Resting Energy Expenditure Relationship with Macronutrients and Gestational Weight Gain: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
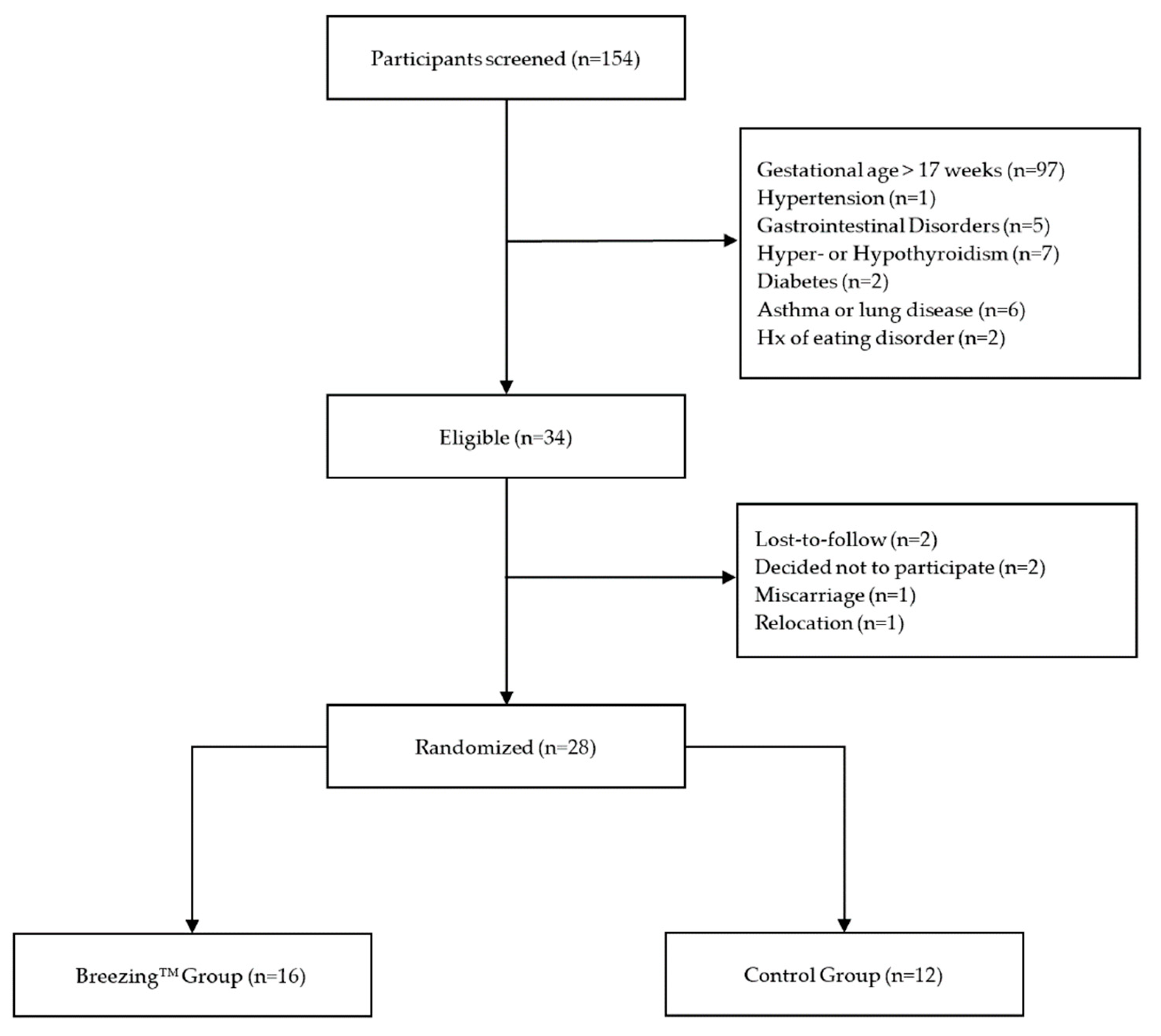
2.1. Study Participants and Procedures
2.2. Instruments
2.2.1. Demographic and Health Data
2.2.2. Anthropometric Data
2.2.3. Resting Energy Expenditure (REE)
2.2.4. Dietary Data
2.2.5. Statistical Analyses
3. Results
3.1. Demographic and Baseline Data
3.2. Intervention Effect on GWG
3.3. REE
3.4. Association between REE and Rate of GWG
3.5. Macronutrient Composition
3.6. Association between REE and Macronutrient Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Center for Disease Control and Prevention. Table 58 Normal Weight, Overweight, and Obesity among Adults Aged 20 and Over, by Selected Characteristics: United States, Selected Years 1988–1994 through 2013–2016. Available online: https://www.cdc.gov/nchs/hus/contents2017.htm#058. (accessed on 1 December 2019).
- The American College of Obstetricians and Gynecologists ACOG Committee Opinion. Weight Gain During Pregnancy. Obstet. Gynecol. 2013, 121, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (IOM); National Research Council (NRC). Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; The National Academies Press: Washington, DC, USA, 2009.
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Deputy, N.; Sharma, A.J.; Kim, S.Y.; Hinkle, S.N. Prevalence and Characteristics Associated with Gestational Weight Gain Adequacy. Obs. Gynecol 2015, 125, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Shariff, Z.M.; Tabatabaei, S.Z.; Fathollahi, M.S.; Mun, C.Y.; Nazari, M. Relationship between sociodemographics, dietary intake, and physical activity with gestational weight gain among pregnant women in Rafsanjan city, Iran. J. Heal. Popul. Nutr. 2015, 33, 168–176. [Google Scholar]
- Meng, Y.; Groth, S.W.; Stewart, P.; Smith, J.A. An Exploration of the Determinants of Gestational Weight Gain in African American Women: Genetic Factors and Energy Expenditure. Biol. Res. Nurs. 2018, 20, 118–125. [Google Scholar] [CrossRef]
- Chihara, H.; Otsubo, Y.; Araki, T. Resting Energy Expenditure in Pregnant Japanese Women. J. Nippon Med. Sch. 2002, 69, 373–375. [Google Scholar] [CrossRef] [Green Version]
- Melzer, K.; Schutz, Y.; Boulvain, M.; Kayser, B. Pregnancy-related changes in activity energy expenditure and resting metabolic rate in Switzerland. Eur. J. Clin. Nutr. 2009, 63, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Hronek, M.; Doubkova, P.; Hrnciarikova, D.; Zadak, Z. Dietary intake of energy and nutrients in relation to resting energy expenditure and anthropometric parameters of Czech pregnant women. Eur. J. Nutr. 2013, 52, 117–125. [Google Scholar] [CrossRef]
- Berggren, E.K.; O’Tierney-Ginn, P.; Lewis, S.; Presley, L.; De-Mouzon, S.H.; Catalano, P.M. Variations in resting energy expenditure: Impact on gestational weight gain. Am. J. Obstet. Gynecol. 2017, 217, 445.e1–445.e6. [Google Scholar] [CrossRef]
- Shcherbina, A.; Mattsson, C.M.; Waggott, D.; Salisbury, H.; Christle, J.W.; Hastie, T.; Wheeler, M.T.; Ashley, E.A. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J. Pers. Med. 2017, 7, 3. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Whitehead, M.; Sheats, J.Q.; Mastromonico, J.; Hardy, D.; Smith, S.A. Smartphone Applications for Promoting Healthy Diet and Nutrition: A Literature Review. Jacobs J. Food Nutr. 2015, 2, 21–34. [Google Scholar]
- Overdijkink, S.B.; Velu, A.V.; Rosman, A.N.; van Beukering, M.D.; Kok, M.; Steegers-Theunissen, R.P. The Usability and Effectiveness of Mobile Health Technology–Based Lifestyle and Medical Intervention Apps Supporting Health Care During Pregnancy: Systematic Review. JMIR mHealth uHealth 2018, 6, e109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, N.; Groves, A.M.; McIntyre, H.D.; Callaway, L.; Group BAMBINO. Changes in resting and walking energy expenditure and walking speed during pregnancy in obese women. Am. J. Clin. Nutr. 2011, 94, 819–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willommet, L.; Schutz, Y.; Whitehead, R.; Jequier, E.; Fern, E.B. Whole body protein metabolism and resting energy expenditure in pregnant Gambian women. Am. Physiol. Soc. 1992, 263, E624–E631. [Google Scholar] [CrossRef]
- Catalano, P.M.; Roman-Drago, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am. J. Obstet. Gynecol. 1998, 179, 156–165. [Google Scholar] [CrossRef]
- Xian, X.; Quach, A.; Bridgeman, D.; Forzani, E.; Tao, N. Personalized Indirect Calorimeterfor Energy Expenditure (EE) Measurement. Glob. J. Obes. Diabetes Metab. Syndr. 2015, 2, 4–8. [Google Scholar]
- Jackemeyer, D.; Forzani, F.; Whisner, C. Study of Resting Energy Expenditure and Weight Changes during Pregnancy. Glob. J. Obes. Diabetes Metab. Syndr. 2017, 4, 016–023. [Google Scholar]
- Eto, E.; Maki, J.; Tamada, S.; Mitsui, T.; Hayata, K.; Hiramatsu, Y.; Masuyama, H. Assessment of resting energy expenditure and body composition in Japanese pregnant women with diabetes. J. Diabetes Investig. 2018, 9, 959–966. [Google Scholar] [CrossRef]
- Carneiro, I.; Elliott, S.; Siervo, M.; Padwal, R.; Bertoli, S.; Battezzati, A.; Prado, C. Is Obesity Associated with Altered Energy Expenditure? Adv. Nutr. Int. Rev. J. 2016, 7, 476–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaeth, A.; Dinges, D.F.; Goel, N. Resting metabolic rate varies by race and by sleep duration. Obesity 2015, 23, 2349–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrin, C.; Shrivastava, A.; Kelleher, C.; Lifeways, C.C.S.S.G. Maternal macronutrient intake during pregnancy and 5 years postpartum and associations with child weight status aged five. Eur. J. Clin. Nutr. 2013, 67, 670–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.F.-F.; Chia, A.-R.; Colega, M.; Tint, M.-T.; Aris, I.M.; Chong, Y.-S.; Gluckman, P.; Godfrey, K.M.; Kwek, K.; Saw, S.-M.; et al. Maternal protein intake during pregnancy is not associated with offspring birth weight in a multiethnic Asian population. J. Nutr. 2015, 145, 1303–1310. [Google Scholar] [PubMed] [Green Version]
- Hrolfsdottir, L.; Halldorsson, T.I.; Rytter, D.; Bech, B.H.; Birgisdottir, B.E.; Gunnarsdottir, I.; Granström, C.; Henriksen, T.B.; Olsen, S.F.; Maslova, E. Maternal Macronutrient Intake and Offspring Blood Pressure 20 Years Later. J. Am. Heart Assoc. 2017, 6, e005808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.W.; Aris, I.M.; Bernard, J.Y.; Tint, M.T.; Colega, M.; Gluckman, P.D.; Tan, K.H.; Shek, L.P.C.; Chong, Y.S.; Yap, F.; et al. Associations of maternal macronutrient intake during pregnancy with infant BMI peak characteristics and childhood BMI. Am. J. Clin. Nutr. 2017, 105, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.; Smith, R.; Collins, C.E. A systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2012, 70, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Crume, T.L.; Brinton, J.T.; Shapiro, A.; Kaar, J.; Glueck, D.H.; Siega-Riz, A.M.; Dabelea, D. Maternal dietary intake during pregnancy and offspring body composition: The Healthy Start Study. Am. J. Obstet. Gynecol. 2016, 215, 609.e1–609.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-da-Silva, L.; Cabo, C.; Moreira, A.; Virella, D.; Guerra, T.; Camoes, T.; Silva, A.R.; Neves, R.; Ferreira, G.C. The Adjusted Effect of Maternal Body Mass Index, Energy and Macronutrient Intakes during Pregnancy, and Gestational Weight Gain on Body Composition of Full-Term Neonates. Am. J. Perinatol. 2014, 31, 875–882. [Google Scholar]
- Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Dietary Quality during Pregnancy Varies by Maternal Characteristics in Project Viva: A US Cohort. J. Am. Diet. Assoc. 2009, 109, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Ley, S.; Hanley, A.J.; Retnakaran, R.; Sermer, M.; Zinman, B.; O’Connor, D.L. Effect of macronutrient intake during the second trimester on glucose metabolism later in pregnancy. Am. J. Clin. Nutr. 2011, 94, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. 24-h Dietary Recall (24HR) At a Glance | Dietary Assessment Primer. Available online: https://dietassessmentprimer.cancer.gov/profiles/recall/ (accessed on 10 August 2018).
- University of Minnesota NDSR Software-NCC: Nutrition Coordinating Center. Available online: http://www.ncc.umn.edu/products/ (accessed on 5 April 2019).
- Blumfield, M.; Hure, A.; MacDonald-Wicks, L.; Smith, R.; Simpson, S.; Raubenheimer, D.; Collins, C. The association between the macronutrient content of maternal diet and the adequacy of micronutrients during pregnancy in the women and their children’s health (WATCH) study. Nutrients 2012, 4, 1958–1976. [Google Scholar] [CrossRef]
- Shapiro, A.L.; Kaar, J.L.; Crume, T.L.; Starling, A.P.; Siega-Riz, A.M.; Ringham, B.M.; Glueck, D.H.; Norris, J.M.; Barbour, L.A.; Friedman, J.E.; et al. Maternal diet quality in pregnancy and neonatal adiposity: The Healthy State Study. Int. J. Obes. 2016, 40, 1056–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.L.; Siega-Riz, A.M.; Sotres-Alvarez, D.; Robinson, W.R.; Daniels, J.L.; Perrin, E.M.; Stuebe, A.M. Maternal Dietary Patterns during Pregnancy are Associated with Child Growth in the First 3 Years of Life. J. Nutr. 2016, 146, 2281–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talai Rad, N.; Ritterath, C.; Siegmund, T.; Wascher, C.; Siebert, G.; Henrich, W.; Buhling, K.J. Longitudinal analysis of changes in energy intake and macronutrient composition during pregnancy and 6 weeks post-partum. Arch. Gynecol. Obstet. 2011, 283, 185–190. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 28) | Control (n = 12) | Breezing ™ (n = 16) | p-Value | |
|---|---|---|---|---|
| Maternal age, mean ± SD | 29.8 ± 4.9 | 29.6 ± 5.9 | 29.9 ± 4.3 | 0.86 |
| Race/Ethnicity, % (n) | 0.36 | |||
| Non-Hispanic Caucasian | 78.6 (22) | 75.0 (9) | 81.3 (13) | ---- |
| Hispanic Caucasian | 14.3 (4) | 25.0 (3) | 6.25 (1) | ---- |
| Asian | 3.6 (1) | 0 (0) | 6.25 (1) | ---- |
| More than one race | 3.6 (1) | 0 (0) | 6.25 (1) | ---- |
| Employment classification, % (n) | 0.71 | |||
| Service and sales workers | 14.3 (4) | 16.7 (2) | 12.5 (2) | ---- |
| Managerial jobs | 3.6 (1) | 0 (0) | 6.3 (1) | ---- |
| Professional | 39.3 (11) | 50.0 (6) | 31.3 (5) | ---- |
| Clerical support workers | 7.1 (2) | 8.3 (1) | 6.3 (1) | ---- |
| Stay at home mom | 35.7 (10) | 25.0 (3) | 43.8 (7) | ---- |
| Marital status, % (n) | 0.62 | |||
| Single | 3.6 (1) | 0 (0) | 6.3 (1) | ---- |
| Married | 85.7 (24) | 91.7 (11) | 81.3 (13) | ---- |
| Partnered/significant other | 10.7 (3) | 8.3 (1) | 12.5 (2) | ---- |
| Education level, % (n) | 0.17 | |||
| Less than 8th grade | 3.6 (1) | 0 (0) | 6.3 (1) | ---- |
| High school/GED | 10.7 (3) | 25.0 (3) | 0 (0) | ---- |
| Two-year college | 17.9 (5) | 8.3 (1) | 25.0 (4) | ---- |
| Four-year college | 32.1 (9) | 25.0 (3) | 37.5 (6) | ---- |
| Post-graduate | 35.7 (10) | 41.7 (5) | 31.3 (5) | ---- |
| Weight (kgs), mean ± SD | 73.7 ± 16.0 | 70.3 ± 15.8 | 76.2 ± 16.2 | 0.35 |
| Height (cms), mean ± SD | 164.1.5 | 163.5 ± 6.6 | 164.4 ± 6.6 | 0.72 |
| Body Mass Index (kg/m2), mean ± SD | 27.4 ± 5.8 | 26.3 ± 5.8 | 28.2 ± 5.9 | 0.40 |
| Gestational age (weeks), mean ± SD | 14.8 ± 2.2 | 15.3 ± 1.5 | 14.4 ± 2.7 | 0.29 |
| Exercise level, % (n) | 0.32 | |||
| Sedentary | 14.3 (4) | 25.0 (3) | 6.3 (1) | ---- |
| Mild | 57.1 (16) | 50.0 (6) | 63.5 (10) | ---- |
| Occasional vigorous | 21.4 (6) | 25.0 (3) | 18.8 (3) | ---- |
| Regular vigorous | 7.1 (2) | 0 (0) | 12.5 (2) | ---- |
| Medication use, % (n) | 0.69 | |||
| None | 7.1 (2) | 8.3 (1) | 6.3 (1) | ---- |
| 1 to 3 | 71.4 (20) | 66.7 (8) | 75.0 (12) | ---- |
| 4 to 6 | 17.9 (5) | 16.7 (2) | 18.8 (3) | ---- |
| More than 6 | 3.6 (1) | 8.3 (1) | 0 (0) | ---- |
| Prenatal vitamin use, % (n) | 82.1 (23) | 83.3 (10) | 81.3 (13) | 0.89 |
| Control (n = 12) | Breezing™ (n = 16) | Total (n = 28) | Cohen’s d | p-Value | |
|---|---|---|---|---|---|
| Rate of GWG (kg/week) | |||||
| Overall Changes | 0.5 ± 0.3 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.4 | 0.25 |
| Early Changes | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.4 | 0.41 |
| Late Changes | 0.4 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.3 | 1.1 | 0.02 |
| Total GWG (kg) | |||||
| Overall Changes | 7.2 ± 2.6 | 8.1 ± 2.9 | 7.7 ± 2.8 | 0.4 | 0.29 |
| Early Changes | 4.2 ± 1.7 | 3.6 ± 2.0 | 3.9 ± 1.9 | 0.3 | 0.47 |
| Late Changes | 3.0 ± 1.4 | 4.5 ± 2.0 | 3.9 ± 1.9 | 0.9 | 0.05 |
| Total (n = 28) | Control (n = 12) | Breezing™ (n = 16) | p-Value | |
|---|---|---|---|---|
| Total Energy (kcal) | 1690.2 ± 495.7 | 1751.1 ± 493.8 | 1644.6 ± 508.2 | 0.58 |
| Total Fat (g) | 68.8 ± 29.3 | 71.0 ± 29.0 | 67.2 ± 30.3 | 0.75 |
| Saturated Fat (g) a | 22.2 (13.1, 33.4) | 18.8 (11.3, 56.6) | 26.1 (7.8, 40.7) | 1.00 |
| Monounsaturated Fat (g) | 25.3 ± 13.0 | 27.3 ± 14.3 | 23.8 ± 12.2 | 0.49 |
| Polyunsaturated Fat (g) | 13.0 ± 7.0 | 12.5 ± 5.7 | 13.4 ± 8.0 | 0.73 |
| Cholesterol (mg) a | 178.9 (94.5, 283.3) | 185.2 (89.4, 273.5) | 166.1 (103.3, 292.0) | 0.85 |
| Total Carbohydrate (g) | 207.8 ± 58.2 | 219.2 ± 55.9 | 199.2 ± 60.3 | 0.38 |
| Total Dietary Fiber (g) | 19.6 ± 9.6 | 22.1 ± 11.2 | 17.7 ± 8.1 | 0.23 |
| Soluble Fiber (g) | 5.9 ± 2.9 | 6.6 ± 3.5 | 5.4 ± 2.3 | 0.29 |
| Insoluble Fiber (g) | 13.6 ± 7.5 | 15.4 ± 8.5 | 12.3 ± 6.7 | 0.28 |
| Total Protein (g) | 68.3 ± 24.0 | 68.6 ± 24.0 | 68.0 ± 24.7 | 0.95 |
| Caffeine (mg) a | 3.1 (0.0, 47.4) | 2.9 (0.0, 88.5) | 3.5 (0.0, 44.5) | 0.69 |
| Total Sugars (g) a | 69.6 (51.7, 107.2) | 78.7 (66.0, 104.5) | 62.3 (42.9, 121.1) | 0.33 |
| Added Sugars (g) | 38.9 ± 29.4 | 32.0 ± 20.7 | 44.0 ± 34.4 | 0.26 |
| Variable | Control (n = 12) | Breezing™ (n = 16) | Cohen’s d | p-Value |
|---|---|---|---|---|
| Energy (kcal) | ||||
| Overall Changes | 191.8 ± 422.2 | −143.8 ± 339.2 | 0.18 | 0.03 |
| Early Changes | −43.0 ± 766.1 | 32.2 ± 642.6 | 0.00 | 0.79 |
| Late Changes | 207.1 ± 416.5 | −155.3 ± 309.2 | 0.23 | 0.01 |
| Protein (g) | ||||
| Overall Changes | 13.3 ± 31.0 | −9.9 ± 24.0 | 0.15 | 0.05 |
| Early Changes | −9.9 ± 19.9 | 7.4 ± 30.8 | 0.14 | 0.06 |
| Late Changes | 13.4 ± 33.0 | −10.0 ± 22.9 | 0.15 | 0.05 |
| Carbohydrates (g) | ||||
| Overall Changes | 19.3 ± 57.8 | −14.5 ± 58.7 | 0.10 | 0.12 |
| Early Changes | −2.4 ± 73.6 | 1.8 ± 87.9 | 0.00 | 0.86 |
| Late Changes | 19.3 ± 58.0 | −14.4 ± 55.8 | 0.11 | 0.11 |
| Fat (g) | ||||
| Overall Changes | 7.4 ± 25.5 | −5.5 ± 14.9 | 0.10 | 0.11 |
| Early Changes | 0.5 ± 60.8 | −0.4 ± 28.0 | 0.00 | 0.87 |
| Late Changes | 7.8 ± 25.2 | −5.8 ± 14.5 | 0.11 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Wyst, K.B.; Buman, M.P.; Shaibi, G.Q.; Petrov, M.E.; Reifsnider, E.; Whisner, C.M. Resting Energy Expenditure Relationship with Macronutrients and Gestational Weight Gain: A Pilot Study. Nutrients 2020, 12, 450. https://doi.org/10.3390/nu12020450
Vander Wyst KB, Buman MP, Shaibi GQ, Petrov ME, Reifsnider E, Whisner CM. Resting Energy Expenditure Relationship with Macronutrients and Gestational Weight Gain: A Pilot Study. Nutrients. 2020; 12(2):450. https://doi.org/10.3390/nu12020450
Chicago/Turabian StyleVander Wyst, Kiley B., Matthew P. Buman, Gabriel Q. Shaibi, Megan E. Petrov, Elizabeth Reifsnider, and Corrie M. Whisner. 2020. "Resting Energy Expenditure Relationship with Macronutrients and Gestational Weight Gain: A Pilot Study" Nutrients 12, no. 2: 450. https://doi.org/10.3390/nu12020450





