FADS Genetic Variants in Taiwanese Modify Association of DHA Intake and Its Proportions in Human Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects Recruitment
2.2. Selection of SNPs
2.3. DNA Extraction and SNP Genotyping
2.4. Dietary Information
2.5. Milk Collection and Fatty Acid Analysis
2.6. Statistical Analyses
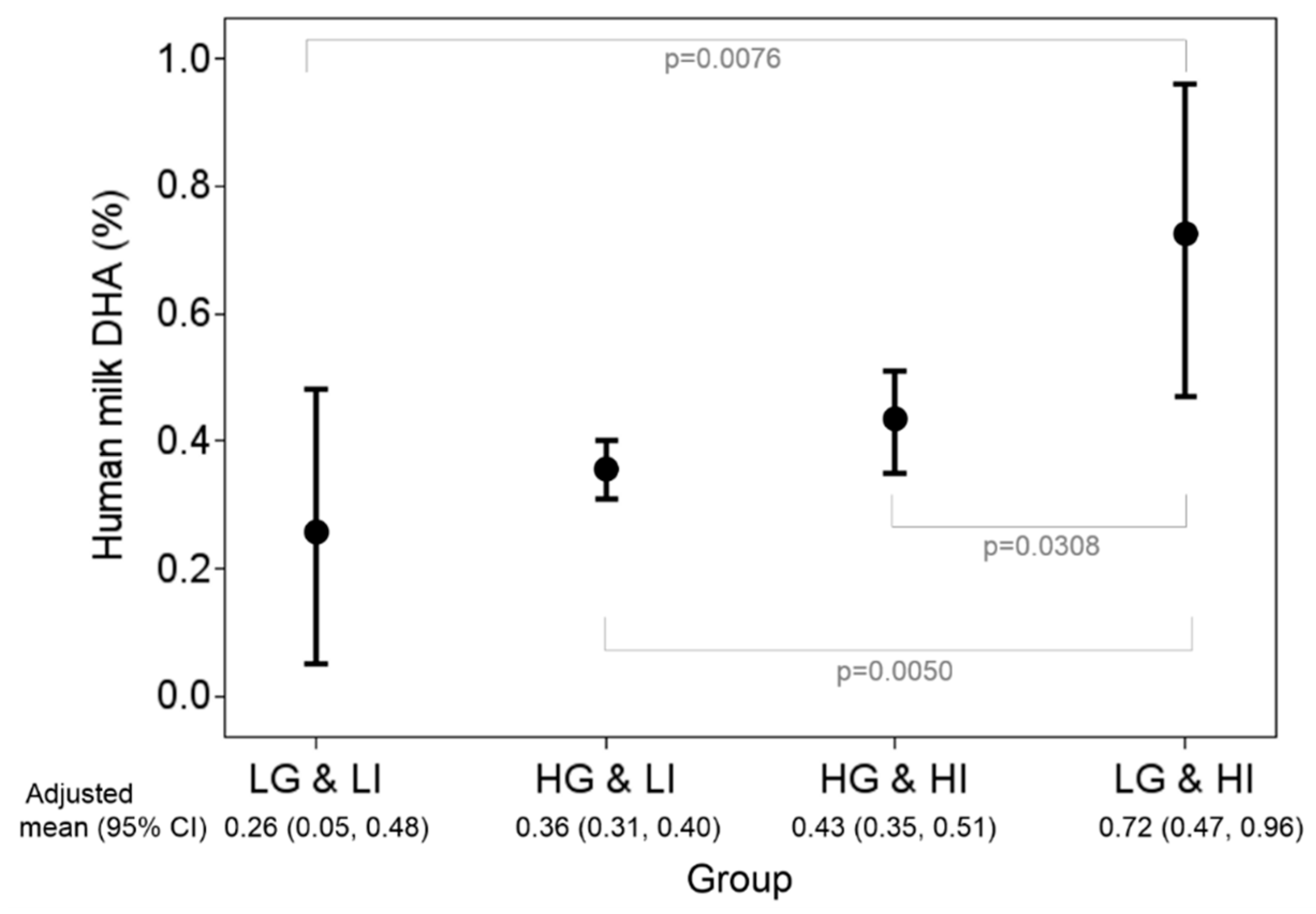
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Molloy, C.; Doyle, L.W.; Makrides, M.; Anderson, P.J. Docosahexaenoic acid and visual functioning in preterm infants: A review. Neuropsychol. Rev. 2012, 22, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Valenzuela, R.; Catalina Hernandez-Rodas, M.; Valenzuela, A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot. Essent. Fatty Acids 2017, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, CD000376. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/nutrition/topics/exclusive_breastfeeding/en/ (accessed on 10 Oct 2019).
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef]
- Innis, S.M. Human milk: Maternal dietary lipids and infant development. Proc. Nutr. Soc. 2007, 66, 397–404. [Google Scholar] [CrossRef]
- Koletzko, B.; Boey, C.C.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef]
- Innis, S.M.; Gilley, J.; Werker, J. Are human milk long-chain polyunsaturated fatty acids related to visual and neural development in breast-fed term infants? J. Pediatr. 2001, 139, 532–538. [Google Scholar] [CrossRef]
- Jensen, C.L.; Voigt, R.G.; Prager, T.C.; Zou, Y.L.; Fraley, J.K.; Rozelle, J.C.; Turcich, M.R.; Llorente, A.M.; Anderson, R.E.; Heird, W.C. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am. J. Clin. Nutr. 2005, 82, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; Tanaka, T.; Tang, W.; Manichaikul, A.; Foy, M.; Kabagambe, E.K.; Nettleton, J.A.; King, I.B.; Weng, L.C.; Bhattacharya, S.; et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011, 7, e1002193. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shen, J.; Abecasis, G.R.; Kisialiou, A.; Ordovas, J.M.; Guralnik, J.M.; Singleton, A.; Bandinelli, S.; Cherubini, A.; Arnett, D.; et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI study. PLoS Genet. 2009, 5, e1000338. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Heinrich, J.; Klopp, N.; Schaeffer, L.; Hoff, S.; Wolfram, G.; Illig, T.; Linseisen, J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br. J. Nutr. 2009, 101, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Innis, S.M. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Plat, J.; Mensink, R.P.; Müller, A.; Jansen, E.; Zeegers, M.P.; Thijs, C. FADS1FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am. J. Clin. Nutr. 2010, 91, 1368–1376. [Google Scholar] [CrossRef]
- Lattka, E.; Rzehak, P.; Szabó, É.; Jakobik, V.; Weck, M.; Weyermann, M.; Grallert, H.; Rothenbacher, D.; Heinrich, J.; Brenner, H.; et al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am. J. Clin. Nutr. 2011, 93, 382–391. [Google Scholar]
- Ding, Z.; Liu, G.L.; Li, X.; Chen, X.Y.; Wu, Y.X.; Cui, C.C.; Zhang, X.; Yang, G.; Xie, L. Association of polyunsaturated fatty acids in breast milk with fatty acid desaturase gene polymorphisms among Chinese lactating mothers. Prostaglandins Leukot. Essent. Fatty Acids 2016, 109, 66–71. [Google Scholar] [CrossRef]
- Moltó-Puigmartí, C.; Castellote, A.I.; López-Sabater, M.C. Conjugated linoleic acid determination in human milk by fast-gas chromatography. Anal. Chim. Acta 2007, 602, 122–130. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, F.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Harsløf, L.B.; Larsen, L.H.; Ritz, C.; Hellgren, L.I.; Michaelsen, K.F.; Vogel, U.; Lauritzen, L. FADS genotype and diet are important determinants of DHA status: A cross-sectional study in Danish infants. Am. J. Clin. Nutr. 2013, 97, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lattka, E.; Zeilinger, S.; Illig, T.; Steer, C.D. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: Findings from the Avon Longitudinal Study of Parents and Children. Am. J. Clin. Nutr. 2011, 93, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.D.; Hibbeln, J.R.; Golding, J.; Davey Smith, G. Polyunsaturated fatty acid levels in blood during pregnancy, at birth and at 7 years: Their associations with two common FADS2 polymorphisms. Hum. Mol. Genet. 2012, 21, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yang, J.H.; Chiang, C.W.; Hsiung, C.N.; Wu, P.E.; Chang, L.C.; Chu, H.W.; Chang, J.; Song, I.W.; Yang, S.L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Zhang, Y.; Zhang, Q.C.; Li, H.J.; Cui, Y.Q.; Xu, Z.; Jin, L.; Zhou, H.; Zhu, H. Ancient DNA reveals that the genetic structure of the northern Han Chinese was shaped prior to 3,000 years ago. PLoS ONE 2015, 10, e0125676. [Google Scholar] [CrossRef]
- Merino, D.M.; Johnston, H.; Clarke, S.; Roke, K.; Nielsen, D.; Badawi, A.; El-Sohemy, A.; Ma, D.W.; Mutch, D.M. Polymorphisms in FADS1 and FADS2 alter desaturase activity in young Caucasian and Asian adults. Mol. Genet. Metab. 2011, 103, 171–178. [Google Scholar] [CrossRef]
- Park, W.J.; Kothapalli, K.S.; Reardon, H.T.; Lawrence, P.; Qian, S.B.; Brenna, J.T. A novel FADS1 isoform potentiates FADS2-mediated production of eicosanoid precursor fatty acids. J. Lipid Res. 2012, 53, 1502–1512. [Google Scholar] [CrossRef]
- Wu, T.C.; Lau, B.H.; Chen, P.H.; Wu, L.T.; Tang, R.B. Fatty acid composition of Taiwanese human milk. J. Chin. Med. Assoc. 2010, 73, 581–588. [Google Scholar] [CrossRef]
- Huang, H.L.; Chuang, L.T.; Li, H.H.; Lin, C.P.; Glew, R.H. Docosahexaenoic acid in maternal and neonatal plasma phospholipids and milk lipids of Taiwanese women in Kinmen: Fatty acid composition of maternal blood, neonatal blood and breast milk. Lipids Health Dis. 2013, 12, 27. [Google Scholar] [CrossRef]
- Yuhas, R.; Pramuk, K.; Lien, E.L. Human milk fatty acid composition from nine countries varies most in DHA. Lipids 2006, 41, 851–858. [Google Scholar] [CrossRef]
- Calder, P.C.; Krauss-Etschmann, S.; de Jong, E.C.; Dupont, C.; Frick, J.S.; Frokiaer, H.; Heinrich, J.; Garn, H.; Koletzko, S.; Lack, G.; et al. Early nutrition and immunity-progress and perspectives. Br. J. Nutr. 2006, 96, 774–790. [Google Scholar] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Heim, T.; Swyer, P.R.; Chance, GW. Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum. Dev. 1981, 5, 355–366. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davies, N.M.; Horta, B.L.; Ahluwalia, T.S.; Bisgaard, H.; Bønnelykke, K.; Caspi, A.; Moffitt, T.E.; Poulton, R.; Sajjad, A.; et al. Effect modification of FADS2 polymorphisms on the association between breastfeeding and intelligence: Results from a collaborative meta-analysis. Int. J. Epidemiol. 2019, 48, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 28–40. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Williams, B.; Kim-Cohen, J.; Craig, I.W.; Milne, B.J.; Poulton, R.; Schalkwyk, L.C.; Taylor, A.; Werts, H.; Moffitt, T.E. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 18860–18865. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.D.; Davey, S.G.; Emmett, P.M.; Hibbeln, J.R.; Golding, J. FADS2 polymorphisms modify the effect of breastfeeding on child IQ. PLoS ONE 2010, 5, e11570. [Google Scholar] [CrossRef]
- Morales, E.; Bustamante, M.; Gonzalez, J.R.; Guxens, M.; Torrent, M.; Mendez, M.; Garcia-Esteban, R.; Julvez, J.; Forns, J.; Vrijheid, M.; et al. Genetic variants of the FADS gene cluster and ELOVL gene family, colostrums LC-PUFA levels, breastfeeding, and child cognition. PLoS ONE 2011, 6, e17181. [Google Scholar] [CrossRef]
- Valentine, C.J.; Morrow, G.; Pennell, M.; Morrow, A.L.; Hodge, A.; Haban-Bartz, A.; Collins, K.; Rogers, L.K. Randomized controlled trial of docosahexaenoic acid supplementation in Midwestern U.S. human milk donors. Breastfeed Med. 2013, 8, 86–91. [Google Scholar] [CrossRef]
- Valentine, C.J.; Dingess, K.A.; Kleiman, J.; Morrow, A.L.; Rogers, L.K. A randomized trial of maternal docosahexaenoic acid supplementation to reduce inflammation in extremely preterm infants. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 388–392. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All (n = 164) |
|---|---|
| Maternal characteristics | |
| Age (year) | 32.7 ± 4.0 |
| Height (cm) | 160 ± 5 |
| BMI (kg/m2) (before pregnancy) | 23.6 ± 3.8 |
| Parity | |
| Multiparous | 76 (46.34) |
| Primiparous | 88 (53.66) |
| Smoking during pregnancy | |
| No | 160 (98.77) |
| Yes | 2 (1.23) |
| Alcohol consumption during pregnancy | |
| No | 151 (98.69) |
| Yes | 2 (1.31) |
| Fish intake (serving/wk) 3 | 4.5 ± 2.4 |
| DHA supplements | |
| No | 108 (65.85) |
| Yes | 56 (34.15) |
| Total DHA intake (mg/d) 4 | 143 ± 126 |
| Low < 200 mg/d | 120 (73.17) |
| High ≥ 200 mg/d | 44 (26.83) |
| Birth characteristics | |
| Gestational age (wk) | 38.6 ± 1.1 |
| Gender | |
| Female | 83 (51.23) |
| Male | 79 (48.77) |
| Birth weight (g) | 3135 ± 445 |
| Birth length (cm) | 50.2 ± 2.4 |
| SNP | Gene | Minor/Major Allele (m/M) | Genotype | HWE p-Value | MAF (%) | ||
|---|---|---|---|---|---|---|---|
| mm | mM | MM | |||||
| rs1535 | FADS2 | A/G | 28 (17.07) | 90 (54.88) | 46 (28.05) | 0.206 | 44.5 |
| rs174448 | FADS2/3 | G/A | 14 (8.54) | 51 (31.10) | 99 (60.37) | 0.057 | 24.1 |
| Fatty Acid | All (n = 164) | SNP | Genotype | p-Value | ||
|---|---|---|---|---|---|---|
| mm | mM | MM | ||||
| 10:0 | 1.94 ± 0.63 | rs1535 | 1.83 ± 0.63 | 1.91 ± 0.64 | 2.08 ± 0.63 | 0.180 |
| rs174448 | 2.03 ± 0.66 | 1.97 ± 0.69 | 1.92 ± 0.61 | 0.785 | ||
| 12:0 | 6.91 ± 2.51 | rs1535 | 7.06 ± 2.65 | 6.74 ± 2.50 | 7.13 ± 2.45 | 0.650 |
| rs174448 | 7.38 ± 2.61 | 6.96 ± 2.54 | 6.81 ± 2.49 | 0.725 | ||
| 14:0 | 5.1 ± 1.9 | rs1535 | 5.45 ± 2.23 | 5.03 ± 1.83 | 5.03 ± 1.84 | 0.583 |
| rs174448 | 5.51 ± 2.16 | 5.16 ± 1.86 | 5.02 ± 1.90 | 0.638 | ||
| 16:0 | 18.87 ± 2.36 | rs1535 | 19.78 ± 2.8 | 18.81 ± 2.25 | 18.42 ± 2.16 | 0.050 |
| rs174448 | 19.07 ± 2.24 | 18.82 ± 2.44 | 18.86 ± 2.35 | 0.941 | ||
| 16:1 | 2.77 ± 0.61 | rs1535 | 2.83 ± 0.63 | 2.74 ± 0.59 | 2.80 ± 0.64 | 0.724 |
| rs174448 | 2.69 ± 0.57 | 2.78 ± 0.54 | 2.78 ± 0.66 | 0.870 | ||
| 17:0 | 0.25 ± 0.42 | rs1535 | 0.24 ± 0.08 | 0.28 ± 0.56 | 0.21 ± 0.07 | 0.672 |
| rs174448 | 0.20 ± 0.07 | 0.21 ± 0.07 | 0.28 ± 0.53 | 0.534 | ||
| 18:0 | 5.44 ± 1.2 | rs1535 | 5.37 ± 1.31 | 5.41 ± 1.35 | 5.55 ± 0.78 | 0.786 |
| rs174448 | 5.63 ± 0.97 | 5.57 ± 1.17 | 5.35 ± 1.25 | 0.485 | ||
| 18:1 | 30.51 ± 7.81 | rs1535 | 29.06 ± 8.36 | 30.39 ± 8.50 | 31.63 ± 5.77 | 0.381 |
| rs174448 | 29.61 ± 9.15 | 30.67 ± 7.74 | 30.56 ± 7.72 | 0.899 | ||
| 18:2n-6 | 22.8 ± 3.84 | rs1535 | 22.39 ± 3.84 | 22.97 ± 4.07 | 22.71 ± 3.41 | 0.773 |
| rs174448 | 22.31 ± 3.09 | 22.63 ± 4.46 | 22.95 ± 3.61 | 0.788 | ||
| 18:3n-3 | 1.64 ± 4.97 | rs1535 | 2.04 ± 5.52 | 1.88 ± 5.47 | 0.92 ± 3.37 | 0.508 |
| rs174448 | 2.15 ± 6.34 | 1.60 ± 4.85 | 1.58 ± 4.87 | 0.922 | ||
| 20:0 | 0.42 ± 0.35 | rs1535 | 0.51 ± 0.38 | 0.41 ± 0.30 | 0.39 ± 0.40 | 0.341 |
| rs174448 | 0.44 ± 0.32 | 0.42 ± 0.40 | 0.42 ± 0.32 | 0.984 | ||
| 20:1n-9 | 0.51 ± 0.16 | rs1535 | 0.59 ± 0.16b | 0.52 ± 0.15b | 0.43 ± 0.12a | <0.001 |
| rs174448 | 0.50 ± 0.22 | 0.49 ± 0.15 | 0.52 ± 0.15 | 0.596 | ||
| 20:2n-6 | 1.06 ± 0.29 | rs1535 | 1.05 ± 0.24 | 1.07 ± 0.32 | 1.03 ± 0.25 | 0.707 |
| rs174448 | 0.99 ± 0.25 | 1.03 ± 0.25 | 1.08 ± 0.31 | 0.394 | ||
| 20:3n-6 | 0.26 ± 0.16 | rs1535 | 0.28 ± 0.16 | 0.25 ± 0.16 | 0.26 ± 0.16 | 0.630 |
| rs174448 | 0.24 ± 0.14 | 0.22 ± 0.14 | 0.28 ± 0.17 | 0.127 | ||
| 20:4n-6 | 0.36 ± 0.44 | rs1535 | 0.30 ± 0.23 | 0.42 ± 0.54 | 0.27 ± 0.22 | 0.151 |
| rs174448 | 0.33 ± 0.34 | 0.28 ± 0.25 | 0.40 ± 0.52 | 0.292 | ||
| 22:1n-9 | 0.84 ± 0.33 | rs1535 | 0.85 ± 0.29 | 0.82 ± 0.34 | 0.88 ± 0.33 | 0.604 |
| rs174448 | 0.74 ± 0.25 | 0.81 ± 0.34 | 0.87 ± 0.33 | 0.243 | ||
| 22:6n-3 | 0.39 ± 0.26 | rs1535 | 0.41 ± 0.33 | 0.39 ± 0.25 | 0.36 ± 0.22 | 0.683 |
| rs174448 | 0.36 ± 0.14 | 0.41 ± 0.35 | 0.38 ± 0.21 | 0.818 | ||
| Variable | β (SE) | p-Value |
|---|---|---|
| Genetic risk (high vs. low) | −0.28 (0.13) | 0.031 |
| Total DHA intake (low vs. high) | −0.45 (0.17) | 0.008 |
| Gene–diet interaction | 0.38 (0.17) | 0.032 |
| Age (year) | <0.01 (0.01) | 0.457 |
| BMI (kg/m2) | <0.01 (0.01) | 0.980 |
| Parity (multiparous vs. primiparous) | 0.01 (0.04) | 0.883 |
| Gestational weeks | 0.01 (0.02) | 0.716 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-C.; Lin, H.-C.; Liao, W.-L.; Tsai, Y.-Y.; Chen, A.-C.; Chen, H.-C.; Lin, H.-Y.; Liao, L.-N.; Chao, P.-M. FADS Genetic Variants in Taiwanese Modify Association of DHA Intake and Its Proportions in Human Milk. Nutrients 2020, 12, 543. https://doi.org/10.3390/nu12020543
Wu W-C, Lin H-C, Liao W-L, Tsai Y-Y, Chen A-C, Chen H-C, Lin H-Y, Liao L-N, Chao P-M. FADS Genetic Variants in Taiwanese Modify Association of DHA Intake and Its Proportions in Human Milk. Nutrients. 2020; 12(2):543. https://doi.org/10.3390/nu12020543
Chicago/Turabian StyleWu, Wen-Chieh, Hung-Chih Lin, Wen-Ling Liao, Yueh-Ying Tsai, An-Chyi Chen, Hsiang-Chun Chen, Hsiang-Yu Lin, Li-Na Liao, and Pei-Min Chao. 2020. "FADS Genetic Variants in Taiwanese Modify Association of DHA Intake and Its Proportions in Human Milk" Nutrients 12, no. 2: 543. https://doi.org/10.3390/nu12020543
APA StyleWu, W.-C., Lin, H.-C., Liao, W.-L., Tsai, Y.-Y., Chen, A.-C., Chen, H.-C., Lin, H.-Y., Liao, L.-N., & Chao, P.-M. (2020). FADS Genetic Variants in Taiwanese Modify Association of DHA Intake and Its Proportions in Human Milk. Nutrients, 12(2), 543. https://doi.org/10.3390/nu12020543





