Dietary Inflammatory Index and S-Klotho Plasma Levels in Middle-Aged Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Dietary Data
2.2.2. Dietary Inflammatory Index (DII)
2.2.3. S-Klotho Plasma Levels
2.2.4. Anthropometric Measurements
2.2.5. Blood Pressure Assessment
2.3. Statistical Analyses
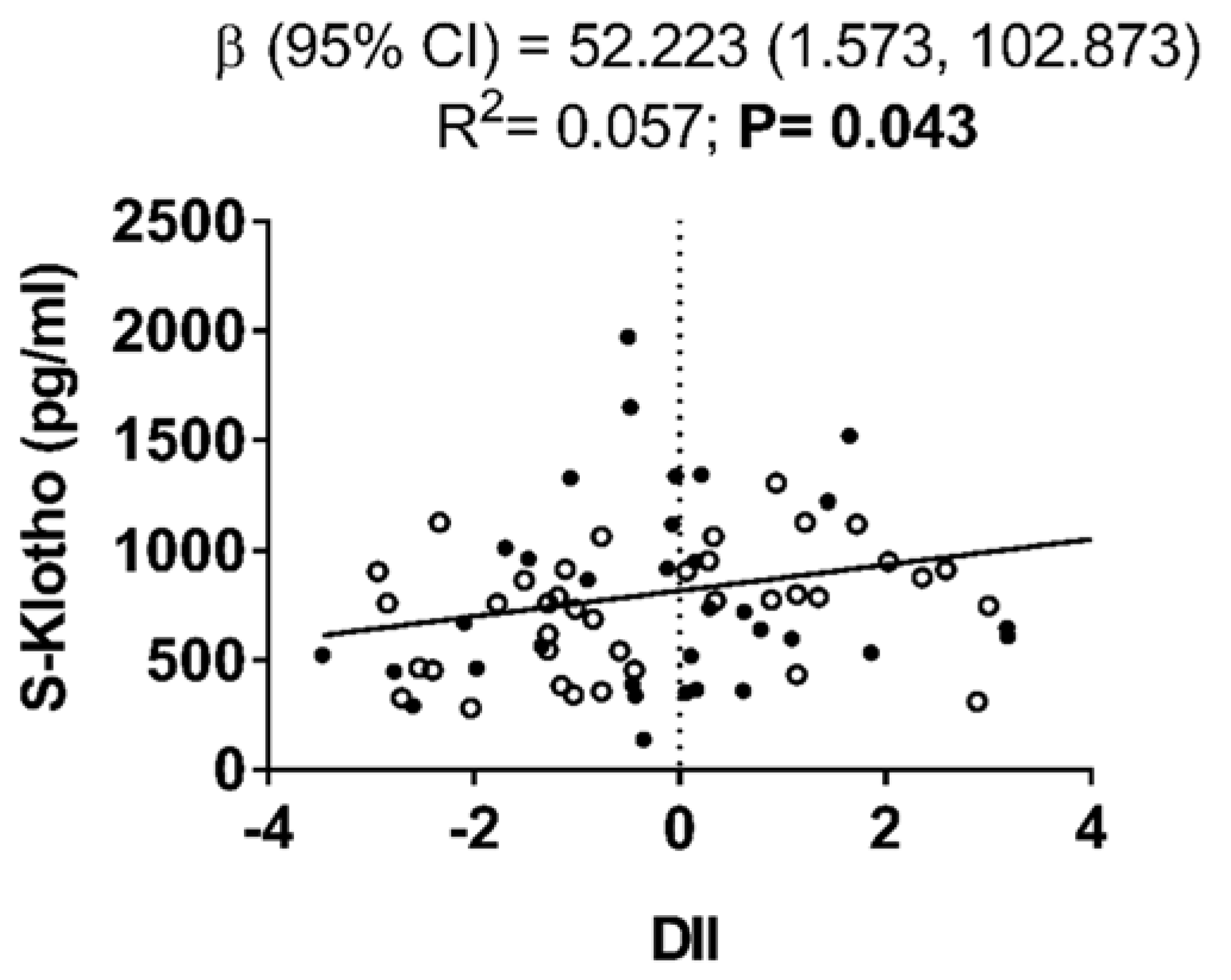
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Zhong, J.; Shi, G. Editorial: Regulation of inflammation in chronic disease. Front. Immunol. 2019, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef]
- Pahwa, R.; Jialal, I. Chronic Inflammation. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 12 December 2019).
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Casas, R.; Estruch, R. Dietary Patterns, Foods, Nutrients and Chronic Inflammatory Disorders. Immunome Res. 2016, 12, 1. [Google Scholar] [CrossRef]
- Shivappa, N. Diet and Chronic Diseases: Is There a Mediating Effect of Inflammation? Nutrients 2019, 11, 1639. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The Effects of Diet on Inflammation. Emphasis on the Metabolic Syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Kotemori, A.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shivappa, N.; Hebert, J.R.; Ishihara, J.; Inoue, M.; Tsugane, S. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: A cross-sectional study of the JPHC-FFQ validation study. Nutrition 2020, 69, 110569. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Shivappa, N.; Davis, L.; Hurley, T.G.; Ortaglia, A.; Drayton, R.; Blair, S.N.; Hébert, J.R. Construct validation of the Dietary Inflammatory Index among African Americans. J. Nutr. Health Aging 2017, 21, 487–491. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Martínez-González, M.A.; Ramallal, R.; Salas-Salvadó, J.; Hébert, J.R.; Corella, D.; Shivappa, N.; Forga, L.; Schröder, H.; Muñoz-Bravo, C.; et al. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin. Nutr. 2019, 38, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Nabeshima, Y.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Cheikhi, A.; Barchowsky, A.; Sahu, A.; Shinde, S.N.; Pius, A.; Clemens, Z.J.; Li, H.; Kennedy, C.A.; Hoeck, J.D.; Franti, M.; et al. Klotho: An Elephant in Aging Research. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho. Pflugers Arch. Eur. J. Physiol. 2010, 459, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011, 59, 1596–1601. [Google Scholar] [CrossRef]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and mortality risk in older community-dwelling adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 794–800. [Google Scholar] [CrossRef]
- Izquierdo, M.C.; Perez-Gomez, M.V.; Sanchez-Niño, M.D.; Sanz, A.B.; Ruiz-Andres, O.; Poveda, J.; Moreno, J.A.; Egido, J.; Ortiz, A. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 6–10. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; Robles-González, L.; Navarro-Lomas, G.; de Haro, T.; Femia, P.; Castillo, M.J.; Gutiérrez, Á. Exercise training as S-Klotho protein stimulator in sedentary healthy adults: Rationale, design, and methodology. Contemp. Clin. Trials Commun. 2018, 11, 10–19. [Google Scholar] [CrossRef]
- Vioque, J.; Navarrete-Muñoz, E.-M.; Gimenez-Monzó, D.; García-de-la-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr. J. 2013, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- López, M.D.R.; Martín-Lagos, R.A. Guía Para Estudios Dietéticos: Álbum Fotográfico de Alimentos; Editorial Universidad de Granada: Granada, Spain, 2010. [Google Scholar]
- Marfell-Jones, M.; Olds, T.; Stewart, A. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2011. [Google Scholar]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la-O, A.; Martinez-Tellez, B.; Ruiz, J.R.; Gutiérrez, Á.; Castillo, M.J. Adherence to the Mediterranean diet, dietary factors, and S-Klotho plasma levels in sedentary middle-aged adults. Exp. Gerontol. 2019, 119, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Fasoli, L.; Amaro-Gahete, F.J.; De-la-O, A.; Gutiérrez, Á.; Castillo, M.J. Alcohol consumption and S-Klotho plasma levels in sedentary healthy middle-aged adults: A cross sectional study. Drug Alcohol Depend. 2019, 194, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Corley, J.; Shivappa, N.; Hébert, J.R.; Starr, J.M.; Deary, I.J. Associations between Dietary Inflammatory Index Scores and Inflammatory Biomarkers Among Older Adults in the Lothian Birth Cohort 1936 Study. J. Nutr. Health Aging 2019, 23, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Bonaccio, M.; Hebert, J.R.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; Pounis, G.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Association of pro-inflammatory diet with low-grade inflammation: Results from the Moli-sani study. Nutrition 2018, 54, 182–188. [Google Scholar] [CrossRef]
- Assmann, K.E.; Adjibade, M.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Touvier, M.; Akbaraly, T.; Hercberg, S.; Galan, P.; Julia, C.; et al. The inflammatory potential of the diet at midlife is associated with later healthy aging in french adults. J. Nutr. 2018, 148, 437–444. [Google Scholar] [CrossRef]
- Dounousi, E.; Torino, C.; Pizzini, P.; Cutrupi, S.; Panuccio, V.; D’Arrigo, G.; Abd Elhafeez, S.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Intact FGF23 and α-klotho during acute inflammation/sepsis in CKD patients. Eur. J. Clin. Investig. 2016, 46, 234–241. [Google Scholar] [CrossRef]
- Abdelmalik, P.A.; Stevens, R.D.; Singh, S.; Skinner, J.; Carhuaopoma, J.R.; Noel, S.; Johns, R.; Fuchs, R.J. Anti-aging factor, serum alpha-Klotho, as a marker of acute physiological stress, and a predictor of ICU mortality, in patients with septic shock. J. Crit. Care 2018, 44, 323–330. [Google Scholar] [CrossRef]
- González-Reimers, E.; Martín-González, C.; Romero-Acevedo, L.; Martínez-Riera, A. Klotho levels and ethanol consumption. Drug Alcohol Depend. 2019, 198, 190–191. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Ambrosius, W.; Messier, S.P.; Miller, G.D.; Penninx, B.W.J.H.; Loeser, R.F.; Palla, S.; Bleecker, E.; Pahor, M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: A randomized controlled clinical trial. Am. J. Clin. Nutr. 2004, 79, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Espuch-Oliver, A.; de Haro, T.; Gutierrez, A.; Ruiz, J.R.; Castillo, M.J. Body composition and S-Klotho plasma levels in middle-aged adults: A cross-sectional study. Rejuvenation Res. 2019, 22, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Trabert, B.; Katki, H.A.; Chaturvedi, A.K.; Kemp, T.J.; Pinto, L.A.; Moore, S.C.; Purdue, M.P.; Wentzensen, N.; Hildesheim, A. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol. Biomarkers Prev. 2008, 23, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
| All (N = 73) | Men (N = 35) | Women (N = 38) | |
|---|---|---|---|
| Age (years) | 53.6 (5.2) | 54.4 (5.3) | 53.0 (5.1) |
| Energy intake (kcal/day) | 2133.9 (688.4) | 2067.7 (497.3) | 2194.8 (828.9) |
| DII | −0.19 (1.66) | −0.07 (1.64) | −0.30 (1.93) |
| BMI (kg/m2) | 26.7 (3.8) | 28.3 (3.6) | 25.2 (3.3) |
| Waist-hip ratio | 0.91 (0.08) | 0.97 (0.07) | 0.86 (0.06) |
| Use of medication % (n) | 37.0 (27) | 31.4 (11) | 42.1 (16) |
| Systolic blood pressure (mmHg) | 127.4 (15.4) | 134.2 (13.6) | 121.7 (14.6) |
| Diastolic blood pressure (mmHg) | 81.5 (11.7) | 85.4 (10.8) | 78.2 (11.7) |
| S-Klotho (pg/mL) | 773.7 (366.0) | 814.1 (452.2) | 737.5 (268.0) |
| S-Klotho Plasma Levels | |||
|---|---|---|---|
| Β (95% CI) | t | p | |
| DII | 40.619 (−16.512, 97.749) | 1.425 | 0.160 |
| Energy Intake | 0.020 (−0.109, 0.149) | 0.311 | 0.757 |
| Sex | 18.719 (−229.935, 267.374) | 0.151 | 0.881 |
| BMI | 33.051 (4.675, 61.427) | 2.334 | 0.023 |
| WHiR | −608.476 (−1994.171, 777.218) | −0.880 | 0.383 |
| Medication use | 7.879 (−179.829, 195.5889 | 0.084 | 0.933 |
| Systolic blood pressure | 3.512 8−7.294, 14.3199 | 0.651 | 0.518 |
| Dyastolic blood pressure | −1.188 (−14.393, 12.017) | −0.180 | 0.858 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado-Fasoli, L.; Castillo, M.J.; Amaro-Gahete, F.J. Dietary Inflammatory Index and S-Klotho Plasma Levels in Middle-Aged Adults. Nutrients 2020, 12, 281. https://doi.org/10.3390/nu12020281
Jurado-Fasoli L, Castillo MJ, Amaro-Gahete FJ. Dietary Inflammatory Index and S-Klotho Plasma Levels in Middle-Aged Adults. Nutrients. 2020; 12(2):281. https://doi.org/10.3390/nu12020281
Chicago/Turabian StyleJurado-Fasoli, Lucas, Manuel J. Castillo, and Francisco J. Amaro-Gahete. 2020. "Dietary Inflammatory Index and S-Klotho Plasma Levels in Middle-Aged Adults" Nutrients 12, no. 2: 281. https://doi.org/10.3390/nu12020281
APA StyleJurado-Fasoli, L., Castillo, M. J., & Amaro-Gahete, F. J. (2020). Dietary Inflammatory Index and S-Klotho Plasma Levels in Middle-Aged Adults. Nutrients, 12(2), 281. https://doi.org/10.3390/nu12020281





