Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of p53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cell Viability Using Sulforhodamin B Assay
2.4. Apoptotic Cell Detection Using Annexin V Staining
2.5. DNA Fragmentation
2.6. Hoechst Staining Assay
2.7. Cell Cycle Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
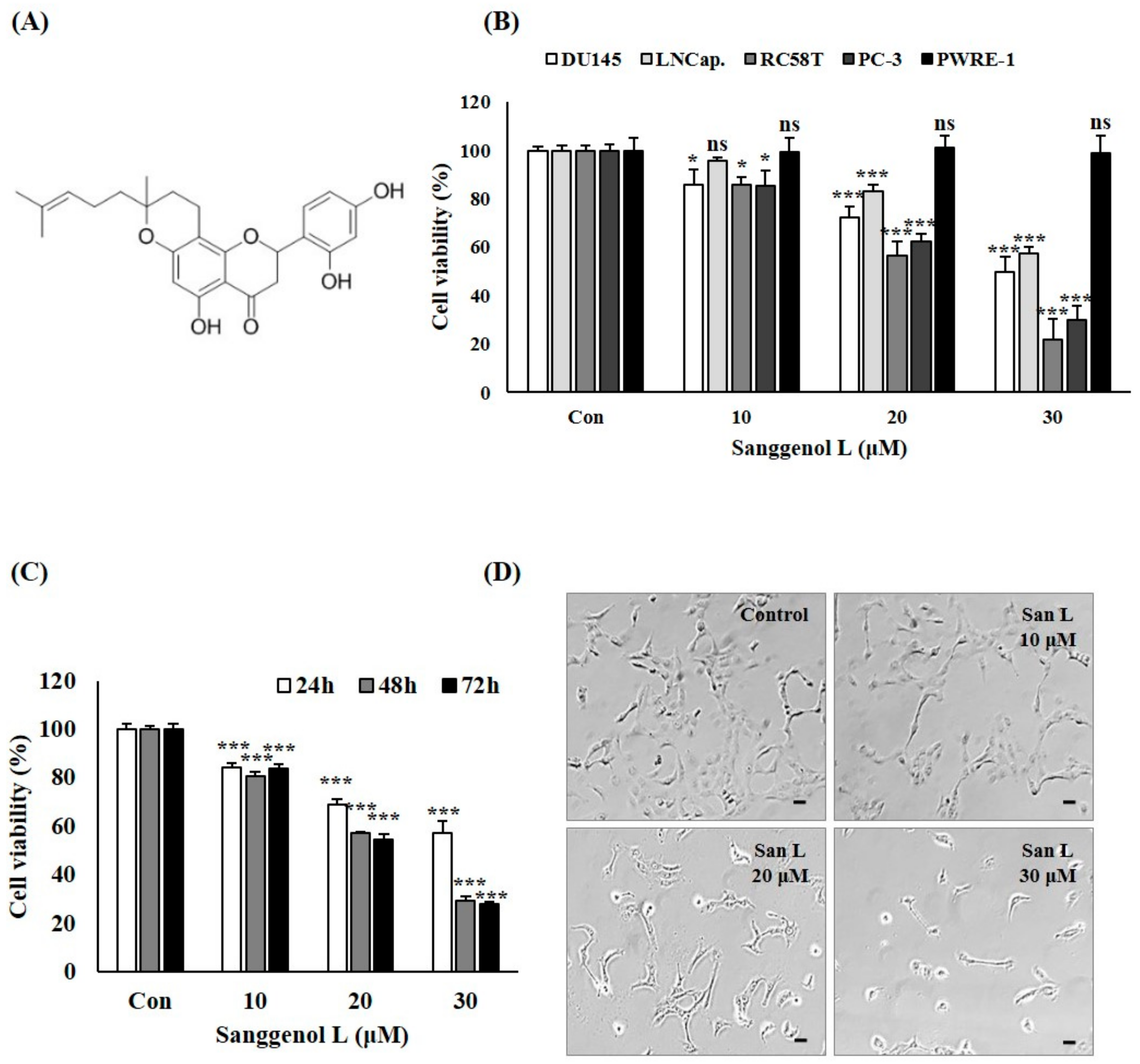
3.1. Sanggenol L Inhibits Cell Growth in DU145, LNCap, RC-58T, and PC-3 Human Prostate Cancer Cells
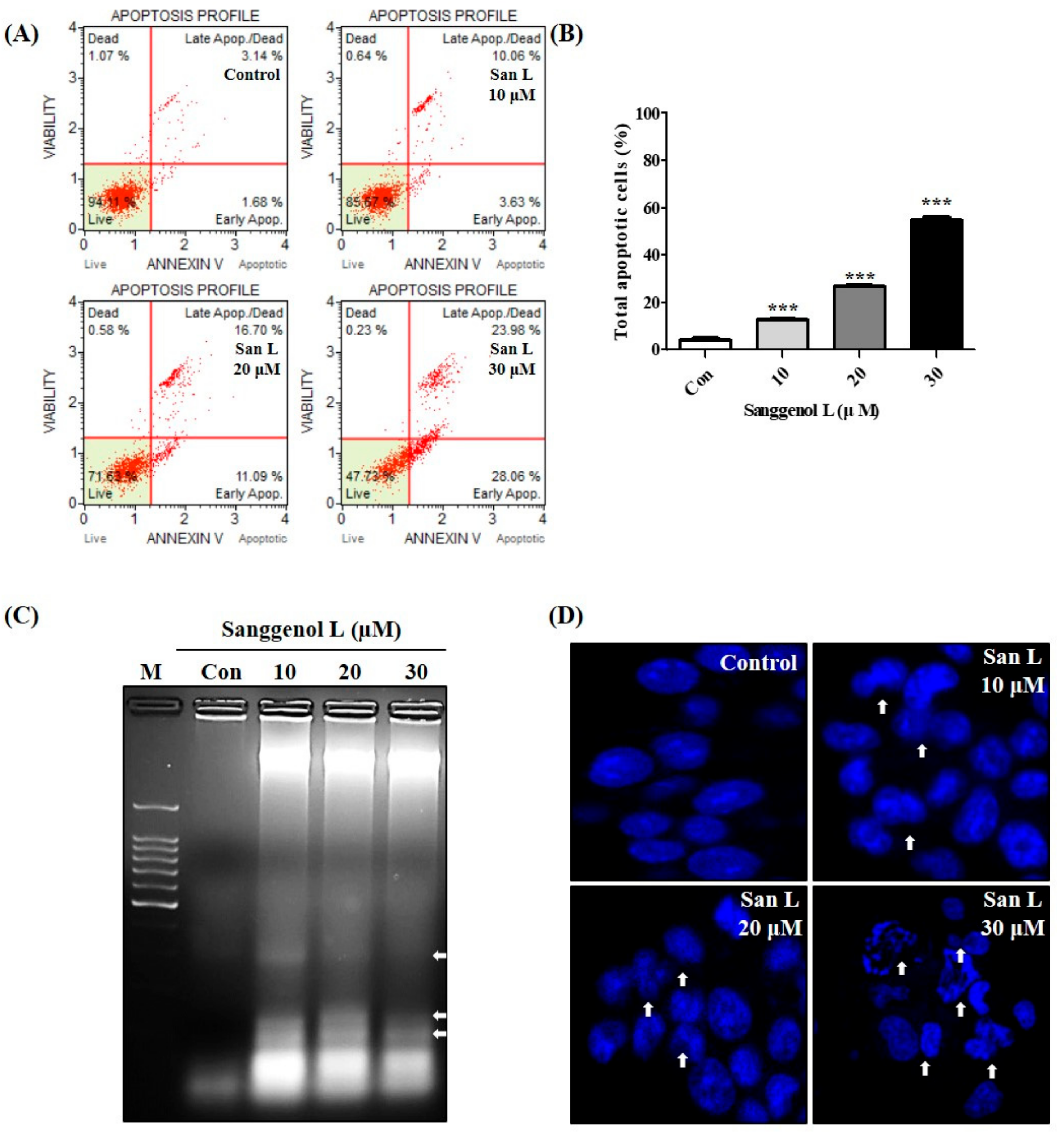
3.2. Sanggenol L Induces Apoptosis in RC-58T Human Prostate Cancer Cells
3.3. Sanggenol L Induces Caspase-Dependent Apoptosis in Prostate Cancer RC-58T Cells
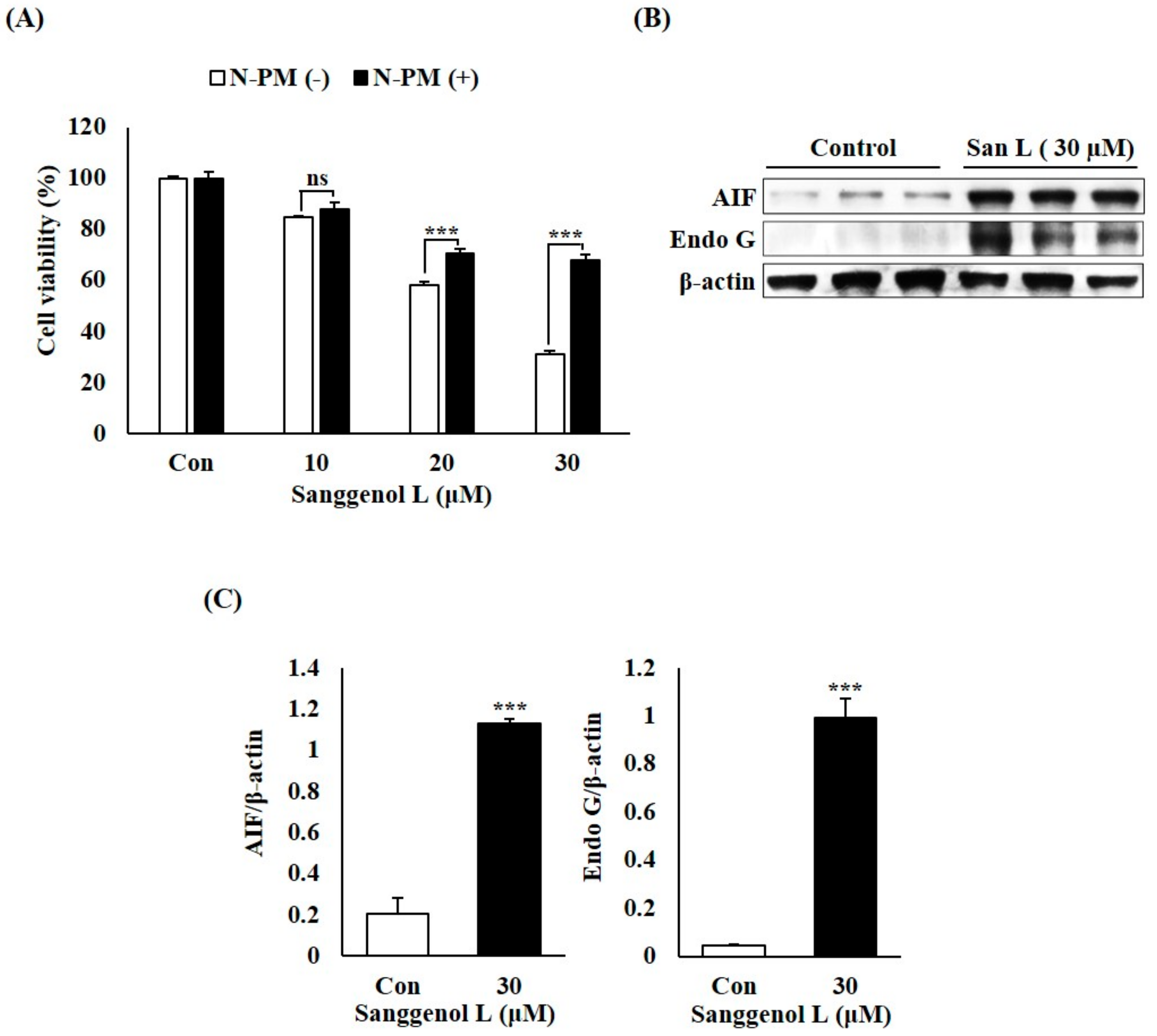
3.4. Sanggenol L Induces Caspase-Independent Apoptosis in Prostate Cancer RC-58T Cells
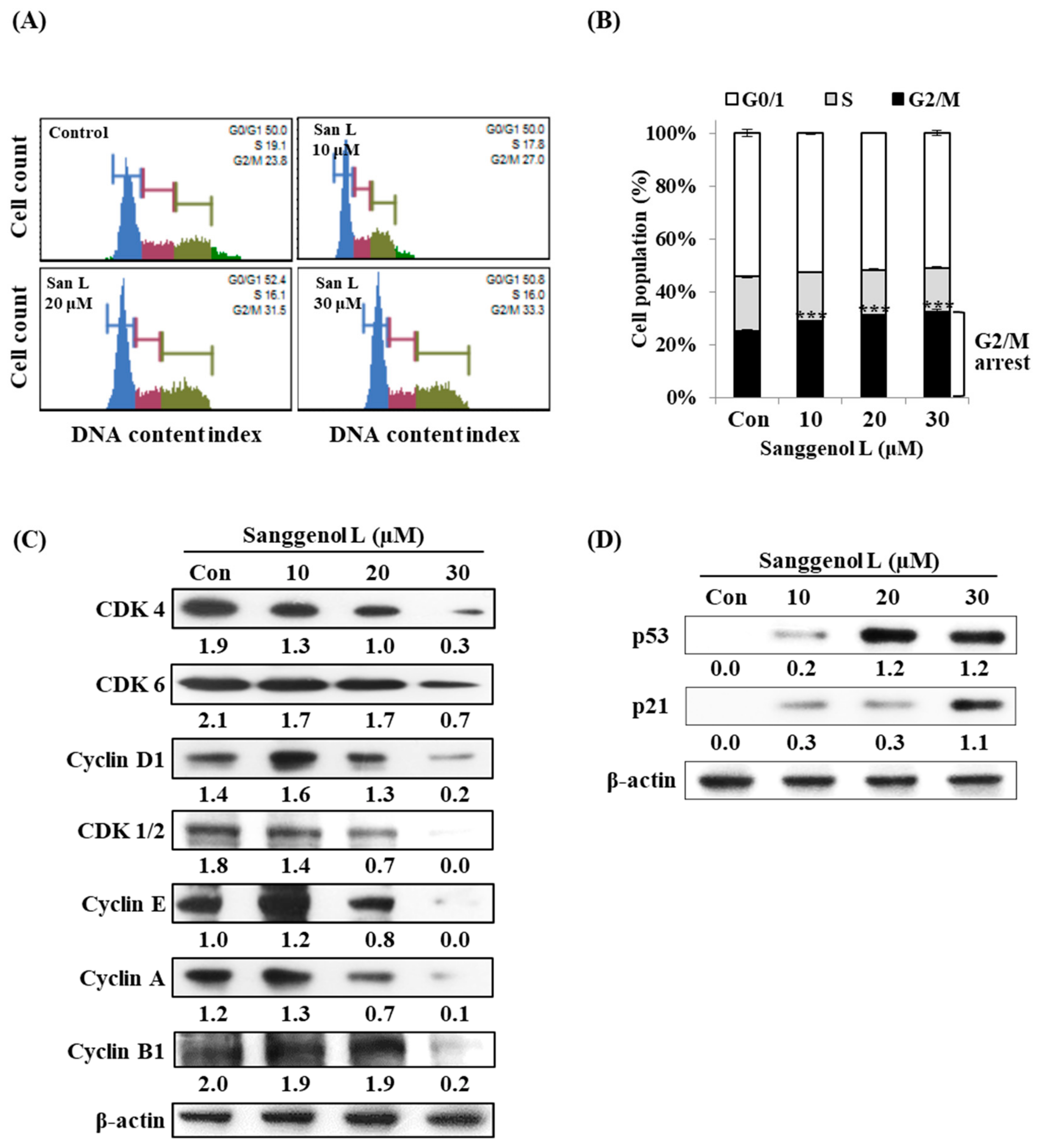
3.5. Sanggenol L Induces Cell Cycle Arrest in RC-58T Human Prostate Cancer Cells
3.6. Sanggenol L Inhibits Cell Growth of Prostate Cancer Cells Through Suppression of the PI3K/Akt/mTOR Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of prostate cancer. World J Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Livingstone, T.L.; Beasy, G.; Mills, R.D.; Plumb, J.; Needs, P.W.; Mithen, R.; Traka, M.H. Plant bioactives and the prevention of prostate cancer: Evidence from human studies. Nutrients 2019, 11, 2245. [Google Scholar] [CrossRef] [PubMed]
- Reynard, J.; Brewster, S.; Biers, S. Oxford Handbook of Urology, 3rd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.; Fuentes, F.; Lee, J.H.; Kong, A.N.T. Plants against cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2012, 12, 1281–1305. [Google Scholar]
- Aires, V.; Colin, D.J.; Doreau, A.; Di Pietro, A.; Heydel, J.M.; Artur, Y.; Latruffe, N.; Delmas, D. P-glycoprotein 1 affects chemoactivities of resveratrol against human colorectal cancer cells. Nutrients 2019, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Zhu, M.; Zheng, Z.; Huang, J.; Ma, X.; Huang, C.; Wu, R.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; et al. Modulation of miR-34a in curcumin-induced antiproliferation of prostate cancer cells. J. Cell Biochem. 2019, 120, 15616–15624. [Google Scholar] [CrossRef]
- Pavese, J.M.; Krishna, S.N.; Bergan, R.C. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am. J. Clin. Nutr. 2014, 100. [Google Scholar] [CrossRef]
- Cho, H.D.; Lee, J.H.; Moon, K.D.; Park, K.H.; Lee, M.K.; Seo, K.I. Auriculasin-induced ROS causes prostate cancer cell death via induction of apoptosis. Food Chem. Toxicol. 2018, 111, 660–669. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, Y.S.; Park, K.H.; Lee, M.K.; Tachibana, H.; Yamada, K.; Seo, K.I. Celastrol inhibits growth and induces apoptotic cell death in melanoma cells via the activation ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT signaling. Apoptosis 2012, 17, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Lee, J.H.; Kim, J.Y.; Park, K.W.; Jeong, I.Y.; Shim, K.H.; Lee, M.K.; Seo, K.I. Decursin from Angelicagigas Nakai induces apoptosis in RC-58T/h/SA#4 primary human prostate cancer cells via a mitochondria-related caspase pathway. Food Chem. Toxicol. 2011, 49, 2517–2523. [Google Scholar] [PubMed]
- Mohammadi, J.; Naik, P.R. Evaluation of hypoglycemic effect of Morus alba in an animal model. Indian J. Pharmacol. 2008, 40, 15–18. [Google Scholar] [PubMed]
- Eo, H.J.; Park, J.H.; Park, G.H.; Lee, M.H.; Lee, J.R.; Koo, J.S.; Jeong, J.B. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement Altern. Med. 2014, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Satoh, K.; Nomura, T.; Sakagami, H. Antinephritis and radical scavenging activity of prenylflavonoids. Fitoterapia 2003, 74, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Lee, D.Y.; Jeong, R.H. Neuroprotective effect of prenylated arylbenzofuran and flavonoids from Morus alba fruits on glutamate-induced oxidative injury in HT22 hippo-campal cells. J. Med. Food 2015, 18, 403–408. [Google Scholar] [CrossRef]
- Nam, M.S.; Jung, D.B.; Seo, K.H.; Kim, B.I.; Kim, J.H.; Kim, J.H.; Kim, B.; Baek, N.I.; Kim, S.H. Apoptotic effect of sanggenol L via caspase activation and inhibition of NF-κB signaling in ovarian cancer cells. Phytother. Res. 2016, 30, 90–96. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Daniel, P.T.; Koert, U.; Schuppan, J. Apoptolidin: Induction of apoptosis by a natural product. Angew. Chem. Int. Ed. 2006, 45, 872–893. [Google Scholar] [CrossRef]
- Arnoult, D.; Gaume, B.; Karbowski, M.; Sharpe, J.C.; Cecconi, F.; Youle, R.J. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003, 22, 4385–4399. [Google Scholar] [CrossRef]
- Won, Y.S.; Seo, K.I. Lupiwighteone induces caspase-dependent and -independent apoptosis on human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. Food Chem. Toxicol. 2019, 135, 110863. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, Y.; Ling, Z.; Liu, X.; Zhao, X.; Yuan, Z.; Nie, C.; Wei, Y. Caspase-3 feedback loop enhances Bid-induced AIF/endoG and Bak activation in Bax and p53-independent manner. Cell Death Dis. 2015, 6, e1919. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, S.; Li, J.; Zhang, Y.; Gao, D.; Zhao, S. Caspase-independent cell death mediated by apoptosis-inducing factor (AIF) nuclear translocation is involved in ionizing radiation induced HepG2 cell death. Biochem. Biophys. Res. Commun. 2016, 472, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Caspase-independent cell death: Leaving the set without the final cut. Oncogene 2008, 27, 6452–6461. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI: J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Jung, J.W.; Ko, W.M.; Park, J.H.; Seo, K.H.; Oh, E.J.; Lee, D.Y.; Lee, D.S.; Kim, Y.C.; Lim, D.W.; Han, D.; et al. Isoprenylated flavonoids from the root bark of Morus Alba and their hepatoprotective and neuroprotective activities. Arch. Pharm. Res. 2015, 38, 2066–2075. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Fukai, T.; Sakagami, H.; Chang, W.J.; Yang, P.Q.; Wang, F.P.; Nomura, T. Cytotoxic flavonoids with isoprenoid groups from Morus mongolica. J. Nat. Prod. 2001, 64, 181–188. [Google Scholar] [CrossRef]
- Florentin, A.; Arama, E. Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J. Cell Biol. 2012, 196, 513–527. [Google Scholar] [CrossRef]
- Krajewska, M.; Zapata, J.M.; Meinhold-Heerlein, I.; Hedayat, H.; Monks, A.; Bettendorf, H.; Shabaik, A.; Bubendorf, L.; Kallioniemi, O.P.; Kim, H.; et al. Expression of Bcl-2 family member Bid in normal and malignant tissues. Neoplasia 2002, 4, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Shelar, S.B.; Kaminska, K.K.; Reddy, S.A.; Kumar, D.; Tan, C.T.; Yu, V.C.; Lu, J.; Holmgren, A.; Hagen, T.; Chew, E.H. Thioredoxin-dependent regulation of AIF-mediated DNA damage. Free Radic. Biol. Med. 2015, 87, 125–136. [Google Scholar] [CrossRef]
- Peyressatre, M.; Prével, C.; Pellerano, M.; Morris, M.C. Targeting cyclin-dependent kinases in human cancers: From small molecules to peptide inhibitors. Cancers 2015, 7, 179–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.A.; Peixoto, D.; Queiroz, M.J.; Soares, R. Antiangiogenic 1-Aryl-3-[3-(thieno[3,2-b] pyridin-7-ylthio) phenyl] ureas inhibit MCF-7 and MDA-MB-231 human breast cancer cell lines through PI3K/Akt and MAPK/Erk pathways. J. Cell. Biochem. 2016, 117, 2791–2799. [Google Scholar] [CrossRef]
- Lin, V.C.; Kuo, P.T.; Lin, Y.C.; Chen, Y.; Hseu, Y.C.; Yang, H.L.; Kao, J.Y.; Ho, C.T.; Way, T.D. Penta-O-galloyl-β-D-glucose suppresses EGF-induced eIF3i expression through inhibition of the PI3K/AKT/mTOR pathway in prostate cancer cells. J. Agric. Food Chem. 2014, 62, 8990–8996. [Google Scholar] [CrossRef]
- Huang, S. Inhibition of PI3K/Akt/mTOR signaling by natural products. Anti-Cancer Agents Med. Chem. 2013, 13, 967–970. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, Y.-S.; Seo, K.-I. Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of p53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells. Nutrients 2020, 12, 488. https://doi.org/10.3390/nu12020488
Won Y-S, Seo K-I. Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of p53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells. Nutrients. 2020; 12(2):488. https://doi.org/10.3390/nu12020488
Chicago/Turabian StyleWon, Yeong-Seon, and Kwon-Il Seo. 2020. "Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of p53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells" Nutrients 12, no. 2: 488. https://doi.org/10.3390/nu12020488
APA StyleWon, Y.-S., & Seo, K.-I. (2020). Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of p53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells. Nutrients, 12(2), 488. https://doi.org/10.3390/nu12020488



