Figure 1.
Schematic representation of the study protocol and groups of animals as well as of the different therapeutic approaches.
Figure 1.
Schematic representation of the study protocol and groups of animals as well as of the different therapeutic approaches.
Figure 2.
Representative examples of the gastrocnemius muscle in animals of the all study groups of mice. Myofibers were stained in green, type I in the top panel and type II in the bottom panel. Hybrid fibers (arrows) are seen in both panels. Definition of abbreviations: MyHC myosin heavy chain; I, immobilization; R, recovery.
Figure 2.
Representative examples of the gastrocnemius muscle in animals of the all study groups of mice. Myofibers were stained in green, type I in the top panel and type II in the bottom panel. Hybrid fibers (arrows) are seen in both panels. Definition of abbreviations: MyHC myosin heavy chain; I, immobilization; R, recovery.
Figure 3.
Representative examples of positively-stained nuclei (arrowheads) and negatively-stained nuclei (arrows) for the TUNEL assay in the gastrocnemius muscles of all the study groups of mice. Definition of abbreviations: I, immobilization; R, recovery.
Figure 3.
Representative examples of positively-stained nuclei (arrowheads) and negatively-stained nuclei (arrows) for the TUNEL assay in the gastrocnemius muscles of all the study groups of mice. Definition of abbreviations: I, immobilization; R, recovery.
Figure 4.
(A) Representative immunoblots of sirtuin-1 protein in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of sirtuin-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of sirtuin-1 activity levels in the gastrocnemius muscle of the different study groups of mice, as measured by fluorescence in arbitrary units (a.u.). Definition of abbreviations: a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of the variable tyrosine release (nmol/mg/2 h) of the gastrocnemius muscle of the different study groups of mice. Definition of abbreviations: nmol, nanomol; mg, milligram; h, hour; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of the variable plasma troponin-I (ng/ml) of the gastrocnemius muscle of the different study groups of mice. Definition of abbreviations: ng, nanogram; ml, milliliter; h, hour; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
Figure 4.
(A) Representative immunoblots of sirtuin-1 protein in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of sirtuin-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of sirtuin-1 activity levels in the gastrocnemius muscle of the different study groups of mice, as measured by fluorescence in arbitrary units (a.u.). Definition of abbreviations: a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of the variable tyrosine release (nmol/mg/2 h) of the gastrocnemius muscle of the different study groups of mice. Definition of abbreviations: nmol, nanomol; mg, milligram; h, hour; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of the variable plasma troponin-I (ng/ml) of the gastrocnemius muscle of the different study groups of mice. Definition of abbreviations: ng, nanogram; ml, milliliter; h, hour; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
![Nutrients 12 00388 g004]()
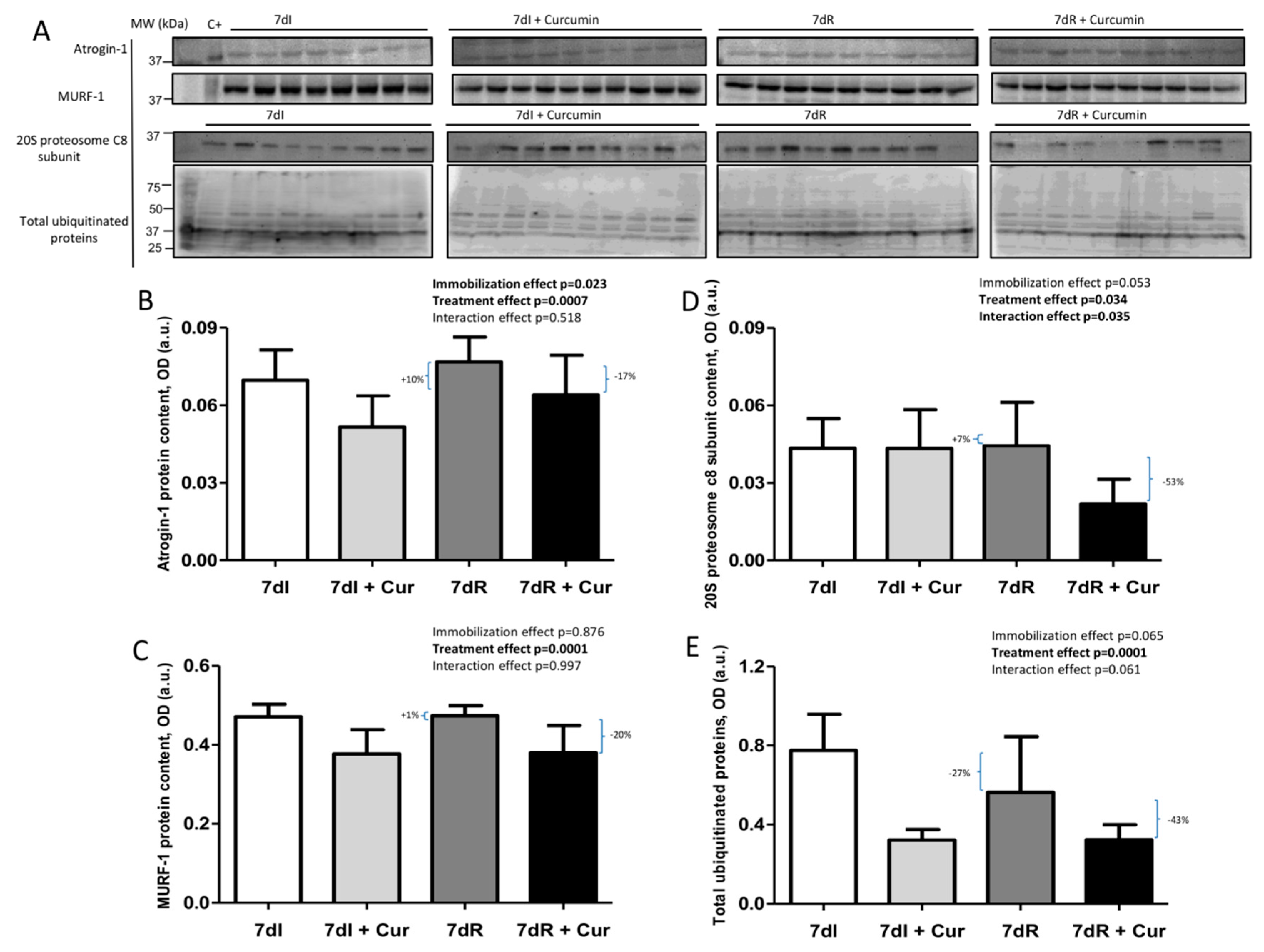
Figure 5.
(A) Representative immunoblots of atrogin-1, MuRF-1, 20S proteasome alpha subunit C8 and total ubiquitinated proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: MuRF-1, muscle RING-finger protein-1; C+, positive control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of atrogin-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of MuRF-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: MuRF-1, muscle ring finger protein 1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of 20S proteasome alpha subunit C8 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of total ubiquitinated proteins content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
Figure 5.
(A) Representative immunoblots of atrogin-1, MuRF-1, 20S proteasome alpha subunit C8 and total ubiquitinated proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: MuRF-1, muscle RING-finger protein-1; C+, positive control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of atrogin-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of MuRF-1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: MuRF-1, muscle ring finger protein 1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of 20S proteasome alpha subunit C8 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of total ubiquitinated proteins content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
![Nutrients 12 00388 g005]()
Figure 6.
(A) Representative immunoblots of MyHC, actin, puromycin-labeled proteins, Akt and phosphorylated Akt proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: MyHC, myosin heavy chain; Akt, Serine/Threonine Kinase 1, p-, phosphorylated; C-, negative control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of MyHC protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: MyHC, myosin heavy chain; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of actin protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of the puromycin-labeled proteins levels in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of the Akt proteins levels in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Akt, Serine/Threonine Kinase 1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (F) Mean values and standard deviation of phosphorylated Akt proteins levels in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: p, phosphorylated; Akt, Serine/Threonine Kinase 1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
Figure 6.
(A) Representative immunoblots of MyHC, actin, puromycin-labeled proteins, Akt and phosphorylated Akt proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: MyHC, myosin heavy chain; Akt, Serine/Threonine Kinase 1, p-, phosphorylated; C-, negative control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of MyHC protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: MyHC, myosin heavy chain; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of actin protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of the puromycin-labeled proteins levels in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of the Akt proteins levels in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Akt, Serine/Threonine Kinase 1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (F) Mean values and standard deviation of phosphorylated Akt proteins levels in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: p, phosphorylated; Akt, Serine/Threonine Kinase 1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
![Nutrients 12 00388 g006a]()
![Nutrients 12 00388 g006b]()
Figure 7.
(A) Representative immunoblots of PGC-1α, acetylated PGC-1α, NF-κB p50 and acetylated NF-κB p50 proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; NF-κB p50, nuclear factor kappa-light-chain-enhancer of activated B cells p50; ac-, acetylated; C+, positive control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of PGC-1α protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of acetylated PGC-1α protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Ac, acetylated; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of NF-κB p50 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: NF-κB p50, nuclear factor kappa-light-chain-enhancer of activated B cells p50; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of acetylated NF-κB p50 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Ac, acetylated; NF-κB p50, nuclear factor kappa-light-chain-enhancer of activated B cells p50; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
Figure 7.
(A) Representative immunoblots of PGC-1α, acetylated PGC-1α, NF-κB p50 and acetylated NF-κB p50 proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; NF-κB p50, nuclear factor kappa-light-chain-enhancer of activated B cells p50; ac-, acetylated; C+, positive control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of PGC-1α protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of acetylated PGC-1α protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Ac, acetylated; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of NF-κB p50 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: NF-κB p50, nuclear factor kappa-light-chain-enhancer of activated B cells p50; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of acetylated NF-κB p50 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Ac, acetylated; NF-κB p50, nuclear factor kappa-light-chain-enhancer of activated B cells p50; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
![Nutrients 12 00388 g007]()
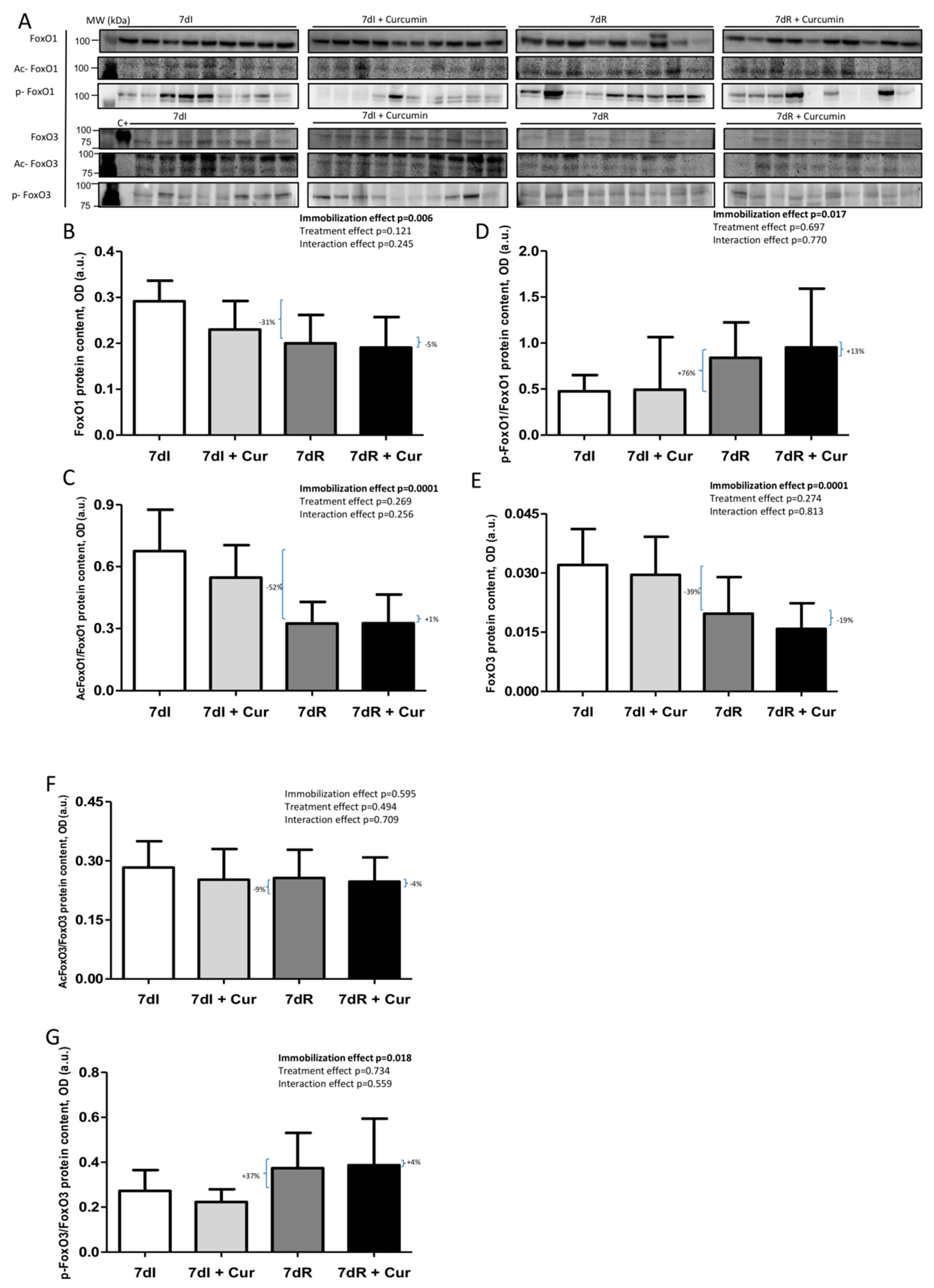
Figure 8.
(A) Representative immunoblots of FoxO1, acetylated FoxO1, phosphorylated FoxO1, FoxO3, acetylated FoxO3 and phosphorylated FoxO3 proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: FoxO1, transcription factor fork-head box O1; FoxO3, transcription factor fork-head box O3; ac-, acetylated; p-, phosphorylated; C+, positive control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of FoxO1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: FoxO1, transcription factor fork-head box O1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of acetylated FoxO1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Ac, acetylated; FoxO1, transcription factor fork-head box O1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of phosphorylated FoxO1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: p, phosphorylated; FoxO1, transcription factor fork-head box O1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of FoxO3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: FoxO3, transcription factor fork-head box O3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (F) Mean values and standard deviation of acetylated FoxO3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: FoxO3, transcription factor fork-head box O3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (G) Mean values and standard deviation of phosphorylated FoxO3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: p, phosphorylated; FoxO3, transcription factor fork-head box O3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
Figure 8.
(A) Representative immunoblots of FoxO1, acetylated FoxO1, phosphorylated FoxO1, FoxO3, acetylated FoxO3 and phosphorylated FoxO3 proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: FoxO1, transcription factor fork-head box O1; FoxO3, transcription factor fork-head box O3; ac-, acetylated; p-, phosphorylated; C+, positive control; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of FoxO1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: FoxO1, transcription factor fork-head box O1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of acetylated FoxO1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: Ac, acetylated; FoxO1, transcription factor fork-head box O1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of phosphorylated FoxO1 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: p, phosphorylated; FoxO1, transcription factor fork-head box O1; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (E) Mean values and standard deviation of FoxO3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: FoxO3, transcription factor fork-head box O3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (F) Mean values and standard deviation of acetylated FoxO3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: FoxO3, transcription factor fork-head box O3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (G) Mean values and standard deviation of phosphorylated FoxO3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: p, phosphorylated; FoxO3, transcription factor fork-head box O3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
![Nutrients 12 00388 g008]()
Figure 9.
(A) Representative immunoblots of HDAC3, HDAC4 and HDAC6 proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: HDAC3, histone deacetylase 3; HDAC4, histone deacetylase 3; HDAC6, histone deacetylase 6; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of HDAC3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: HDAC3, Histone deacetylase 3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of HDAC4 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: HDAC4, Histone deacetylase 4; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of HDAC6 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: HDAC6, Histone deacetylase 6; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
Figure 9.
(A) Representative immunoblots of HDAC3, HDAC4 and HDAC6 proteins in the gastrocnemius muscle of all study groups of mice. Definition of abbreviations: HDAC3, histone deacetylase 3; HDAC4, histone deacetylase 3; HDAC6, histone deacetylase 6; MW, molecular weight; kDa, kilodalton; I, immobilization; R, recovery. (B) Mean values and standard deviation of HDAC3 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: HDAC3, Histone deacetylase 3; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (C) Mean values and standard deviation of HDAC4 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: HDAC4, Histone deacetylase 4; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable. (D) Mean values and standard deviation of HDAC6 protein content in the gastrocnemius muscle of the different study groups of mice, as measured by optical densities in arbitrary units (OD, a.u.). Definition of abbreviations: HDAC6, Histone deacetylase 6; OD, optical densities; a.u., arbitrary units; I, immobilization; R, recovery; Cur, curcumin. The statistical analyses (two-way ANOVA test) of the effect of immobilization, treatment and interaction effects, are also indicated as actual P values for each variable. The percentage of change between: 1) 7dR and 7dI and 2) 7dR + Curcumin and 7dR are also indicated for each variable.
![Nutrients 12 00388 g009]()
Table 1.
Physiological parameters in all experimental groups of mice.
Table 1.
Physiological parameters in all experimental groups of mice.
| Physiological Parameters | 7dI (N = 10) | 7dI + Curcumin (N = 10) | 7dR (N = 10) | 7dR + Curcumin (N = 10) | Immobilization Effect p-value | Treatment Effect p-value | Interaction Effect p-value |
|---|
| Food intake (g/24 h) | 3.18 (0.24) | 3.37 (0.09) | 3.32 (0.23), +5% | 3.34 (0.12), 1% | 0.199 | 0.09 | 0.08 |
| Total body weight gain (%) | −5.94 (2.92) | −5.00 (3.24) | +0.99 (2.86), +119% | +3.37 (4.16), +223% | 0.0001 | 0.135 | 0.471 |
| Gastrocnemius weight (g) | 0.092 (0.010) | 0.095 (0.014) | 0.098 (0.010), +7% | 0.100 (0.009), +2% | 0.195 | 0.847 | 0.250 |
| Limb strength gain (%) | −10.85 (12.57) | −2.04 (16.26) | +10.92 (11.47), +200% | +14.10 (10.28), +29% | 0.001 | 0.253 | 0.588 |
Table 2.
Structural characteristics of the gastrocnemius muscle in the study groups.
Table 2.
Structural characteristics of the gastrocnemius muscle in the study groups.
| Structural Characteristics | 7dI (N = 10) | 7dI + Curcumin (N = 10) | 7dR (N = 10) | 7dR + Curcumin (N = 10) | Immobilization Effect (p-value) | Treatment Effect (p-value) | Interaction Effect (p-value) |
|---|
| Muscle fiber type, % | | | | | | | |
| Type I fibers | 16.88 (2.70) | 18.45 (5.50) | 16.38 (6.78), −3% | 15.12 (4.36), −8% | 0.190 | 0.722 | 0.904 |
| Type II fibers | 83.12 (2.70) | 81.55 (5.50) | 83.62 (6.78), +1% | 84.88 (4.36), +2% | 0.310 | 0.963 | 0.455 |
| Muscle fiber size (CSA) | | | | | | | |
| Cross-sectional area, type I fibers | 872.28 (241.46) | 843.59 (133.10) | 911.63 (187.08), +5% | 996.27 (274.75), +9% | 0.223 | 0.717 | 0.467 |
| Cross-sectional area, type II fibers | 922.02 (212.89) | 948.48 (115.00) | 1079.92 (119.03), +17% | 1120.61 (146.98), +4% | 0.0134 | 0.591 | 0.908 |
| Muscle hybrid fiber, % | 3.55 (1.61) | 2.60 (0.67) | 3.23 (3.22), −9% | 1.82 (1.27), −43% | 0.461 | 0.589 | 0.683 |
| Cross-sectional area, hybrid fibers | 881.97 (270.88) | 543.23 (180.00) | 715.92 (187.11), −19% | 948.60 (328.35), +33% | 0.292 | 0.635 | 0.0191 |
| Number of apoptotic nuclei (TUNEL) % | 68.90 (5.58) | 51.69 (5.34) | 47.42 (3.36), −31% | 43.68 (3.09), −9% | 0.0001 | 0.0001 | 0.0004 |