Wheat Extract Oil (WEO) Attenuates UVB-Induced Photoaging via Collagen Synthesis in Human Keratinocytes and Hairless Mice
Abstract
1. Introduction
2. Material and Method
2.1. Preparation of WEO
2.2. Test Material Preparation
2.3. In Vivo Experiments
2.3.1. Experimental Animals and UVB Irradiation
2.3.2. Skin Hydration and Transepidermal Water Loss (TEWL) Measurement
2.3.3. Skin Elasticity and Wrinkle Measurement
2.3.4. Ceramide Content of Skin Tissue
2.3.5. Histopathological Assessment of Skin Tissue
2.4. Cell Culture Experiments
2.4.1. HaCaT Cell Culture and Viability
2.4.2. CCD-986sk Cell Culture and Viability
2.4.3. Collagen Formation Test
2.4.4. Hyaluronic Acid Formation Test
2.4.5. Matrix Metalloproteinase-1 (MMP-1) Formation Test
2.4.6. RNA Isolation and Quantitative RT-PCR
2.5. Statistics
3. Results
3.1. Skin Hydration and Transepidermal Water Loss
3.2. Skin Elasticity and Wrinkles in the Dorsal Epidermis
3.3. Procollagen Type I, Hyaluronic Acid, Ceramide, and Collagen in Skin of Hairless Mice Improved with WEO Administration
3.4. Ceramide Measurement
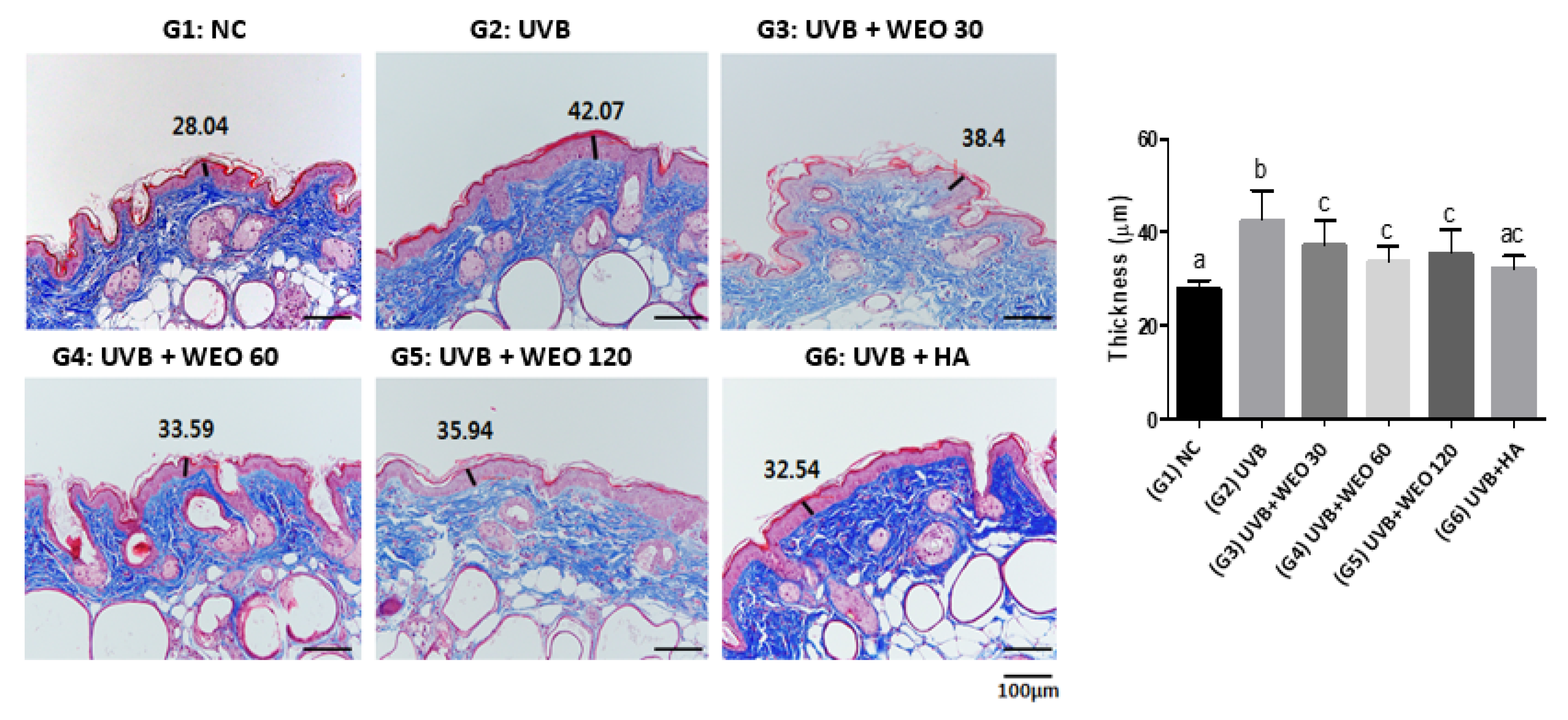
3.5. Epidermal Thickness
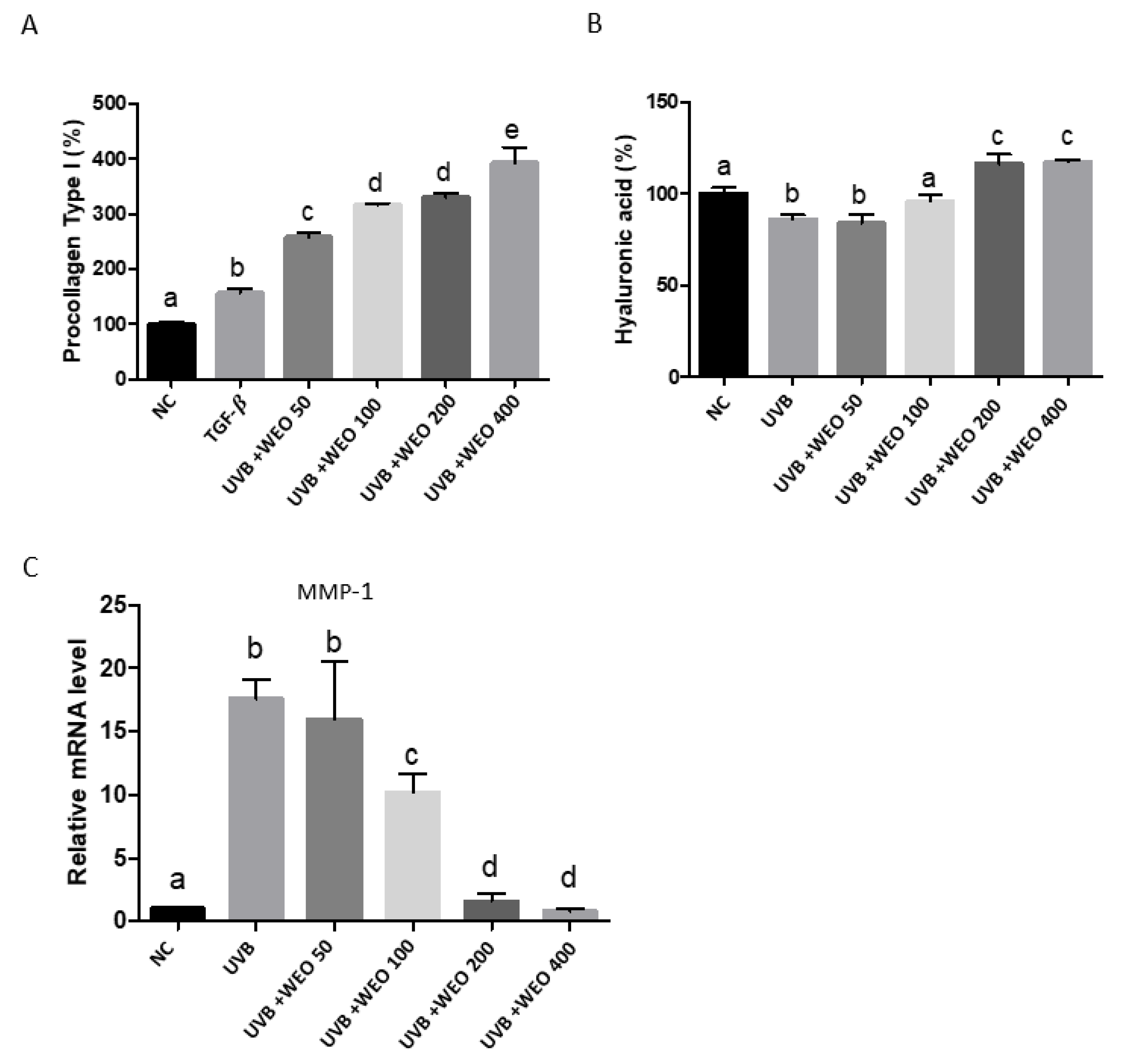
3.6. In Vitro Determination of WEO on Procollagen, Hyaluronic Acid, and Matrix Metalloproteinase-1 Synthesis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chambers, E.S.; Vukmanovic-Stegic, M. Skin barrier immunity and aging. Immunology 2019. [Google Scholar] [CrossRef] [PubMed]
- Barco, D.; Gimenez-Arnau, A. Xerosis: A dysfunction of the epidermal barrier. Actas Dermo-Sifiliogr. 2008, 99, 671–682. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Ceramides of pig epidermis: Structure determination. J. Lipid Res. 1983, 24, 759–765. [Google Scholar] [PubMed]
- Martini, M.C. Biochemical analysis of epidermal lipids. Pathol. Biol. 2003, 51, 267–270. [Google Scholar] [CrossRef]
- Claudy, A. Cutaneous lipids: From physiology to the clinical. Pathol. Biol. 2003, 51, 260–263. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef]
- Li, Q.; Fang, J.; Dang, E.; Wang, G. The role of ceramides in skin homeostasis and inflammatory skin diseases. J. Dermatol. Sci. 2019. [Google Scholar] [CrossRef]
- Jungersted, J.M.; Hellgren, L.I.; Jemec, G.B.; Agner, T. Lipids and skin barrier function—A clinical perspective. Contact Dermat. 2008, 58, 255–262. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Abramovits, W. Ceramides and the stratum corneum: Structure, function, and new methods to promote repair. Int. J. Dermatol. 2003, 42, 256–259. [Google Scholar] [CrossRef]
- Rawlings, A.V. Trends in stratum corneum research and the management of dry skin conditions. Int. J. Cosmet. Sci. 2003, 25, 63–95. [Google Scholar] [CrossRef]
- Yilmaz, E.; Borchert, H.H. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema—An in vivo study. Int. J. Pharm. 2006, 307, 232–238. [Google Scholar] [CrossRef]
- Imokawa, G.; Akasaki, S.; Minematsu, Y.; Kawai, M. Importance of intercellular lipids in water-retention properties of the stratum corneum: Induction and recovery study of surfactant dry skin. Arch. Dermatol. Res. 1989, 281, 45–51. [Google Scholar] [CrossRef]
- Bak, H.; Hong, S.P.; Jeong, S.K.; Choi, E.H.; Lee, S.E.; Lee, S.H.; Ahn, S.K. Altered epidermal lipid layers induced by long-term exposure to suberythemal-dose ultraviolet. Int. J. Dermatol. 2011, 50, 832–837. [Google Scholar] [CrossRef]
- Kim, H.; Oh, I.; Park, K.H.; Kim, N.M.; Do, J.H.; Cho, Y. Stimulatory effect of dietary red ginseng on epidermal hydration and ceramide levels in ultraviolet-irradiated hairless mice. J. Med. Food 2009, 12, 746–754. [Google Scholar] [CrossRef]
- Jin, K.; Higaki, Y.; Takagi, Y.; Higuchi, K.; Yada, Y.; Kawashima, M.; Imokawa, G. Analysis of beta-glucocerebrosidase and ceramidase activities in atopic and aged dry skin. Acta Derm. Venereol. 1994, 74, 337–340. [Google Scholar]
- Nilsson, A.; Duan, R.D. Absorption and lipoprotein transport of sphingomyelin. J. Lipid Res. 2006, 47, 154–171. [Google Scholar] [CrossRef]
- Sugawara, T.; Kinoshita, M.; Ohnishi, M.; Nagata, J.; Saito, M. Digestion of maize sphingolipids in rats and uptake of sphingadienine by Caco-2 cells. J. Nutr. 2003, 133, 2777–2782. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Schmelz, E.M.; Wang, E.; Dillehay, D.L.; Rice, L.G.; Meredith, F.; Riley, R.T. Importance of sphingolipids and inhibitors of sphingolipid metabolism as components of animal diets. J. Nutr. 1997, 127, 830S–833S. [Google Scholar] [CrossRef]
- Pappas, A.; Liakou, A.; Zouboulis, C.C. Nutrition and skin. Rev. Endocr. Metab. Disord. 2016, 17, 443–448. [Google Scholar] [CrossRef]
- Lorencini, M.; Brohem, C.A.; Dieamant, G.C.; Zanchin, N.I.; Maibach, H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014, 15, 100–115. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef] [PubMed]
- Djedour, A.; Lafforgue, C.; Marty, J.P.; Grossiord, J.L. A very promising new glucolipidic surfactant: Lipowheat. Int. J. Cosmet. Sci. 2005, 27, 301–308. [Google Scholar] [CrossRef]
- Abreu, S.; Solgadi, A.; Chaminade, P. Optimization of normal phase chromatographic conditions for lipid analysis and comparison of associated detection techniques. J. Chromatogr. A 2017, 1514, 54–71. [Google Scholar] [CrossRef]
- Guillou, S.; Ghabri, S.; Jannot, C.; Gaillard, E.; Lamour, I.; Boisnic, S. The moisturizing effect of a wheat extract food supplement on women’s skin: A randomized, double-blind placebo-controlled trial. Int. J. Cosmet. Sci. 2011, 33, 138–143. [Google Scholar] [CrossRef]
- Fotinos, S. Compositions Rich in Lipids Obtained from Vegetable Sources by Solvent Extraction, Suitable for Use in Cosmetics, Pharmaceuticals, and Foodstuffs [Internet]. FR2785806 (A1). 2000. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20000519&DB=&locale=en_EP&CC=FR&NR=2785806A1&KC=A1&ND=4 (accessed on 15 December 2019).
- Bellamy, G.; Bornstein, P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc. Natl. Acad. Sci. USA 1971, 68, 1138–1142. [Google Scholar] [CrossRef]
- Ghersetich, I.; Lotti, T.; Campanile, G.; Grappone, C.; Dini, G. Hyaluronic acid in cutaneous intrinsic aging. Int. J. Dermatol. 1994, 33, 119–122. [Google Scholar] [CrossRef]
- Akimoto, K.; Yoshikawa, N.; Higaki, Y.; Kawashima, M.; Imokawa, G. Quantitative analysis of stratum corneum lipids in xerosis and asteatotic eczema. J. Dermatol. 1993, 20, 1–6. [Google Scholar] [CrossRef]
- Imokawa, G.; Kuno, H.; Kawai, M. Stratum corneum lipids serve as a bound-water modulator. J. Investig. Dermatol. 1991, 96, 845–851. [Google Scholar] [CrossRef]
- Boisnic, S.; Keophiphath, M.; Serandour, A.L.; Branchet, M.C.; Le Breton, S.; Lamour, I.; Gaillard, E. Polar lipids from wheat extract oil improve skin damages induced by aging: Evidence from a randomized, placebo-controlled clinical trial in women and an ex vivo study on human skin explant. J. Cosmet. Dermatol. 2019, 18, 2027–2036. [Google Scholar] [CrossRef]
- Jiang, S.J.; Chu, A.W.; Lu, Z.F.; Pan, M.H.; Che, D.F.; Zhou, X.J. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp. Dermatol. 2007, 16, 985–992. [Google Scholar] [CrossRef]
- Haratake, A.; Uchida, Y.; Schmuth, M.; Tanno, O.; Yasuda, R.; Epstein, J.H.; Elias, P.M.; Holleran, W.M. UVB-induced alterations in permeability barrier function: Roles for epidermal hyperproliferation and thymocyte-mediated response. J. Investig. Dermatol. 1997, 108, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Sevrain, S.; Bonte, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Simon, L.S.; Villanueva, A.R.; Krane, S.M. Procollagen type I carboxy-terminal extension peptide in serum as a marker of collagen biosynthesis in bone. Correlation with Iliac bone formation rates and comparison with total alkaline phosphatase. J. Bone Miner Res. 1987, 2, 427–436. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choic, H.R.; Park, K.C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Yang, B.; Ji, C.; Kang, J.; Chen, W.; Bi, Z.; Wan, Y. Trans-Zeatin inhibits UVB-induced matrix metalloproteinase-1 expression via MAP kinase signaling in human skin fibroblasts. Int. J. Mol. Med. 2009, 23, 555–560. [Google Scholar]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-kappaB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar]
- Park, H.S.; Ha, H.Y.; Kim, H.T. An Experimental Study on the Effect of Angelica gigas Ethanol Extract on Hyaluronic Acid Synthesis. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2018, 31, 32–41. [Google Scholar]
- Kim, K.H.; Kim, K.T.; Kim, Y.H.; Kim, J.G.; Han, C.S.; Park, S.H.; Lee, B.Y. Preparation of Oligo Hyaluronic Acid by Hydrolysis and Its Application as a Cosmetic Ingredient. J. Soc. Cosmet. Sci. Korea 2007, 33, 189–196. [Google Scholar]


| Group | Volume (mg/kg) | |
|---|---|---|
| G1 | Normal control group | 0 |
| G2 | UVB control group | 0 |
| G3 | UVB + WEO | 30 |
| G4 | 60 | |
| G5 | 120 | |
| G6 | UVB + Positive control (HA) | 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, D.J.; Jung, J.C.; Choi, Y.M.; Ryu, H.Y.; Lee, S.; Davis, B.A. Wheat Extract Oil (WEO) Attenuates UVB-Induced Photoaging via Collagen Synthesis in Human Keratinocytes and Hairless Mice. Nutrients 2020, 12, 300. https://doi.org/10.3390/nu12020300
Son DJ, Jung JC, Choi YM, Ryu HY, Lee S, Davis BA. Wheat Extract Oil (WEO) Attenuates UVB-Induced Photoaging via Collagen Synthesis in Human Keratinocytes and Hairless Mice. Nutrients. 2020; 12(2):300. https://doi.org/10.3390/nu12020300
Chicago/Turabian StyleSon, Dong Ju, Jae Chul Jung, Yong Min Choi, Hyeon Yeol Ryu, Somin Lee, and Barbara A. Davis. 2020. "Wheat Extract Oil (WEO) Attenuates UVB-Induced Photoaging via Collagen Synthesis in Human Keratinocytes and Hairless Mice" Nutrients 12, no. 2: 300. https://doi.org/10.3390/nu12020300
APA StyleSon, D. J., Jung, J. C., Choi, Y. M., Ryu, H. Y., Lee, S., & Davis, B. A. (2020). Wheat Extract Oil (WEO) Attenuates UVB-Induced Photoaging via Collagen Synthesis in Human Keratinocytes and Hairless Mice. Nutrients, 12(2), 300. https://doi.org/10.3390/nu12020300






