Potency Assessment of CBD Oils by Their Effects on Cell Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolated CBD and CBD Oils
2.2. Cannabinoid and Terpene Profiles of CBD Oils
2.3. GC-MS Analysis of Terpene Profiles
2.4. HPLC Analysis of Cannabinoid Profiles
2.5. Cell Line and Culturing Condition
2.6. Treatment Condition
2.7. Cell Proliferation and Cytotoxicity Assays
2.8. Preparation of Cell Lysates
2.9. Antibodies and Biomarker Proteins
2.10. cIEF Immunoassays
2.11. Capillary Western Immunoassays
2.12. Data Analysis
2.13. Statistical Analysis
2.14. Sample Availability
3. Results
3.1. Physical Appearances and Chemical Profiles of Six Full-Spectrum CBD Oils
3.2. Cytotoxicity of Isolated CBD to Cultured Neuronal Cells
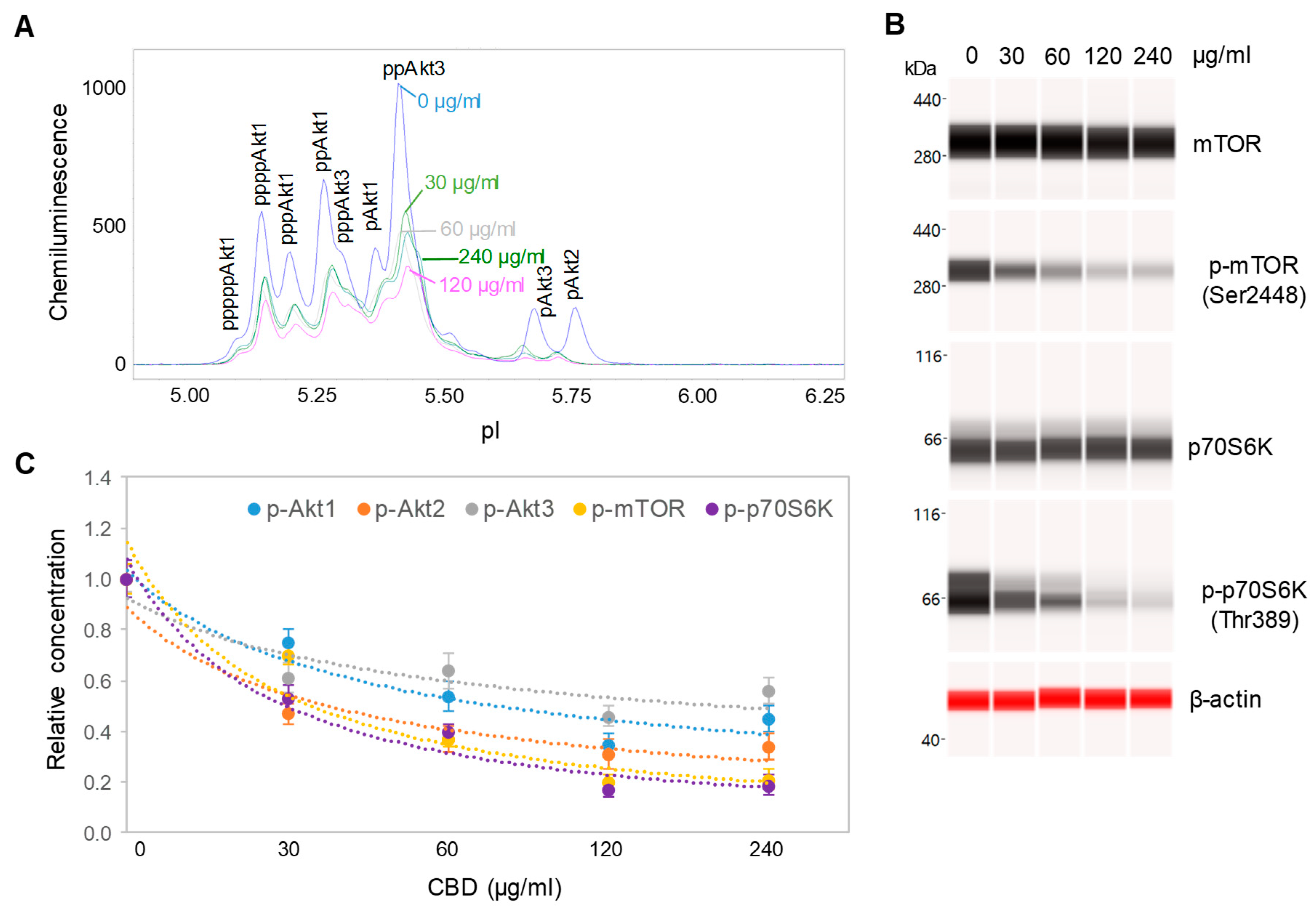
3.3. Downregulation of the PI3K/Akt/mTOR Signaling Pathway
3.4. Dose- and Time-dependent Suppression of the PI3K/Akt/mTOR Signaling Pathway
3.5. Negative Regulation of Neuronal Signaling Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mead, A. Legal and regulatory issues governing cannabis and cannabis-derived products in the United States. Front. Plant Sci. 2019, 10, 697. [Google Scholar] [CrossRef]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ guide to cannabidiol and hemp oils. Mayo. Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. A Review of human studies sssessing cannabidiol’s (CBD) therapeutic actions and potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Bonn-Miller, M.O.; Loflin, M.J.E.; Thomas, B.F.; Marcu, J.P.; Hyke, T.; Vandrey, R. Labeling accuracy of cannabidiol extracts sold online. JAMA 2017, 318, 1708–1709. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the bell-shaped dose-response of cannabidiol by using cannabis extract enriched in cannabidiol. Pharmacol. Pharm. 2015, 6, 75–85. [Google Scholar] [CrossRef]
- Russo, E.B. The case for the entourage effect and conventional breeding of clinical cannabis: No “strain,” no gain. Front. Plant Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; ElSohly, M.A.; Loflin, M.J.E.; Chandra, S.; Vandrey, R. Cannabis and cannabinoid drug development: Evaluating botanical versus single molecule approaches. Int. Rev. Psychiatry 2018, 30, 277–284. [Google Scholar] [CrossRef]
- Horth, R.Z.; Crouch, B.; Horowitz, B.Z.; Prebish, A.; Slawson, M.; McNair, J.; Elsholz, C.; Gilley, S.; Robertson, J.; Risk, I.; et al. Notes from the field: Acute poisonings from a synthetic cannabinoid sold as cannabidiol - Utah, 2017-2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 587–588. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Urasaki, Y.; Fiscus, R.R.; Le, T.T. Molecular classification of fatty liver by high-throughput profiling of protein post-translational modifications. J. Pathol. 2016, 238, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, Y.; Zhang, C.; Cheng, J.X.; Le, T.T. Quantitative assessment of liver steatosis and affected pathways with molecular imaging and proteomic profiling. Sci. Rep. 2018, 8, 3606. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, Y.; Fiscus, R.R.; Le, T.T. Detection of the cell cycle-regulated negative feedback phosphorylation of mitogen-activated protein kinases in breast carcinoma using nanofluidic proteomics. Sci. Rep. 2018, 8, 9991. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, Y.; Le, T.T. Differentiation of essential oils using nanofluidic protein post-translational modification profiling. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Iacovides, D.C.; Johnson, A.B.; Wang, N.; Boddapati, S.; Korkola, J.; Gray, J.W. Identification and quantification of AKT isoforms and phosphoforms in breast cancer using a novel nanofluidic immunoassay. Mol. Cell Prot. 2013, 12, 3210–3220. [Google Scholar] [CrossRef]
- Chen, J.Q.; Lee, J.H.; Herrmann, M.A.; Park, K.S.; Heldman, M.R.; Goldsmith, P.K.; Wang, Y.; Giaccone, G. Capillary isoelectric-focusing immunoassays to study dynamic oncoprotein phosphorylation and drug response to targeted therapies in non-small cell lung cancer. Mol. Cancer Ther. 2013, 12, 2601–2613. [Google Scholar] [CrossRef]
- Guo, H.; Gao, M.; Lu, Y.; Liang, J.; Lorenzi, P.L.; Bai, S.; Hawke, D.H.; Li, J.; Dogruluk, T.; Scott, K.L.; et al. Coordinate phosphorylation of multiple residues on single AKT1 and AKT2 molecules. Oncogene 2014, 33, 3463–3472. [Google Scholar] [CrossRef]
- Crosbie, P.A.; Crosbie, E.J.; Aspinall-O’Dea, M.; Walker, M.; Harrison, R.; Pernemalm, M.; Shah, R.; Joseph, L.; Booton, R.; Pierce, A.; et al. ERK and AKT phosphorylation status in lung cancer and emphysema using nanocapillary isoelectric focusing. BMJ Open Respir Res. 2016, 3, e000114. [Google Scholar] [CrossRef]
- Sabnis, H.; Bradley, H.L.; Bunting, S.T.; Cooper, T.M.; Bunting, K.D. Capillary nano-immunoassay for Akt 1/2/3 and 4EBP1 phosphorylation in acute myeloid leukemia. J. Transl. Med. 2014, 12, 166. [Google Scholar] [CrossRef]
- Schrotter, S.; Leondaritis, G.; Eickholt, B.J. Capillary isoelectric focusing of Akt isoforms identifies highly dynamic phosphorylation in neuronal cells and brain tissue. J. Biol. Chem. 2016, 291, 10239–10251. [Google Scholar] [CrossRef]
- Fan, A.C.; Deb-Basu, D.; Orban, M.W.; Gotlib, J.R.; Natkunam, Y.; O’Neill, R.; Padua, R.A.; Xu, L.; Taketa, D.; Shirer, A.E.; et al. Nanofluidic proteomic assay for serial analysis of oncoprotein activation in clinical specimens. Nat. Med. 2009, 15, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Padhan, N.; Nordling, T.E.; Sundstrom, M.; Akerud, P.; Birgisson, H.; Nygren, P.; Nelander, S.; Claesson-Welsh, L. High sensitivity isoelectric focusing to establish a signaling biomarker for the diagnosis of human colorectal cancer. BMC Cancer 2016, 16, 683. [Google Scholar] [CrossRef] [PubMed]
- Tikhanovich, I.; Kuravi, S.; Campbell, R.V.; Kharbanda, K.K.; Artigues, A.; Villar, M.T.; Weinman, S.A. Regulation of FOXO3 by phosphorylation and methylation in hepatitis C virus infection and alcohol exposure. Hepatology 2014, 59, 58–70. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.A.; Bhamidipati, A.; Bi, X.; Deb-Basu, D.; Cahill, L.; Ferrante, J.; Gentalen, E.; Glazer, M.; Gossett, J.; Hacker, K.; et al. Isoelectric focusing technology quantifies protein signaling in 25 cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16153–16158. [Google Scholar] [CrossRef]
- Aspinall-O’Dea, M.; Pierce, A.; Pellicano, F.; Williamson, A.J.; Scott, M.T.; Walker, M.J.; Holyoake, T.L.; Whetton, A.D. Antibody-based detection of protein phosphorylation status to track the efficacy of novel therapies using nanogram protein quantities from stem cells and cell lines. Nat. Prot. 2015, 10, 149–168. [Google Scholar] [CrossRef]
- Johlfs, M.G.; Gorjala, P.; Urasaki, Y.; Le, T.T.; Fiscus, R.R. Capillary isoelectric focusing immunoassay for fat cell differentiation proteomics. PLoS ONE 2015, 10, e0132105. [Google Scholar] [CrossRef]
- Urasaki, Y.; Pizzorno, G.; Le, T.T. Chronic uridine administration induces fatty liver and pre-diabetic conditions in mice. PLoS ONE 2016, 11, e0146994. [Google Scholar] [CrossRef]
- Crino, P.B. The mTOR signalling cascade: Paving new roads to cure neurological disease. Nat. Rev. Neurol. 2016, 12, 379–392. [Google Scholar] [CrossRef]
- Lipton, J.O.; Sahin, M. The neurology of mTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. Biomed. Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017, 142, 624–648. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Intl. J. Mol. Sci. 2018, 19. [Google Scholar]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: Observational data meta-analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality traits of “cannabidiol oils”: Cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules 2018, 23. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. A review of methods for the chemical characterization of cannabis natural products. J. Sep. Sci. 2018, 41, 398–415. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G. Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol. 1997, 82, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.S.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Zheng, C.J.; Han, L.Y.; Xie, B.; Jia, J.; Cao, Z.W.; Li, Y.X.; Chen, Y.Z. Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov. Today 2009, 14, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. Botanical drugs, synergy, and network pharmacology: Forth and back to intelligent mixtures. Planta. Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef]
- Balmes, J.R. Vaping-induced acute lung injury: An epidemic that could have been prevented. Am. J. Respir. Crit. Care Med. 2019. [Google Scholar] [CrossRef]
- Christiani, D.C. Vaping-induced lung injury. N. Engl. J. Medi. 2019. [Google Scholar] [CrossRef]
- Henry, T.S.; Kligerman, S.J.; Raptis, C.A.; Mann, H.; Sechrist, J.W.; Kanne, J.P. Imaging findings of vaping-associated lung injury. AJR Am. J. Roentgenol. 2019. [Google Scholar] [CrossRef]
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - preliminary report. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef]
- Maddock, S.D.; Cirulis, M.M.; Callahan, S.J.; Keenan, L.M.; Pirozzi, C.S.; Raman, S.M.; Aberegg, S.K. Pulmonary lipid-laden macrophages and vaping. N. Engl. J. Med. 2019, 381, 1488–1489. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mehrad, M.; Dammert, P.; Arrossi, A.V.; Sarda, R.; Brenner, D.S.; Maldonado, F.; Choi, H.; Ghobrial, M. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping). Am. J. Clin. Pathol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Padhan, N.; Yan, J.; Boge, A.; Scrivener, E.; Birgisson, H.; Zieba, A.; Gullberg, M.; Kamali-Moghaddam, M.; Claesson-Welsh, L.; Landegren, U. Highly sensitive and specific protein detection via combined capillary isoelectric focusing and proximity ligation. Sci. Rep. 2017, 7, 1490. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Wakefield, L.M.; Goldstein, D.J. Capillary nano-immunoassays: Advancing quantitative proteomics analysis, biomarker assessment, and molecular diagnostics. J. Transl. Med. 2015, 13, 182. [Google Scholar] [CrossRef] [PubMed]
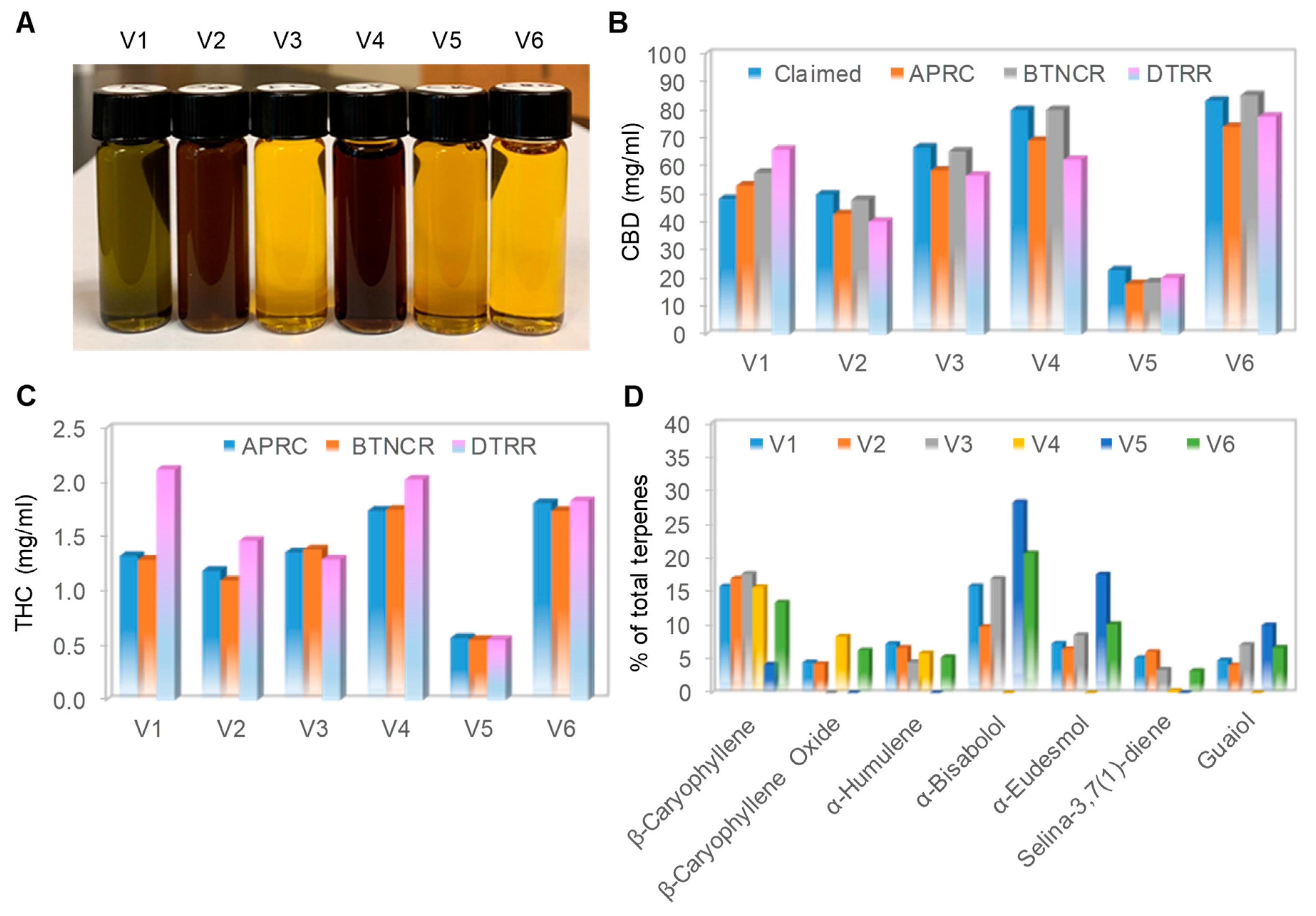






| CBD Oil | Extraction Method | Dilution | Addition of Isolated CBD | Measured Density (mg/mL) | Claimed CBD (mg/mL) | Measured CBD (mg/mL) | Measured THC (mg/mL) | Measured Terpenes (mg/mL) |
|---|---|---|---|---|---|---|---|---|
| V1 | CO2 | No | No | 921.10 | 48.33 | 53.16–65.86 | 1.29–2.11 | 2.27 |
| V2 | CO2 | HSO | Yes | 915.50 | 50.00 | 40.28–48.05 | 1.10–1.46 | 1.49 |
| V3 | CO2 | MCT | Yes | 922.80 | 66.67 | 56.66–65.24 | 1.29–1.38 | 1.04 |
| V4 | ETHANOL | HSO | Yes | 921.10 | 80.00 | 62.36–80.03 | 1.74–2.03 | 1.56 |
| V5 | ETHANOL | OO | Yes | 922.70 | 23.00 | 18.09–20.21 | 0.55–0.57 | 0.17 |
| V6 | ETHANOL | MCT | Yes | 915.10 | 83.33 | 74.02–85.29 | 1.74–1.83 | 1.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urasaki, Y.; Beaumont, C.; Workman, M.; Talbot, J.N.; Hill, D.K.; Le, T.T. Potency Assessment of CBD Oils by Their Effects on Cell Signaling Pathways. Nutrients 2020, 12, 357. https://doi.org/10.3390/nu12020357
Urasaki Y, Beaumont C, Workman M, Talbot JN, Hill DK, Le TT. Potency Assessment of CBD Oils by Their Effects on Cell Signaling Pathways. Nutrients. 2020; 12(2):357. https://doi.org/10.3390/nu12020357
Chicago/Turabian StyleUrasaki, Yasuyo, Cody Beaumont, Michelle Workman, Jeffery N. Talbot, David K. Hill, and Thuc T. Le. 2020. "Potency Assessment of CBD Oils by Their Effects on Cell Signaling Pathways" Nutrients 12, no. 2: 357. https://doi.org/10.3390/nu12020357
APA StyleUrasaki, Y., Beaumont, C., Workman, M., Talbot, J. N., Hill, D. K., & Le, T. T. (2020). Potency Assessment of CBD Oils by Their Effects on Cell Signaling Pathways. Nutrients, 12(2), 357. https://doi.org/10.3390/nu12020357





