Effects of Coriandrum sativum Seed Extract on Aging-Induced Memory Impairment in Samp8 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Administration of the CSSE Extract
2.3. BW and Evaluation of Senescence
2.4. Barnes Maze Test
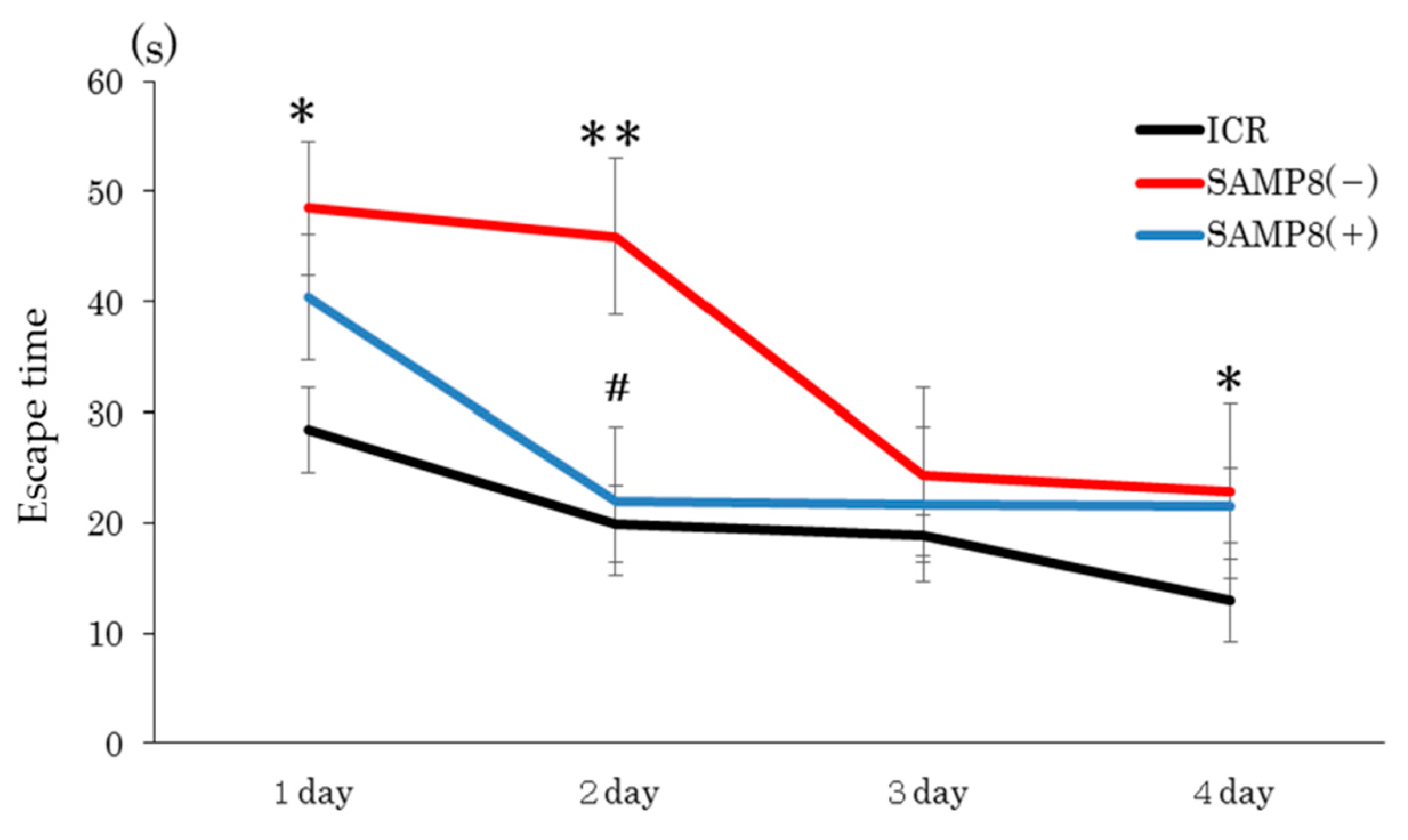
2.4.1. Spatial Memory Test
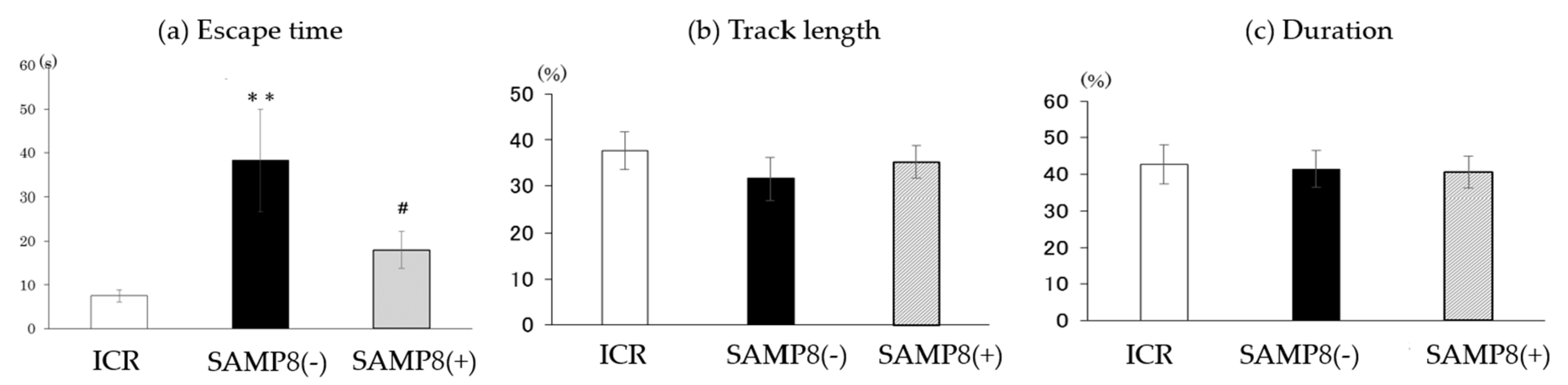
2.4.2. Probe Trial
2.5. Animal Sacrifice and Sample Collection
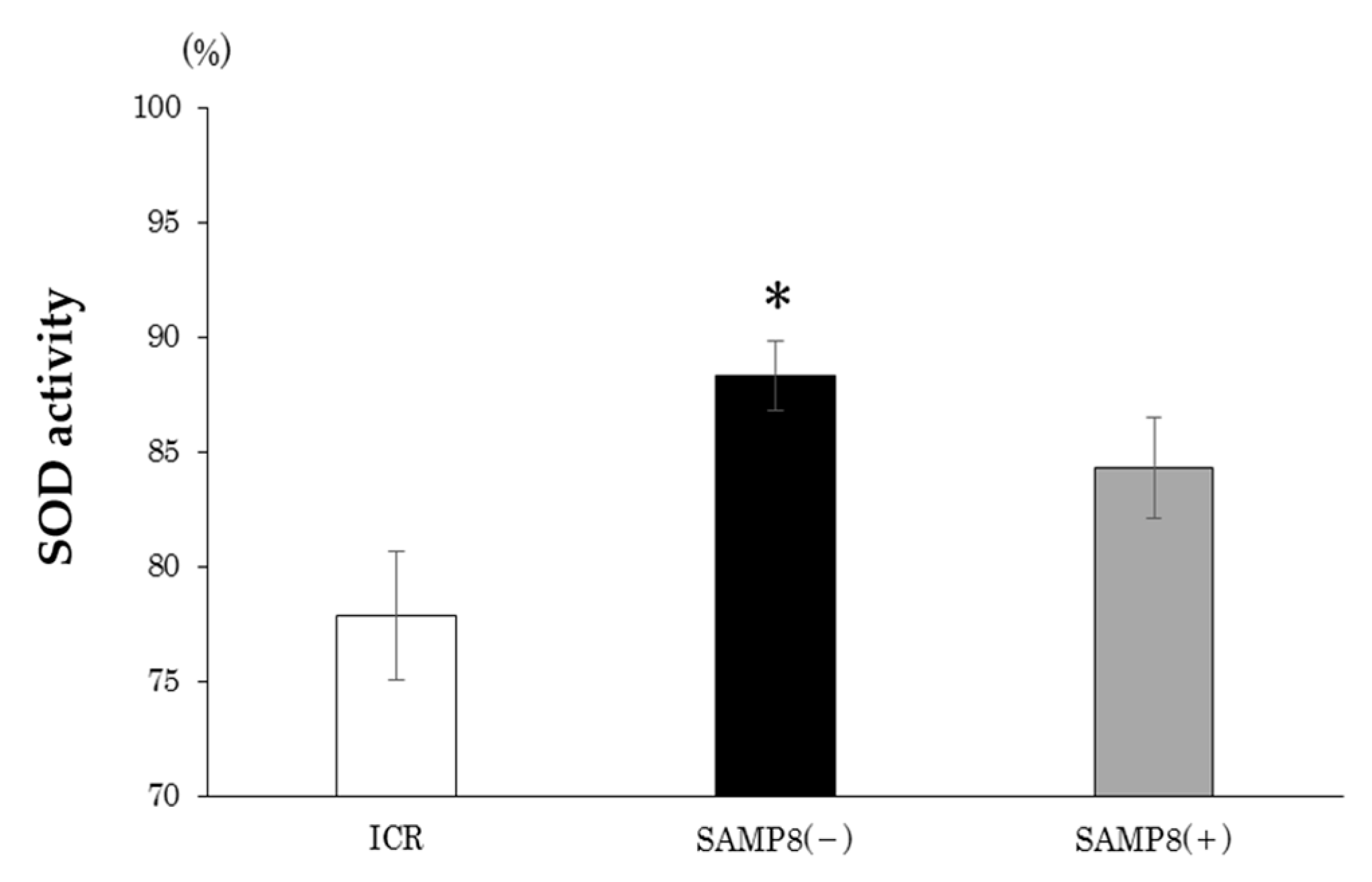
2.5.1. Plasma Superoxide Dismutase (SOD) Activity
2.5.2. Total Messenger (m)RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (QPCR)
2.6. Statistical Analysis
3. Results
3.1. Final BW and Appearance Changes
3.2. Performance on the Barnes Maze Test
3.2.1. Spatial Memory Test
3.2.2. Probe Trial
3.3. Plasma SOD activity
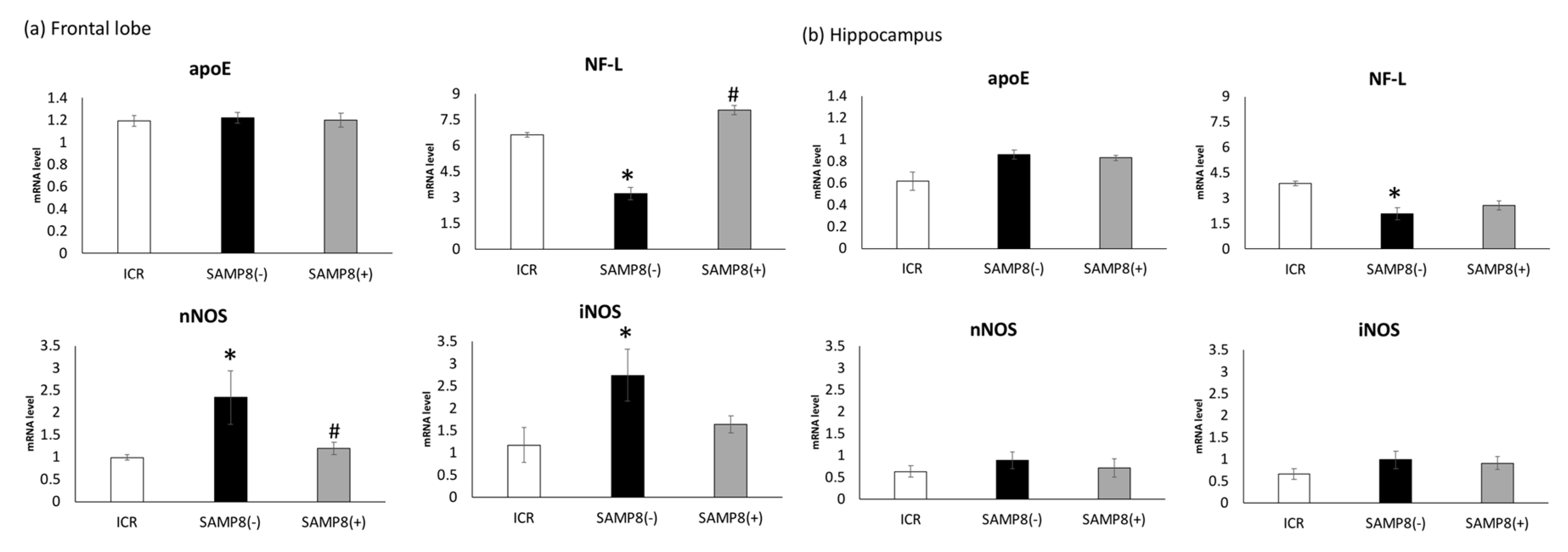
3.4. Gene mRNA Levels in the Frontal Lobe and Hippocampus
4. Discussion
4.1. SAMP8 Mouse Model
4.2. CSSE and Appearance Changes of Senescence
4.3. CSSE and Brain Function
4.3.1. Spatial Memory Test and Probe Trial
4.3.2. mRNA Levels of Related Protein Biomarkers in the Brain
4.3.3. Oxidative Stress
4.4. Dosage and Effective Components of CSSE
4.5. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization, US National Institute of Aging. Global Health and Ageing; World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.who.int/ageing/publications/global_health/en/ (accessed on 1 August 2019).
- Lee, R.; Zhou, Y. Does fertility or mortality drive contemporary population aging? The revisionist view revisited. Popul. Dev. Rev. 2017, 43, 285–301. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lobello, K.; Ryan, J.M.; Liu, E.; Rippon, G.; Black, R. Targeting betaamyloid: A clinical review of immunotherapeutic approaches in Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.J.; Dubey, A.; Dawson, K.M.; Stutts, W.A.; Lal, H.; Sohal, R.S. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA 1996, 93, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S. The Free radical hypothesis of aging: An appraisal of the current status. Aging Clin. Exp. Res. 1993, 5, 3–17. [Google Scholar] [CrossRef]
- Agarwal, S.; Sohal, R.S. Relationship between susceptibility to protein oxidation, aging, and maximum life span potential of different species. Exp. Gerontol. 1996, 31, 365–372. [Google Scholar] [CrossRef]
- Harsha, S.N.; Anilakumar, K.R. In vitro free radical scavenging and DNA damage protective property of Coriandrum sativum L. leaves extract. J. Food Sci. Technol. 2014, 51, 1533–1539. [Google Scholar] [CrossRef]
- Gray, A.M.; Flatt, P.R. Insulin-releasing and insulin-like activity of traditional anti-diabetic plant Coriandrum sativum. Br. J. Nutr. 1999, 81, 203–209. [Google Scholar] [CrossRef]
- Chithra, V.; Leelamma, S. Coriandrum sativum-mechanism of hypoglycemic action. Food Chem. 1999, 67, 229–231. [Google Scholar] [CrossRef]
- Chithra, V.; Leelamma, S. Coriandrum sativum: Effect on lipid metabolism in 1,2-dimethylhydrazine induced colon cancer. J. Ethnopharmacol. 2000, 71, 457–463. [Google Scholar] [CrossRef]
- Emamghoreishi, M.; Khasaki, M.; Fath-Aazam, M. Coriandrum sativum: Evaluation of its anxiolytic effect in the elevated plus-maze. J. Ethnopharmacol. 2005, 96, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Ripa, F.A.; Hamid, K. Comparative antioxidant activity study of some commonly used spices in Bangladesh. Pak. J. Biol. Sci. 2010, 13, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Anilakumar, K.R.; Saritha, V.; Khanum, F.; Bawa, A.S. Ameliorative effect of ajwain extract on hexachlorocyclohexane induced lipid peroxidation in rat liver. Food Chem. Toxicol. 2009, 47, 279–282. [Google Scholar] [CrossRef]
- Anilakumar, K.R.; Khanum, F.; Bawa, A.S. Effect of Coriander seeds powder on 1, 2 dimethyl hydrazine induced changes in antioxidant enzymes system and lipid peroxidation formation in rats. J. Diet Suppl. 2010, 7, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Izumo, N.; Wanadabe, Y. Effect of Coriandrum Sativum L. leaf extract on the brain GABA neurons in mice. J. Nutr. Health Food Sci. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Mani, V.; Parte, M.; Ramasamy, K.; Abdul Majid, A.B. Reversal of memory deficits by Coriandrum sativum leaves in mice. J. Sci. Food Agric. 2001, 91, 186–192. [Google Scholar] [CrossRef]
- Hozumi, E.; Kato, D.; Murakami, H.; Yokoyama, T.; Ito, Y.; Maeda, H.; Kameyama, Y. The effect of age-related changes and loss of molar teeth on learning and memory in senescence-accelerated mouse (SAM). J. J. Gerodont. 2001, 16, 196–202. [Google Scholar]
- Cortegano, I.; Rodríguez, M.; Martín, I.; Prado, M.C.; Ruíz, C.; Hortigüela, R.; Alía, M.; Vilar, M.; Mira, H.; Cano, E.; et al. Altered marginal zone and innate-like B cells in aged senescence-accelerated SAMP8 mice with defective IgG1 responses. Cell Death Dis. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Liu, H.Y.; Huang, C.F.; Li, C.H.; Tsai, C.Y.; Chen, W.H.; Wei, H.J.; Wang, M.F.; Kuo, Y.H.; Cheong, M.L.; Deng, W.P. Osteoporosis Recovery by Antrodia camphorata Alcohol Extracts through Bone Regeneration in SAMP8 Mice. Evid. Based Complement. Altern. Med. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Nakanishi, H.; Oka, M.; Yamamoto, K. Increased expression of cathepsins E and D in reactive microglial cells associated with spongiform degeneration in the brain stem of senescence-accelerated mouse. Exp. Neurol. 1995, 136, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Irino, M.; Matsushita, T.; Katoh, S.; Umezawa, M.; Tsuboyama, T.; Hosokawa, M.; Akiguchi, I.; Tokunaga, R.; Takeda, T. Spontaneous spongy degeneration of the brain stem in SAM-P/8 mice, a newly developed memory-deficient strain. J. Neuropathol. Exp. Neurol. 1989, 48, 577–590. [Google Scholar] [CrossRef]
- Marie, A.; Larroze-Chicot, P.; Cosnier-Pucheu, S.; Gonzalez-Gonzalez, S. Senescence-accelerated mouse prone 8 (SAMP8) as a model of age-related hearing loss. Neurosci. Lett. 2017, 656, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.Y.; Leung, K.S.; Siu, P.M.F.; Qin, J.H.; Chow, S.K.H.; Qin, L.; Li, C.Y.; Cheung, W.H. Muscle mass, structural and functional investigations of senescence-accelerated mouse P8 (SAMP8). Exp. Anim. 2015, 64, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Kiyota, Y.; Yamazaki, N.; Nagaoka, A.; Matsuo, T.; Nagawa, Y.; Takeda, T. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 1986, 3, 399–406. [Google Scholar] [CrossRef]
- Miyamoto, M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp. Gerontol. 1997, 32, 139–148. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kiyota, Y.; Nishiyama, M.; Nagaoka, A. Senescence-accelerated mouse (SAM): Age-related reduced anxiety-like behavior in the SAM-P/8 strain. Physiol. Behav. 1992, 51, 979–985. [Google Scholar] [CrossRef]
- Sergiev, P.V.; Dontsova, O.A.; Berezkin, G.V. Theories of aging: An ever-evolving field. Acta. Naturae 2015, 7, 9–18. [Google Scholar] [CrossRef]
- Deepa, B.; Anuradha, C.V. Antioxidant potential of Coriandrum sativum L. seed extract. Indian J. Exp. Biol. 2011, 49, 30–38. [Google Scholar]
- Li, Q.; Zhao, H.F.; Zhang, Z.F.; Liu, Z.G.; Pei, X.R.; Wang, J.B.; Li, Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta1-42 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience 2009, 163, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Chauhan, B.C.; Chauhan, N.B. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J. Alzheimer Dis. 2014, 39, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The Role of Apolipoprotein E in Alzheimer’s Disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Leuzy, A.; Lim, Y.M.; Troakes, C.; Hortobágyi, T.; Höglund, K.; Aarsland, D.; Lovestone, S.; Schöll, M.; Blennow, K.; et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Alzheimers Res. Ther. 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Cinelli, M.A.; Kang, S.; Silverman, R.B. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem. Soc. Rev. 2014, 43, 6814–6838. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol. Life Sci. 2003, 60, 1158–1171. [Google Scholar] [CrossRef]
- Evans, K.C.; Berger, E.P.; Cho, C.G.; Weisgraber, K.H.; Lansbury, P.T., Jr. Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: Implications for the pathogenesis and treatment of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1995, 92, 763–767. [Google Scholar] [CrossRef]
- Kramer, J.H.; Rosen, H.J.; Du, A.T.; Schuff, N.; Hollnagel, C.; Weiner, M.W.; Miller, B.L.; Delis, D.C. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology 2005, 19, 799–805. [Google Scholar] [CrossRef]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef]
- Bajo, M.; Yoo, B.C.; Cairns, N.; Gratzer, M.; Lubec, G. Neurofilament proteins NF-L, NF-M and NF-H in brain of patients with Down syndrome and Alzheimer’s disease. Amino Acids 2001, 21, 293–301. [Google Scholar] [CrossRef]
- Kawano, T.; Zoga, V.; Kimura, M.; Liang, M.Y.; Wu, H.E.; Gemes, G.; McCallum, J.B.; Kwok, W.M.; Hogan, Q.H.; Sarantopoulos, C.D. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: Action by direct S-nitrosylation. Mol. Pain 2009, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Choi, Y.B.; Pan, Z.H.; Lei, S.Z.; Chen, H.S.; Sucher, N.J.; Loscalzo, J.; Singel, D.J.; Stamler, J.S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 1993, 364, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Desai, S.; Devkar, R.; Ramachandran, A.V. Acute and sub-chronic toxicological evaluation of hydro-methanolic extract of Coriandrum Sativum L. seeds. EXCLI J. 2012, 11, 566–575. [Google Scholar] [PubMed]
- Emamghoreishi, M.; Heidari-Hamedani, G. Sedative-Hypnotic Activity of Extracts and Essential Oil of Coriander Seeds. Iran. J. Med. Sci. 2006, 31, 22–27. [Google Scholar]
- Alshahrani, A.M. In vivo neurological assessment of sedative hypnotic effect of Coriandrum Sativum L. seeds in mice. Int. J. Phytomed. 2016, 8, 113–116. [Google Scholar]
- Linck, V.M.; da Silva, A.L.; Figueiró, M.; Piato, A.L.; Herrmann, A.P.; Dupont Birck, F.; Caramão, E.B.; Nunes, D.S.; Moreno, P.R.; Elisabetsky, E. Inhaled linalool-induced sedation in mice. Phytomedicine 2009, 16, 303–307. [Google Scholar] [CrossRef]
- Latha, K.; Rammohan, B.; Sunanda, B.P.V.; Maheswari, M.S.U.; Mohan, S.K. Evaluation of anxiolytic activity of aqueous extract of Coriandrum sativum Linn. in mice: A preliminary experimental study. Pharmacogn. Res. 2015, 7, S47–S51. [Google Scholar] [CrossRef]
| Gene | Forward 5’→3’ | Reverse 5’→3’ | Universal Probe Library |
|---|---|---|---|
| GAPDH | AGCTTGTCATCAACGGGAAG | TTTGATGTTAGTGGGGTCTCG | #9 |
| apoE | TAGAGGAGGTGCGTGAGCA | ATTTGCTGGGTCTGTTCCTC | #25 |
| NF-L | GGAAAGAGCCGAGCAGACATC | GCCGTTCTGCTTACAGTGG | #17 |
| nNOS | GGCGTTCGTGATTACTGTGA | TCTTCCTCATGTCCAAATCCA | #69 |
| iNOS | CTTTGCCACGGACGAGAC | TCATTGTACTCTGAGGGCTGAC | #13 |
| ICR | SAMP8(−) | SAMP8(+) | |
|---|---|---|---|
| Final Body Weight (g) | 45.2 ± 0.9 | 26.0 ± 0.6# | 27.5 ± 0.5 * |
| Behavior | 1.1 ± 0.1 | 3.5 ± 0.5# | 3.4 ± 0.4 |
| Skin and Hair | 3.9 ± 0.9 | 6.8 ± 0.9# | 7.3 ± 0.8 # |
| Spine (Lordokyphosis) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mima, Y.; Izumo, N.; Chen, J.-R.; Yang, S.-C.; Furukawa, M.; Watanabe, Y. Effects of Coriandrum sativum Seed Extract on Aging-Induced Memory Impairment in Samp8 Mice. Nutrients 2020, 12, 455. https://doi.org/10.3390/nu12020455
Mima Y, Izumo N, Chen J-R, Yang S-C, Furukawa M, Watanabe Y. Effects of Coriandrum sativum Seed Extract on Aging-Induced Memory Impairment in Samp8 Mice. Nutrients. 2020; 12(2):455. https://doi.org/10.3390/nu12020455
Chicago/Turabian StyleMima, Yurina, Nobuo Izumo, Jiun-Rong Chen, Suh-Ching Yang, Megumi Furukawa, and Yasuo Watanabe. 2020. "Effects of Coriandrum sativum Seed Extract on Aging-Induced Memory Impairment in Samp8 Mice" Nutrients 12, no. 2: 455. https://doi.org/10.3390/nu12020455
APA StyleMima, Y., Izumo, N., Chen, J.-R., Yang, S.-C., Furukawa, M., & Watanabe, Y. (2020). Effects of Coriandrum sativum Seed Extract on Aging-Induced Memory Impairment in Samp8 Mice. Nutrients, 12(2), 455. https://doi.org/10.3390/nu12020455





