlak, M.E.; Uğuz, F.F.; Verberne, K.E.S.A.; van Swelm, R.P.L.; Klaver, S.; de Haan, A.F.J.; Pitts, K.R.; et al. Provisional standardization of hepcidin assays: Creating a traceability chain with a primary reference material, candidate reference method and a commutable secondary reference material. Clin. Chem. Lab. Med. 2019, 57, 864–872. [Google Scholar] [CrossRef]
- Laarakkers, C.M.M.; Wiegerinck, E.T.; Klaver, S.; Kolodziejczyk, M.; Gille, H.; Hohlbaum, A.M.; Tjalsma, H.; Swinkels, D.W. Improved Mass Spectrometry Assay for Plasma Hepcidin: Detection and Characterization of a Novel Hepcidin Isoform. PLoS ONE 2013, 8, e75518. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banzet, S.; Sanchez, H.; Chapot, R.; Bigard, X.; Vaulont, S.; Koulmann, N. Interleukin-6 contributes to hepcidin mRNA increase in response to exercise. Cytokine 2012, 58, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Janse de Jonge, X.A.K.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sport. Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Alis, R.; Ibañez-Sania, S.; Basterra, J.; Sanchis-Gomar, F.; Romagnoli, M. Effects of an acute high-intensity interval training protocol on plasma viscosity. J. Sports Med. Phys. Fit. 2015, 55, 647–653. [Google Scholar]
- Sim, M.; Dawson, B.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Townsend, M.-A.; Trinder, D.; Peeling, P. The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur. J. Appl. Physiol. 2012, 112, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.E.; Dawson, B.; Cox, G.R.; Laarakkers, C.M.; Swinkels, D.W.; Peeling, P. Timing of post-exercise carbohydrate ingestion: Influence on IL-6 and hepcidin responses. Eur. J. Appl. Physiol. 2015, 115, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Case, M.W.; Soukup, J.M. Heme oxygenase activity increases after exercise in healthy volunteers. Free Radic. Res. 2018, 52, 267–272. [Google Scholar] [CrossRef]
- Marro, S.; Chiabrando, D.; Messana, E.; Stolte, J.; Turco, E.; Tolosano, E.; Muckenthaler, M.U. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica 2010, 95, 1261–1268. [Google Scholar] [CrossRef] [Green Version]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [Green Version]
- Zacharski, L.R.; Ornstein, D.L.; Woloshin, S.; Schwartz, L.M. Association of age, sex, and race with body iron stores in adults: Analysis of NHANES III data. Am. Heart J. 2000, 140, 98–104. [Google Scholar] [CrossRef]
- Hinton, P.S. Iron and the endurance athlete. Appl. Physiol. Nutr. Metab. 2014, 39, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Lundby, C. Regulation of Red Blood Cell Volume with Exercise Training. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2018; Volume 9, pp. 149–164. [Google Scholar] [CrossRef]
- Díaz, B.B.; González, D.A.; Gannar, F.; Pérez, M.C.R.; de León, A.C. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol. Lett. 2018, 203, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bay, M.L.; Pedersen, B.K. Muscle-Organ Crosstalk: Focus on Immunometabolism. Front. Physiol. 2020, 11, 567881. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]

| Age (years) | 51.5 ± 3.89 |
| Height (cm) | 161.8 ± 4.9 |
| Body mass (kg) | 55.9 ± 3.6 |
| Body fat (%) | 24.7 ± 4.2 |
| O2peak (mL/kg/minute) | 42.4 ± 4.0 |
| Endurance training experience (years) | 7.2 ± 3.1 |
| Training volume during the last 6 months (minute/week) | 255 ± 106 |
| 17β-estradiol (pg/mL) | 28.13 ± 46.95 |
| Progesterone (ng/mL) | 0.21 ± 0.13 |
| LH (mIU/mL) | 50.32 ± 20.21 |
| FSH (mIU/mL) | 90.93 ± 44.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro-Magallanes, V.M.; Benito, P.J.; Rael, B.; Barba-Moreno, L.; Romero-Parra, N.; Cupeiro, R.; Swinkels, D.W.; Laarakkers, C.M.; Peinado, A.B.; on behalf of the IronFEMME Study Group. Menopause Delays the Typical Recovery of Pre-Exercise Hepcidin Levels after High-Intensity Interval Running Exercise in Endurance-Trained Women. Nutrients 2020, 12, 3866. https://doi.org/10.3390/nu12123866
Alfaro-Magallanes VM, Benito PJ, Rael B, Barba-Moreno L, Romero-Parra N, Cupeiro R, Swinkels DW, Laarakkers CM, Peinado AB, on behalf of the IronFEMME Study Group. Menopause Delays the Typical Recovery of Pre-Exercise Hepcidin Levels after High-Intensity Interval Running Exercise in Endurance-Trained Women. Nutrients. 2020; 12(12):3866. https://doi.org/10.3390/nu12123866
Chicago/Turabian StyleAlfaro-Magallanes, Víctor M., Pedro J. Benito, Beatriz Rael, Laura Barba-Moreno, Nuria Romero-Parra, Rocío Cupeiro, Dorine W. Swinkels, Coby M. Laarakkers, Ana B. Peinado, and on behalf of the IronFEMME Study Group. 2020. "Menopause Delays the Typical Recovery of Pre-Exercise Hepcidin Levels after High-Intensity Interval Running Exercise in Endurance-Trained Women" Nutrients 12, no. 12: 3866. https://doi.org/10.3390/nu12123866
APA StyleAlfaro-Magallanes, V. M., Benito, P. J., Rael, B., Barba-Moreno, L., Romero-Parra, N., Cupeiro, R., Swinkels, D. W., Laarakkers, C. M., Peinado, A. B., & on behalf of the IronFEMME Study Group. (2020). Menopause Delays the Typical Recovery of Pre-Exercise Hepcidin Levels after High-Intensity Interval Running Exercise in Endurance-Trained Women. Nutrients, 12(12), 3866. https://doi.org/10.3390/nu12123866






