The Elapsed Time between Dinner and the Midpoint of Sleep Is Associated with Adiposity in Young Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Parameters
2.3. Sleep and Circadian Related Variables
- I.
- Sleep duration (in hours) was calculated for each day as the difference between bedtime and wakeup timing. A total weekly sleep duration was calculated as follows: [(5 × sleep duration on weekdays) + (2 × sleep duration on weekends)]/7 [19].
- II.
- III.
- Sleep timing behavior was categorized using the median splits of the time in which each volunteer went to bed and woke up during the week [7]. First, bedtime was classified as follows: “Early-bedtime” (<23:48 h) and “Late-bedtime” (≥23:48 h). Second, for each bedtime group, we used median splits of wakeup timing. Early-bedtime subjects were divided into “Early-rise” (wakeup time <7:12 h) and “Late-rise” (wakeup time ≥ 7:12 h). Subsequently, “Late-bedtime” subjects were divided into “Early-rise” (wakeup time <7:52 h) and “Late-rise” (wakeup time ≥ 7:52 h). Accordingly, four sleep timing behavior categories were defined: early-bedtime/early-rise (EE), early-bedtime/late-rise (EL), late-bedtime/early-rise (LE), and late-bedtime/late-rise (LL).
- IV.
- Sleep quality was measured using the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) [20]. Scores range from 0 to 21, where the higher the score, the worse the sleep quality.
- V.
2.4. Meal Timing
2.5. Time Elapsed between Dinner and the Midpoint of Sleep TDM
2.6. Dietary Intake
2.7. Physical Activity
2.8. Sample Size Calculation
2.9. Statistical Analyses
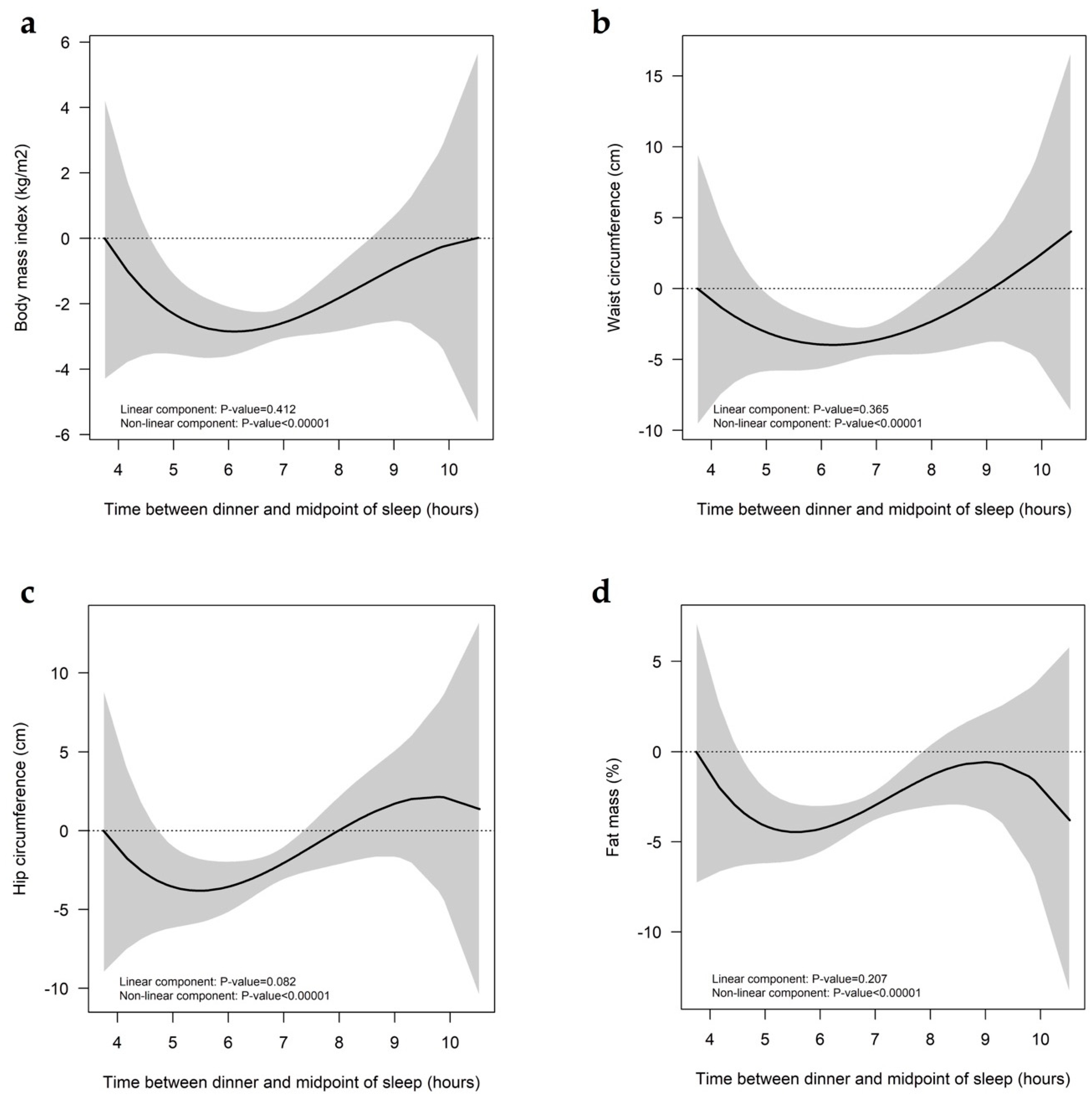
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Panda, S. Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol. Metab. 2016, 27, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Mchill, A.W.; Phillips, A.J.K.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag—A (self-) critical review. Biology 2019, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Zerón-Rugerio, M.F.; Cambras, T.; Izquierdo-Pulido, M. Social Jet Lag Associates Negatively with the Adherence to the Mediterranean Diet and Body Mass Index among Young Adults. Nutrients 2019, 11, 1756. [Google Scholar] [CrossRef]
- Quick, V.; Byrd-bredbenner, C.; White, A.A.; Brown, O.; Colby, S. Eat, sleep, work, play: Associations of weight status and health-related behaviors among young adult college students. Am. J. Health Promot. 2014, 29, 64–73. [Google Scholar] [CrossRef]
- Olds, T.S.; Maher, C.A.; Matricciani, L. Sleep Duration or Bedtime? Exploring the Relationship between Sleep Habits and Weight Status and Activity Patterns. Sleep 2011, 34, 1299–1307. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.J.L.; Jacques, P.F.; Lamon-Fava, S.; Ordovas, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef]
- Golley, R.K.; Maher, C.A.; Matricciani, L.; Olds, T.S. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int. J. Obes. 2013, 37, 546–551. [Google Scholar] [CrossRef]
- Ferranti, R.; Marventano, S.; Castellano, S.; Giogianni, G.; Nolfo, F.; Rametta, S.; Matalone, M.; Mistretta, A. Sleep quality and duration is related with diet and obesity in young adolescent living in Sicily, Southern Italy. Sleep Sci. 2016, 9, 117–122. [Google Scholar] [CrossRef]
- Xiao, Q.; Garaulet, M.; Scheer, F.A.J.L. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Imano, H.; Muraki, I.; Yamada, K.; Iso, H. The Association of Having a Late Dinner or Bedtime Snack and Skipping Breakfast with Overweight in Japanese Women. J. Obes. 2019, 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Suwa, K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J. Diabetes Metab. Disord. 2015, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 2018, 37, 1133–1140. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef]
- Terman, J.S.; Terman, M.; Lo, E.S.; Cooper, T.B. Circadian time of morning light administration and therapeutic response in winter depression. Arch. Gen. Psychiatry 2001, 58, 69–75. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.; Heymsfield, S.B. Pathophisiology and management of obesity. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Waist Circumference and Waist–Hip Ratio. Report of a WHO Expert Consultation; WHO Document Production Services: Geneva, Switzerland, 2008; ISBN 978-92-4-150149-1. [Google Scholar]
- Garaulet, M.; Sánchez-Moreno, C.; Smith, C.E.; Lee, Y.C.; Nicolás, F.; Ordovás, J.M. Ghrelin, sleep reduction and evening preference: Relationships to clock 3111 T/C SNP and weight loss. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Royuela, A.; Macías, J. Propiedades clinimétricas de la versión castellana del cuestionario de Pittsburg. Vigilia-Sueño 1997, 9, 81–94. [Google Scholar]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Perez, A.; Palacios, B.; Castro, A.; Flores, I. Sistema Mexicano de Alimentos Equivalentes, 4th ed.; Fomento de Nutricion y Salud: Ciudad de México, Mexico, 2014; ISBN 9786070079283. [Google Scholar]
- Muñoz, M.; Chavez, A. Tablas de Uso Práctico de los Alimentos de Mayor Consumo, 3rd ed.; McGraw Hill: Contadero, Mexico, 2014; ISBN 9786071509697. [Google Scholar]
- Ramos, E.; Ramírez, E.; Salas, R.; Núñez, G.; Villarreal, J. Calidad del patron de consumo alimentario del noreste de Mexico. Respyn 2016, 15, 8–15. [Google Scholar]
- Román-Viñas, B.; Ribas-Barba, L.; Ngoa, J.; Serra-Majem, L. Validación en población catalana del cuestionario internacional de actividad física. Gac. Sanit. 2013, 27, 254–257. [Google Scholar] [CrossRef] [PubMed]
- R Core Team and Contributors World Wide GLM. Fitting Generalized Linear Models. Available online: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/glm.html (accessed on 31 July 2019).
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Wu, D.; Patel, S.R.; Loredo, J.S.; Redline, S.; Cai, J.; Gallo, L.C.; Mossavar-Rahmani, Y.; Ramos, A.R.; Teng, Y.; et al. Association Between Sleep Timing, Obesity, Diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Cohort Study. Sleep 2017, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J.; Kim, T.; Van Horn, L.; Attarian, H.; Wolfe, L.; Siddique, J.; Santostasi, G.; Zee, P.C. Circadian timing and alignment in healthy adults: Associations with BMI, body fat, caloric intake and physical activity. Int. J. Obes. 2017, 41, 203–209. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 1–25. [Google Scholar] [CrossRef]
- Morris, C.J.; Garcia, J.I.; Myers, S.; Yang, J.N.; Trienekens, N.; Scheer, F.A.J.L. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity 2015, 23, 2053–2058. [Google Scholar] [CrossRef]
- Zitting, K.M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human Resting Energy Expenditure Varies with Circadian Phase. Curr. Biol. 2018, 28, 3685–3690. [Google Scholar] [CrossRef]
- Wilms, B.; Chamorro, R.; Hallschmid, M.; Trost, D.; Forck, N.; Schultes, B.; Mölle, M.; Sayk, F.; Lehnert, H.; Schmid, S.M. Timing modulates the effect of sleep loss on glucose homeostasis. J. Clin. Endocrinol. Metab. 2019, 104, 2801–2808. [Google Scholar] [CrossRef]
- Eckel, R.H.; Depner, C.M.; Perreault, L.; Markwald, R.R.; Smith, M.R.; McHill, A.; Higgins, J.; Melanson, E.L.; Wright, K.P.J. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr. Biol. 2015, 25, 3004–3010. [Google Scholar] [CrossRef]
- Mossavar-Rahmani, Y.; Weng, J.; Wang, R.; Shaw, P.A.; Jung, M.; Sotres-Alvarez, D.; Castañeda, S.F.; Gallo, L.C.; Gellman, M.D.; Qi, Q.; et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueño ancillary study. J. Sleep Res. 2017, 26, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Schnepp, J.; Tucker, R. Increased Hunger, Food Cravings, Food Reward, and Portion Size Selection after Sleep Curtailment in Women Without Obesity. Nutrients 2019, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P. Sleep-obesity relation: Underlying mechanisms and consequences for treatment. Obes. Rev. 2017, 18, 34–39. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2018. [Google Scholar]
| EE | LE | EL | LL | p-Value a | p-Trend b | |
|---|---|---|---|---|---|---|
| n | 34 | 33 | 33 | 33 | ||
| Sleep Parameters | ||||||
| Wakeup time, hh:mm | 06:32 (00:56) bc | 07:00 (00:52) de | 07:49 (00:33) bf | 08:39 (00:51) cef | <0.001 | <0.001 |
| Bedtime, hh:mm | 23:00 (00:37) ac | 00:30 (00:31) ade | 23:18 (00:31) df | 01:12 (00:45) cef | <0.001 | <0.001 |
| Midpoint of sleep, hh:mm | 02:49 (00:25) abc | 03:44 (00:27) ae | 03:52 (00:19) bf | 04:56 (00:30) cef | <0.001 | <0.001 |
| Sleep duration, h | 6.1 (0.9) bc | 5.7 (1.1) de | 7.2 (0.7) bd | 6.8 (0.9) ce | <0.001 | <0.001 |
| Sleep quality | 6.6 (2.5) | 6.4 (2.8) | 6.1 (2.9) | 5.8 (3.0) | 0.068 | 0.229 |
| Social jet lag, h | 1.1 (0.8) | 1.1 (0.9) | 1.1 (1.0) | 1.3 (0.8) | 0.350 | 0.372 |
| Meal Timing | ||||||
| Breakfast, hh:mm | 08:34 (01:13) c | 08:23 (01:08) e | 08:58 (00:57) f | 9:46 (00:54) cef | <0.001 | <0.001 |
| Lunch, hh:mm | 15:30 (00:57) | 15:36 (01:01) | 15:18 (00:58) | 15:06 (00:52) | 0.159 | 0.060 |
| Dinner, hh:mm | 21:06 (00:49) | 21:18 (00:58) | 20:54 (00:51) | 21:18 (00:53) | 0.286 | 0.286 |
| TDM, h | 5.8 (0.9) abc | 6.6 (1.2) ae | 6.9 (0.9) bf | 7.6 (1.0) cef | <0.001 | 0.011 |
| Anthropometric Parameters | ||||||
| BMI, kg/m2 | 25.4 (4.0) a | 23.8 (4.5) | 23.0 (3.0) | 22.5 (3.8) a | 0.021 | 0.002 |
| Fat mass, % | 32.2 (7.4) | 31.5 (7.8) | 30.5 (5.3) | 29.5 (6.4) | 0.387 | 0.082 |
| Waist, cm | 78.6 (8.8) | 76.2 (9.7) | 74.9 (8.4) | 72.8 (7.4) | 0.057 | 0.006 |
| Hip, cm | 99.5 (7.7) | 97.3 (10.7) | 96.3 (6.8) | 95.2 (7.3) | 0.194 | 0.033 |
| Dietary Intake | ||||||
| Diet quality | 57.9 (6.8) ab | 60.7 (8.1) c | 64.0 (9.8) b | 67.3 (9.4) ac | <0.001 | <0.001 |
| Total energy intake, kcal/day | 1517 (404) | 1596 (425) | 1555 (412) | 1676 (420) | 0.452 | 0.179 |
| Breakfast,% of kcal | 24.8 (10.4) | 26.9 (10.4) | 26.5 (6.9) | 22.8 (8.3) | 0.258 | 0.381 |
| Lunch, % of kcal | 31.3 (7.5) | 29.5 (10.2) | 33.7 (10.5) | 30.9 (9.6) | 0.364 | 0.722 |
| Dinner, % of kcal | 18.0 (10.4) | 18.6 (9.8) | 20.7 (9.1) | 23.5 (11.3) | 0.123 | 0.020 |
| Physical Activity, METs | 1050 [1006; 2654] | 1036 [462; 2026] | 1040 [546; 2038] | 1029 [447; 1893] | 0.602 | 0.457 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerón-Rugerio, M.F.; Longo-Silva, G.; Hernáez, Á.; Ortega-Regules, A.E.; Cambras, T.; Izquierdo-Pulido, M. The Elapsed Time between Dinner and the Midpoint of Sleep Is Associated with Adiposity in Young Women. Nutrients 2020, 12, 410. https://doi.org/10.3390/nu12020410
Zerón-Rugerio MF, Longo-Silva G, Hernáez Á, Ortega-Regules AE, Cambras T, Izquierdo-Pulido M. The Elapsed Time between Dinner and the Midpoint of Sleep Is Associated with Adiposity in Young Women. Nutrients. 2020; 12(2):410. https://doi.org/10.3390/nu12020410
Chicago/Turabian StyleZerón-Rugerio, María Fernanda, Giovana Longo-Silva, Álvaro Hernáez, Ana Eugenia Ortega-Regules, Trinitat Cambras, and Maria Izquierdo-Pulido. 2020. "The Elapsed Time between Dinner and the Midpoint of Sleep Is Associated with Adiposity in Young Women" Nutrients 12, no. 2: 410. https://doi.org/10.3390/nu12020410
APA StyleZerón-Rugerio, M. F., Longo-Silva, G., Hernáez, Á., Ortega-Regules, A. E., Cambras, T., & Izquierdo-Pulido, M. (2020). The Elapsed Time between Dinner and the Midpoint of Sleep Is Associated with Adiposity in Young Women. Nutrients, 12(2), 410. https://doi.org/10.3390/nu12020410








