Gestational Diabetes Triggers Oxidative Stress in Hippocampus and Cerebral Cortex and Cognitive Behavior Modifications in Rat Offspring: Age- and Sex-Dependent Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Behavioral Test
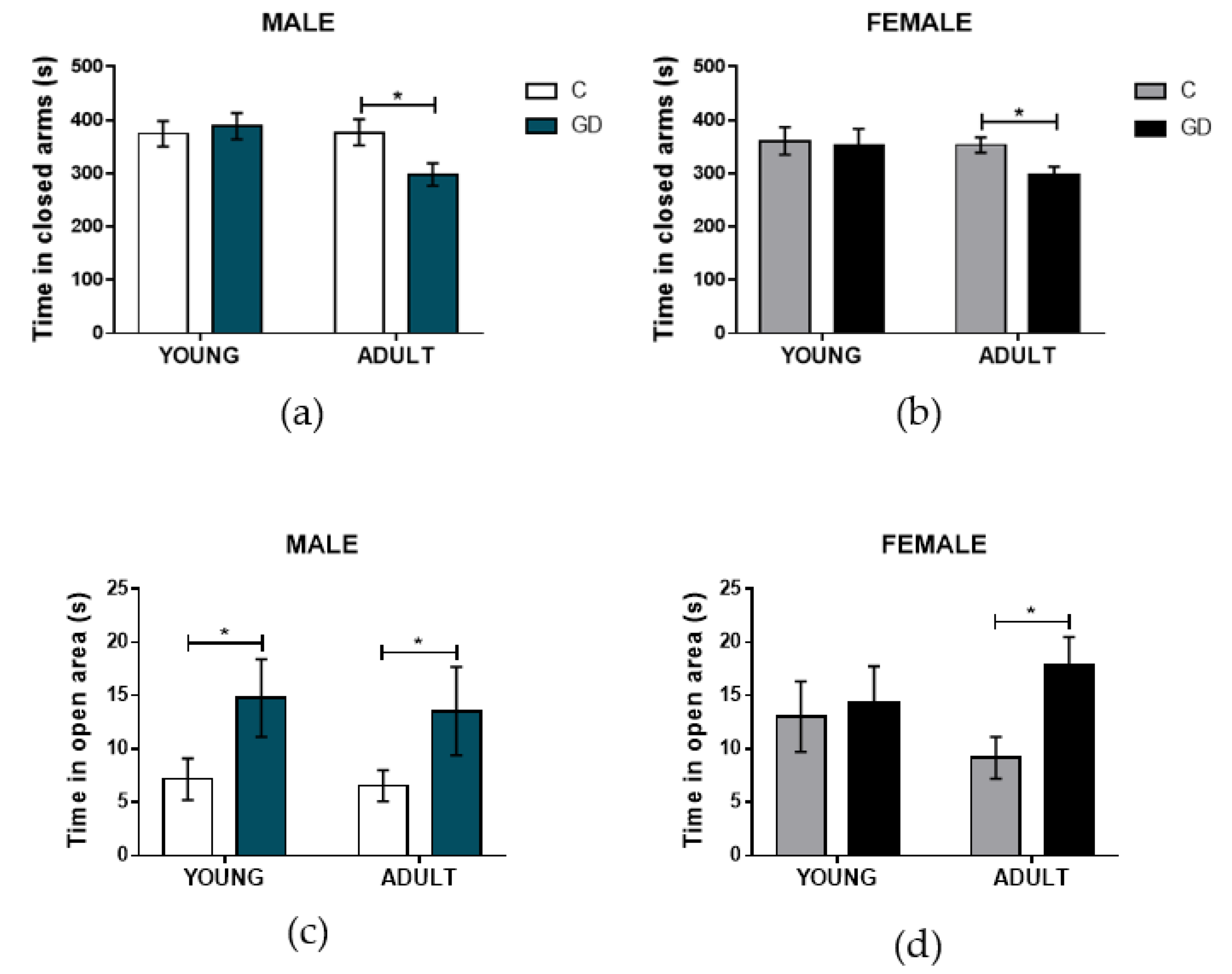
2.2.1. Elevated Plus Maze
2.2.2. Open Field Test
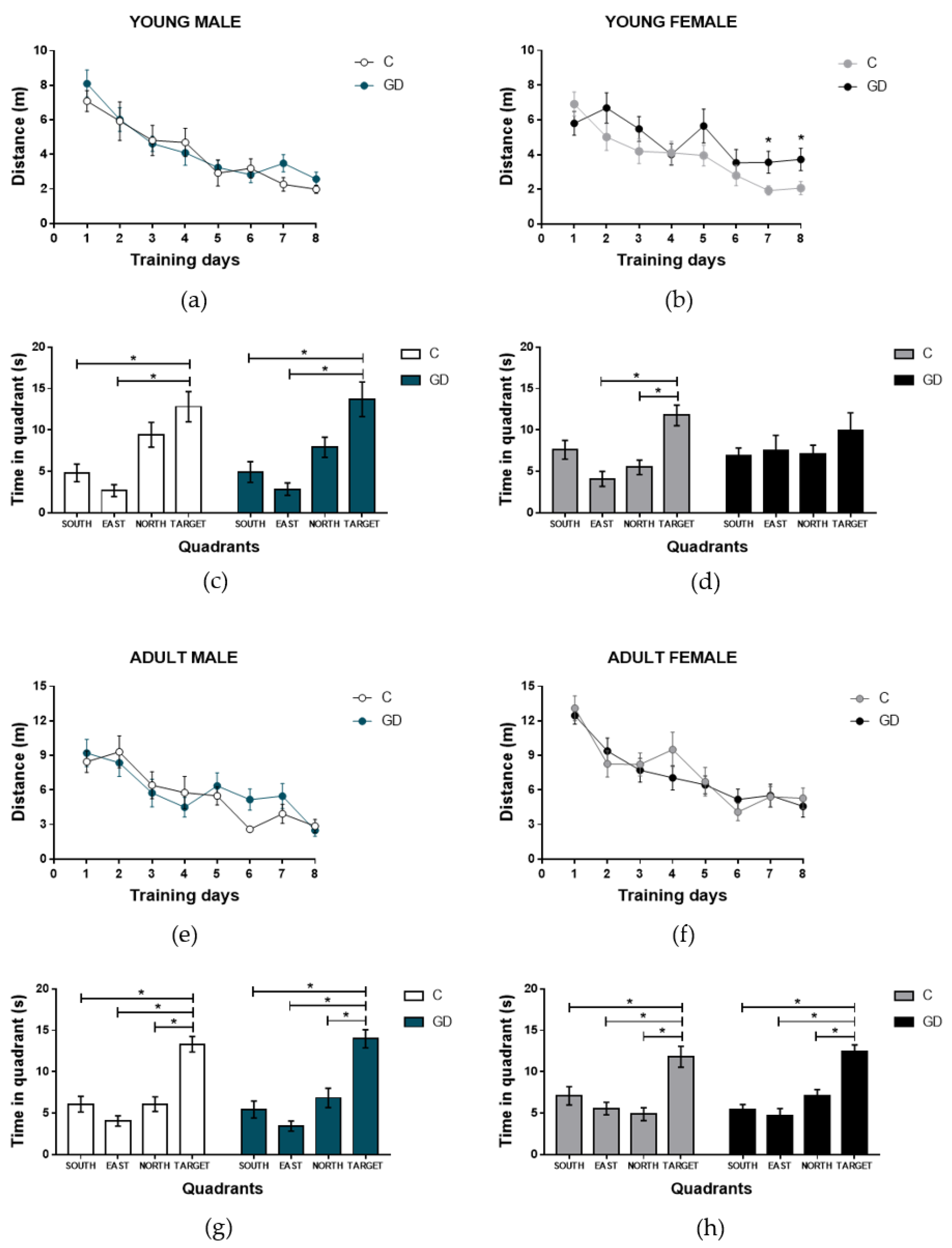
2.2.3. Morris Water Maze
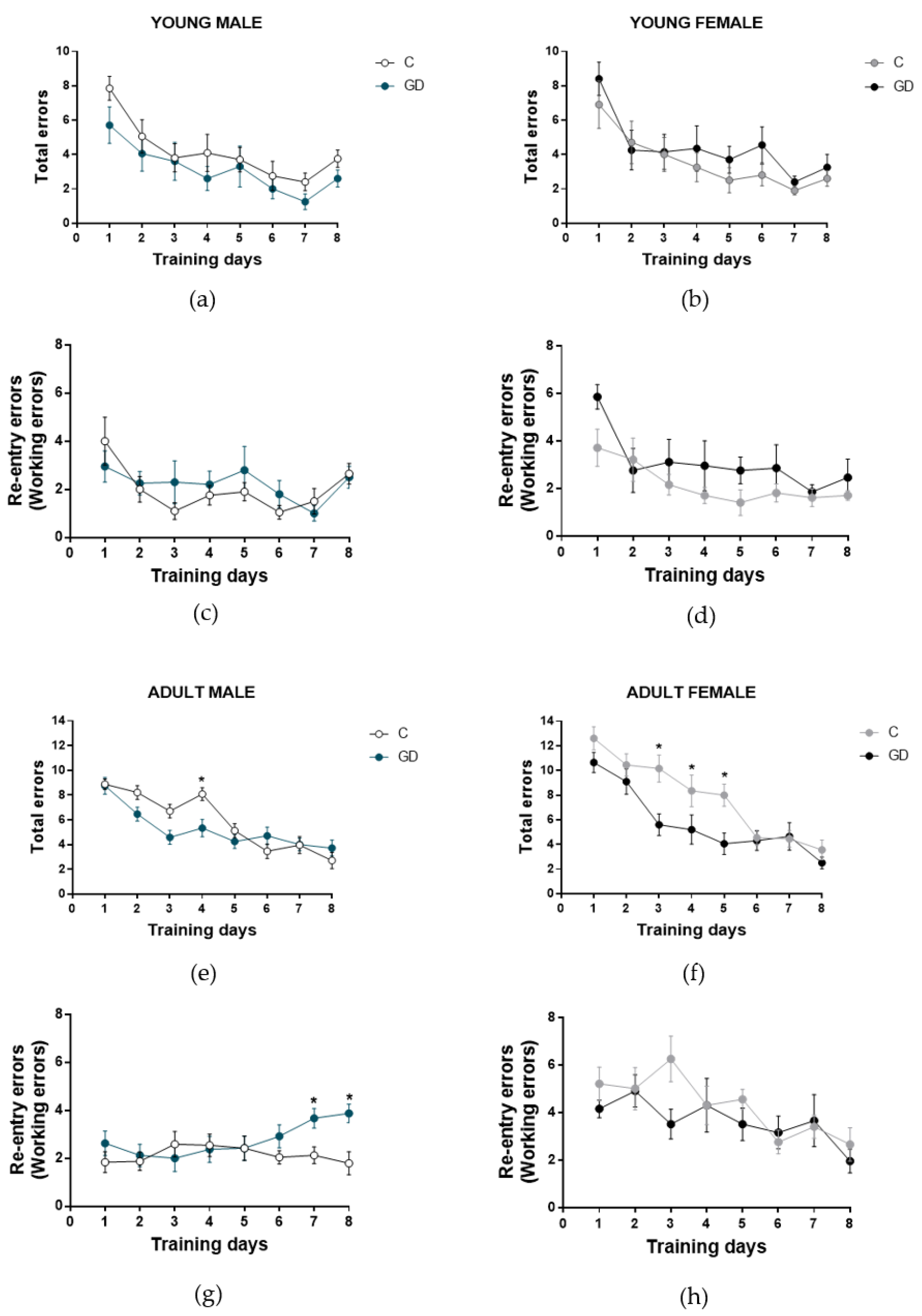
2.2.4. Radial Maze
2.3. Tissue Preparation
2.4. Oxidative Stress Biomarkers
2.4.1. Determination of Lipid Peroxidation
2.4.2. Determination of ROS
2.4.3. Determination of Glutathione Status
2.4.4. Determination of Catalase and SOD activities
2.5. Biochemical Serum Analyses
2.6. Statistical Analysis
3. Results
3.1. Effects of STZ on Pregnant Female Rats (F0)
3.2. Effects of GD on the Body Weight of First-Generation Offspring
3.3. Effects of GD on the Anxiety-Like Behavior of First-Generation Offspring
3.4. Effects of GD on the Spatial Learning of First-Generation Offspring
3.5. Effects of GD on the Working Memory of First-Generation Offspring
3.6. Effects of GD on Oxidative Stress Biomarkers and ROS Production in Hippocampus and Cortex of First-Generation Offspring
3.7. Effects of GD on the Antioxidant Enzymes Activities in First-Generation Offspring
3.8. Effects of GD on the Glutathione Status of First-Generation Offspring
3.9. Effects of GD on the Biochemical Serum Parameters of First-Generation Offspring
3.10. Correlation Between Behavior Parameters and Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Reproductive Health Library. WHO recommendation on the diagnosis of gestational diabetes in pregnancy. (December 2016). The WHO Reproductive Health Library, World Health Organization: Geneva, Switzerland. Available online: https://extranet.who.int/rhl/topics/preconception-pregnancy-childbirth-and-postpartum-care/antenatal-care/who-recommendation-diagnosis-gestational-diabetes-pregnancy-0 (accessed on 21 May 2019).
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; pp. 1–150. Available online: http://www.diabetesatlas.org (accessed on 21 May 2019).
- Aerts, L.; Van Assche, F.A. Animal evidence for the transgenerational development of diabetes mellitus. Int. J. Biochem. Cell Biol. 2006, 38, 894–903. [Google Scholar] [CrossRef]
- Seki, Y.; Williams, L.; Vuguin, P.M.; Charron, M.J. Minireview: Epigenetic programming of diabetes and obesity: Animal models. Endocrinology 2012, 153, 1031–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.W.; Mahmood, S.; Srinivasan, M.; Smiraglia, D.J.; Patel, M.S. Developmental programming in skeletal muscle in response to over nourishment in the immediate postnatal life in rats. J. Nutr. Biochem. 2013, 24, 1859–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.E. Obesity and gestational diabetes mellitus pathways for programming in mouse, monkey, and man—where do we go next? The 2014 Norbert Freinkel Award Lecture. Diabetes Care. 2015, 38, 1402–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrıguez, R.R.; Renauld, A.; Celener, D.; Pérez, R.L.; Susemihl, M.C. Offspring of streptozotocin diabetic rats: Size changes in Langerhans islets with time after birth. Diabetes Res. Clin. Prac. 1998, 41, 95–100. [Google Scholar] [CrossRef]
- Catalano, P.M.; Thomas, A.; Huston-Presley, L.; Amini, S.B. Increased fetal adiposity: A very sensitive marker of abnormal in utero development. Am. J. Obstet. Gynecol. 2003, 189, 1698–1704. [Google Scholar] [CrossRef]
- Blondeau, B.; Joly, B.; Perret, C.; Prince, S.; Bruneval, P.; Lelièvre-Pégorier, M.; Van Huyen, J.P.D. Exposure in utero to maternal diabetes leads to glucose intolerance and high blood pressure with no major effects on lipid metabolism. Diabetes Metab. 2011, 37, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Dalley, J.W.; Cardinal, R.N.; Robbins, T.W. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004, 28, 771–784. [Google Scholar] [CrossRef]
- DeBoer, T.; Wewerka, S.; Bauer, P.J.; Georgieff, M.K.; Nelson, C.A. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev. Med. Child Neurol. 2005, 47, 525–531. [Google Scholar] [CrossRef]
- Cai, S.; Qiu, A.; Broekman, B.F.; Wong, E.Q.; Gluckman, P.D.; Godfrey, K.M.; Saw, M.S.; Soh, S.E.; Kwek, K.; Chong, K.; et al. The influence of gestational diabetes on neurodevelopment of children in the first two years of life: A prospective study. PLoS ONE 2016, 11, e0162113. [Google Scholar] [CrossRef]
- Ornoy, A.; Ratzon, N.; Greenbaum, C.; Wolf, A.; Dulitzky, M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J. Pediatric Endocrinol. Metab. 2001, 14, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.H.; Song, Y.F.; Yao, Y.M.; Yin, J.; Wang, D.G.; Gao, L.P. Retardation of fetal dendritic development induced by gestational hyperglycemia is associated with brain insulin/IGF-I signals. Int. J. Dev. Neurosci. 2014, 37, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-like growth factor 1: At the crossroads of brain development and aging. Front Cell Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golalipour, M.J.; Kafshgiri, S.K.; Ghafari, S. Gestational diabetes induced neuronal loss in CA1 and CA3 subfields of rat hippocampus in early postnatal life. Folia Morphol. 2012, 71, 71–77. [Google Scholar]
- Raza, H.; John, A. Glutathione metabolism and oxidative stress in neonatal rat tissues from streptozotocin-induced diabetic mothers. Diabetes Metab. Res. Rev. 2004, 20, 72–78. [Google Scholar] [CrossRef]
- Kamel, M.A.; Helmy, M.H.; Hanafi, M.Y.; Mahmoud, S.A.; Elfetooh, H.A. Effect of maternal diabetes on pre-and post-natal redox status of F1 rat offspring. Open J. Endocr. Metab. Dis. 2014, 4, 111. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Rego, A.C.; Oliveira, C.R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: Implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003, 28, 1563–1574. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, E.O.; Beauquis, J.; Revsin, Y.; Banzan, A.M.; Roig, P.; De Nicola, A.F.; Saravia, F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain. Res. 2009, 198, 224–230. [Google Scholar] [CrossRef]
- Suge, R.; Shimazu, T.; Hasegawa, H.; Inoue, I.; Hayashibe, H.; Nagasaka, H.; Watanabe, S.I. Cerebral antioxidant enzyme increase associated with learning deficit in type 2 diabetes rats. Brain Res. 2012, 1481, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Solleiro, D.; Roldán-Roldán, G.; Díaz, D.; Velasco, M.; Larqué, C.; Rico-Rosillo, G.; Vega-Robledo, G.; Zambrano, E.; Hiriart, M.; Pérez de la Mora, M. Increased anxiety-like behavior is associated with the metabolic syndrome in non-stressed rats. PLoS ONE 2017, 12, e0176554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemdegs, J.; Quesseveur, G.; Jarriault, D.; Pénicaud, L.; Fioramonti, X.; Guiard, B.P. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. British J. Pharmacol. 2016, 173, 2095–2110. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Ortiz-Avila, O.; Sámano-García, C.A.; Calderón-Cortés, E.; Pérez-Hernández, I.H.; Mejía-Zepeda, R.; Rodríguez-Orozco, A.R.; Saavedra-Molina, A.; Cortés-Rojo, C. Dietary avocado oil supplementation attenuates the alterations induced by type I diabetes and oxidative stress in electron transfer at the complex II-complex III segment of the electron transport chain in rat kidney mitochondria. J. Bioenerg. Biomemb. 2013, 45, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, T.P.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar]
- Jeulin, C.; Soufir, J.C.; Weber, P.; Laval-Martin, D.; Calvayrac, R. Catalase activity in human spermatozoa and seminal plasma. Gamete. Res. 1989, 24, 185–196. [Google Scholar] [CrossRef]
- Smith, N.H.; Ozanne, S.E. Intrauterine origins of metabolic disease. Rev. Gynecol. Peri. Pract. 2006, 6, 211–217. [Google Scholar] [CrossRef]
- Vickers, M. Early life nutrition, epigenetics and programming of later life disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef]
- Han, J.; Xu, J.; Long, Y.S.; Epstein, P.N.; Liu, Y.Q. Rat maternal diabetes impairs pancreatic β-cell function in the offspring. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E228–E236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, L.; Holemans, K.; Van Assche, F.A. Maternal diabetes during pregnancy: Consequences for the offspring. Diabetes Metab. Rev. 1990, 6, 147–167. [Google Scholar] [CrossRef] [PubMed]
- López-Soldado, I.; Herrera, E. Different diabetogenic response to moderate doses of streptozotocin in pregnant rats, and its long-term consequences in the offspring. J. Diabetes Res. 2003, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fetita, L.S.; Sobngwi, E.; Serradas, P.; Calvo, F.; Gautier, J.F. Consequences of fetal exposure to maternal diabetes in offspring. J. Clin. Endocrinol. Metab. 2006, 91, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.V.; Segabinazi, E.; de Meireles, A.L.F.; Mega, F.; de Figueiredo Spindler, C.; Augustin, O.A.; Salvalaggio, G.D.S.; Achaval, M.; Kruse, M.S.; Coirini, H.; et al. Severe Uncontrolled Maternal Hyperglycemia Induces Microsomia and Neurodevelopment Delay Accompanied by Apoptosis, Cellular Survival, and Neuroinflammatory Deregulation in Rat Offspring Hippocampus. Cell. Mol. Neurobiol. 2019, 39, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.E.; Lacroix, M.C.; Jouin, M.; Voisin, S.; Badonnel, K.; Lemaire, M.; Durieux, D. Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice. Nutrients 2019, 11, 948. [Google Scholar] [CrossRef] [Green Version]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Piccolo, B.D.; Shankar, K. Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci. Rep. 2018, 8, 16502. [Google Scholar] [CrossRef]
- Ramanathan, M.; Jaiswal, A.K.; Bhattacharya, S.K. Hyperglycaemia in pregnancy: Effects on the offspring behavior with special reference to anxiety paradigms. Indian J. Exp. Biol. 2000, 38, 231–236. [Google Scholar]
- Chandna, A.R.; Kuhlmann, N.; Bryce, C.A.; Greba, Q.; Campanucci, V.A.; Howland, J.G. Chronic maternal hyperglycemia induced during mid-pregnancy in rats increases RAGE expression, augments hippocampal excitability, and alters behavior of the offspring. Neuroscience 2015, 303, 241–260. [Google Scholar] [CrossRef]
- Kinney, B.A.; Rabe, M.B.; Jensen, R.A.; Steger, R.W. Maternal hyperglycemia leads to gender-dependent deficits in learning and memory in offspring. Exp. Biol. Med. 2003, 228, 152–159. [Google Scholar] [CrossRef]
- McEwen, B.S.; Alves, S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999, 20, 279–307. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.N. Estradiol and cognitive function: Past, present and future. Horm. Behav. 2014, 66, 602–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, G.; Peleg-Raibstein, D. Maternal overnutrition induces long-term cognitive deficits across several generations. Nutrients 2019, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Sung, Y.H.; Lee, H.H.; Ko, I.G.; Kim, S.E.; Shin, M.S.; Kim, B.K. Postnatal treadmill exercise alleviates short-term memory impairment by enhancing cell proliferation and suppressing apoptosis in the hippocampus of rat pups born to diabetic rats. J. Exerc. Rehabil. 2014, 10, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, D.; Yu, J.; Wu, Y.; Fu, N.; Villela, N.A.; Yang, P. Maternal diabetes triggers DNA damage and DNA damage response in neurulation stage embryos through oxidative stress. Biochem. Biophys. Res. Commun. 2015, 467, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, A.; Serviddio, G.; Calcagnini, S.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Linking lipid peroxidation and neuropsychiatric disorders: Focus on 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2017, 111, 281–293. [Google Scholar] [CrossRef]
- Keller, J.N.; Mark, R.J.; Bruce, A.J.; Blanc, E.; Rothstein, J.D.; Uchida, K.; Waeg, G.; Mattson, P. 4–Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience 1997, 80, 685–696. [Google Scholar] [CrossRef]
- Oswald, M.C.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS lett. 2018, 592, 679–691. [Google Scholar] [CrossRef]
- Poston, L. Intergenerational transmission of insulin resistance and type 2 diabetes. Prog. Biophys. Mol. Biol. 2011, 106, 315–322. [Google Scholar] [CrossRef]
- Pinney, S.E.; Simmons, R.A. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr. Diabetes Rep. 2012, 12, 67–74. [Google Scholar] [CrossRef]
| GC | GD | ||
|---|---|---|---|
| Fasting blood glucose (mg/dL) | Pre-induction (day 9) | 88.5 ± 4.1 | 93.17 ± 4.9 |
| Post-induction (day 11) | 77.0 ± 2.0 | 246.8 ± 48.7 * | |
| Postprandial blood glucose (mg/dL) | Day 12 | 76.0 ± 2.0 | 423.0 ± 25.8 * |
| Day 16 | 87.0 ± 6.8 | 398.8 ± 46.5 * | |
| Day 20 | 88.3 ± 5.2 | 296.8 ± 49.8 * | |
| Body weight (g) | Day 12 | 333.3 ± 13.3 | 338.0 ± 18.0 |
| Day 16 | 381.7 ± 2.8 | 378.0 ± 6.9 | |
| Day 20 | 420.0 ± 8.1 | 404.0 ± 13.8 |
| Body Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age | Male Offspring | n | Female Offspring | n | ||||
| C | GD | C | GD | C | GD | C | GD | |
| Birth | 6.82 ± 0.16 | 5.81 ± 0.14 *● | 28 | 34 | 6.35 ± 0.24 | 5.99 ± 0.22 | 20 | 30 |
| Weaning (21 days) | 37.3 ± 0.93 | 24.86 ± 1.06 *● | 28 | 34 | 36.41 ± 1.36 | 26.71 ± 1.07 *● | 20 | 30 |
| Young (2 months) | 295.2 ± 10.85 | 261.5 ± 14.26 *● | 10 | 10 | 204.0 ± 3.84 | 196.0 ± 4.11 | 10 | 10 |
| Adult (6 months) | 514.4 ± 14.59 | 436.8 ± 20.8 *● | 12 | 12 | 281.4 ± 16.14 | 282.5 ± 5.60 | 10 | 10 |
| Male Offspring | Female Offspring | |||||||
| Cerebral Cortex | C-Young n = 6 | GD-Young n = 6 | C-Adult n = 6 | GD-Adult n = 6 | C-Young n = 5 | GD-Young n = 5 | C-Adult n = 5 | GD-Adult n = 5 |
| GSHt (µmoles/mg protein) | 0.39 ± 0.04 | 0.22 ± 0.04 *● | 0.36 ± 0.04 | 0.42 ± 0.01 | 0.38 ± 0.1 | 0.32 ± 0.02 | 0.69 ± 0.03 | 0.49 ± 0.04 *● |
| GSSG (µmoles/mg protein) | 0.01 ± 0.001 | 0.13 ± 0.02 *● | 0.10 ± 0.01 | 0.15 ± 0.016 | 0.17 ± 0.02 | 0.18 ± 0.03 | 0.08 ± 0.01 | 0.26 ±0.04 *● |
| GSH (µmoles/mg protein) | 0.37 ± 0.04 | 0.11 ± 0.03 *● | 0.25 ± 0.05 | 0.27 ± 0.005 | 0.21 ± 0.02 | 0.13 ± 0.04 | 0.61 ± 0.03 | 0.23 ± 0.07 *● |
| GSH/GSSG ratio | 14.02 ± 2.16 | 1.14 ± 0.33 *● | 2.66 ± 0.68 | 1.75 ± 0.18 | 1.36 ± 0.35 | 0.84 ± 0.37 | 8.44 ± 1.73 | 0.97 ± 0.38 *● |
| ROS (arbitrary units/mg protein) | 27.39 ± 2.56 | 36.1 ± 2.53 *● | 21.85 ± 2.15 | 29.74 ± 1.98 *● | 28.52 ± 0.97 | 34.81 ± 2.23 *● | 28.27 ± 3.01 | 41.19 ± 3.33 *● |
| Lipid peroxidation (nmoles TBARS/mg protein) | 330.0 ± 18.21 | 412.9 ± 29.1 *● | 241.1 ± 15.82 | 329.0 ± 33.18 *● | 328.2 ± 10.14 | 433.7 ± 18.68 *● | 309.7 ± 27.23 | 382.3 ± 17.91 *● |
| Catalase activity (U/mg protein) | 1.58 ± 0.17 | 0.87 ± 0.06 * | 1.24 ± 0.08 | 0.79 ± 0.11 * | 2.00 ± 0.17 | 1.03 ± 0.12 *● | 1.88 ± 0.19 | 1.05 ± 0.100 *● |
| SOD activity (U/mg protein) | 2.96 ± 0.15 | 2.97 ± 0.017 | 1.59 ± 0.166 | 1.90 ± 0.13 | 12.76 ± 0.62 | 8.18 ± 0.44 *● | 7.95 ± 0.38 | 2.57 ± 0.085 *● |
| Hippocampus | C-Young n = 6 | GD-Young n = 6 | C-Adult n = 6 | GD-Adult n = 6 | C-Young n = 5 | GD-Young n = 5 | C-Adult n = 5 | GD-Adult n = 5 |
| GSHt (µmoles/mg protein) | 0.21 ± 0.02 | 0.41 ± 0.02 *● | 0.28 ± 0.03 | 0.38 ± 0.06 | 0.42 ± 0.00 | 0.18 ± 0.03 | 0.68 ± 0.0 | 0.47 ± 0.02 |
| GSSG (µmoles/mg protein) | 0.07 ± 0.004 | 0.13 ± 0.03 | 0.15 ± 0.02 | 0.17 ± 0.02 | 0.053 ± 0.00 | 0.06± 0.00 | 0.081 ± 0.0 | 0.05 ± 0.0 |
| GSH (µmoles/mg protein) | 0.14 ± 0.03 | 0.28 ± 0.02 | 0.13 ± 0.01 | 0.21 ± 0.04 | 0.36 ± 0.01 | 0.12 ± 0.04 *● | 0.60 ± 0.01 | 0.41 ± 0.01 *● |
| GSH/GSSG ratio | 2.10 ± 0.55 | 2.33 ± 0.67 | 0.92 ± 0.24 | 1.22 ± 0.33 | 7.01 ± 0.8 | 2.05 ± 0.96 *● | 8.17 ± 1.84 | 8.60 ± 1.6 |
| ROS (arbitrary units/mg protein) | 17.98 ± 1.24 | 29.9 ± 2.61 *● | 17.08 ± 1.74 | 24.03 ± 0.84 *● | 17.97 ± 2.18 | 24.46 ± 1.03 * | 17.8 ± 2.90 | 25.44 ± 1.96 *● |
| Lipid peroxidation (nmoles TBARS/mg protein) | 348.6 ± 18.15 | 403.0 ± 11.85 *● | 197.7 ± 15.97 | 320.7 ± 33.01 *● | 276.5 ± 18.8 | 393.6 ± 35.93 *● | 288.2 ± 17.75 | 420.3 ± 45.54 *● |
| Catalase activity (U/mg protein) | 1.15 ± 0.17 | 0.74 ± 0.08 | 1.41 ± 0.09 | 0.80 ± 0.04 *● | 1.45 ± 0.16 | 0.81 ± 0.14 *● | 1.33 ± 0.09 | 0.66 ± 0.09 *● |
| SOD activity (U/mg protein) | 7.02 ± 0.23 | 6.95 ± 0.20 | 5.43 ± 0.21 | 4.87 ± 0.02 | 12.76 ± 0.62 | 8.12 ± 0.44 *● | 7.72 ± 0.56 | 3.27 ± 0.04 *● |
| Male Offspring | ||||
| C-Young n = 8 | GD-Young n = 8 | C-Adult n = 7 | GD-Adult n = 7 | |
| Glucose (mg/dL) | 90.27 ± 2.07 | 85.4 ± 3.03 | 91.92 ± 1.63 | 91.35 ± 3.31 |
| Insulin (ng/mL) | 0.42 ± 0.027 | 0.33 ± 0.015 * | 0.44 ± 0.023 | 0.42 ± 0.029 |
| Cholesterol (mg/dL) | 71.84 ± 1.92 | 75.89 ± 2.71 | 73.43 ± 2.18 | 68.2 ± 1.98 |
| Triglycerides (mg/dL) | 33.3 ± 2.84 | 43.46 ± 3.31 * | 40.24 ± 2.55 | 53.94 ± 5.26 * |
| HDL-cholesterol (mg/dL) | 52.72 ± 1.95 | 40.1 ± 3.05 * | 50.63 ± 3.04 | 38.1 ± 1.43 * |
| Female Offspring | ||||
| C-Young n = 7 | GD-Young n = 10 | C-Adult n = 9 | GD-Adult n = 7 | |
| Glucose (mg/dL) | 82.22 ± 3.28 | 82.69 ± 2.49 | 86.44 ± 2.23 | 87.63 ± 2.01 |
| Insulin (ng/mL) | 0.32 ± 0.002 | 0.36 ± 0.014 * | 0.32 ± 0.004 | 0.48 ± 0.064 * |
| Cholesterol (mg/dL) | 85.63 ± 1.61 | 85.44 ± 1.72 | 74.29 ± 2.28 | 80.61 ± 4.8 |
| Triglycerides (mg/dL) | 37.96 ± 1.87 | 43.04 ± 2.46 | 37.74 ± 6.46 | 54.25 ± 2.89 * |
| HDL-cholesterol (mg/dL) | 62.46 ± 1.60 | 49.06 ± 2.18 * | 51.38 ± 3.04 | 34.73 ± 2.24 * |
| Male Offspring | Female offspring | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young | Adult | Young | Adult | |||||||||
| Cerebral cortex | ROS | SOD | CAT | ROS | SOD | CAT | ROS | SOD | CAT | ROS | SOD | CAT |
| Time in closed arms (Elevated plus maze) | - | r = 0.709 P = 0.009 | - | r = −0.660 P = 0.019 | - | - | - | - | - | - | r = 0.614 P = 0.033 | - |
| Time in open area (Open field) | - | - | - | - | - | - | - | - | - | - | r = −0.651 P = 0.021 | r = −0.793 P = 0.002 |
| Distance traveled (Morris water maze) | r = 0.746 P = 0.005 | - | - | - | - | - | - | r = −0.587 P = 0.048 | - | - | - | |
| Hippocampus | ROS | SOD | CAT | ROS | SOD | CAT | ROS | SOD | CAT | ROS | SOD | CAT |
| Time in closed arms (Elevated plus maze) | - | r = 0.815 P = 0.001 | - | - | - | - | - | - | - | - | - | r = 0.741 P = 0.005 |
| Time in open area (Open field) | r = 0.601 P = 0.038 | - | - | - | - | - | - | - | - | - | - | - |
| Distance traveled (Morris water maze) | - | - | - | - | - | - | -- | r = −0.577 P = 0.049 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta-Cervantes, M.; Peña-Montes, D.J.; Montoya-Pérez, R.; Trujillo, X.; Huerta, M.; López-Vázquez, M.Á.; Olvera-Cortés, M.E.; Saavedra-Molina, A. Gestational Diabetes Triggers Oxidative Stress in Hippocampus and Cerebral Cortex and Cognitive Behavior Modifications in Rat Offspring: Age- and Sex-Dependent Effects. Nutrients 2020, 12, 376. https://doi.org/10.3390/nu12020376
Huerta-Cervantes M, Peña-Montes DJ, Montoya-Pérez R, Trujillo X, Huerta M, López-Vázquez MÁ, Olvera-Cortés ME, Saavedra-Molina A. Gestational Diabetes Triggers Oxidative Stress in Hippocampus and Cerebral Cortex and Cognitive Behavior Modifications in Rat Offspring: Age- and Sex-Dependent Effects. Nutrients. 2020; 12(2):376. https://doi.org/10.3390/nu12020376
Chicago/Turabian StyleHuerta-Cervantes, Maribel, Donovan J. Peña-Montes, Rocío Montoya-Pérez, Xóchitl Trujillo, Miguel Huerta, Miguel Ángel López-Vázquez, María Esther Olvera-Cortés, and Alfredo Saavedra-Molina. 2020. "Gestational Diabetes Triggers Oxidative Stress in Hippocampus and Cerebral Cortex and Cognitive Behavior Modifications in Rat Offspring: Age- and Sex-Dependent Effects" Nutrients 12, no. 2: 376. https://doi.org/10.3390/nu12020376
APA StyleHuerta-Cervantes, M., Peña-Montes, D. J., Montoya-Pérez, R., Trujillo, X., Huerta, M., López-Vázquez, M. Á., Olvera-Cortés, M. E., & Saavedra-Molina, A. (2020). Gestational Diabetes Triggers Oxidative Stress in Hippocampus and Cerebral Cortex and Cognitive Behavior Modifications in Rat Offspring: Age- and Sex-Dependent Effects. Nutrients, 12(2), 376. https://doi.org/10.3390/nu12020376






