Sarcopenia and Liver Cirrhosis—Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
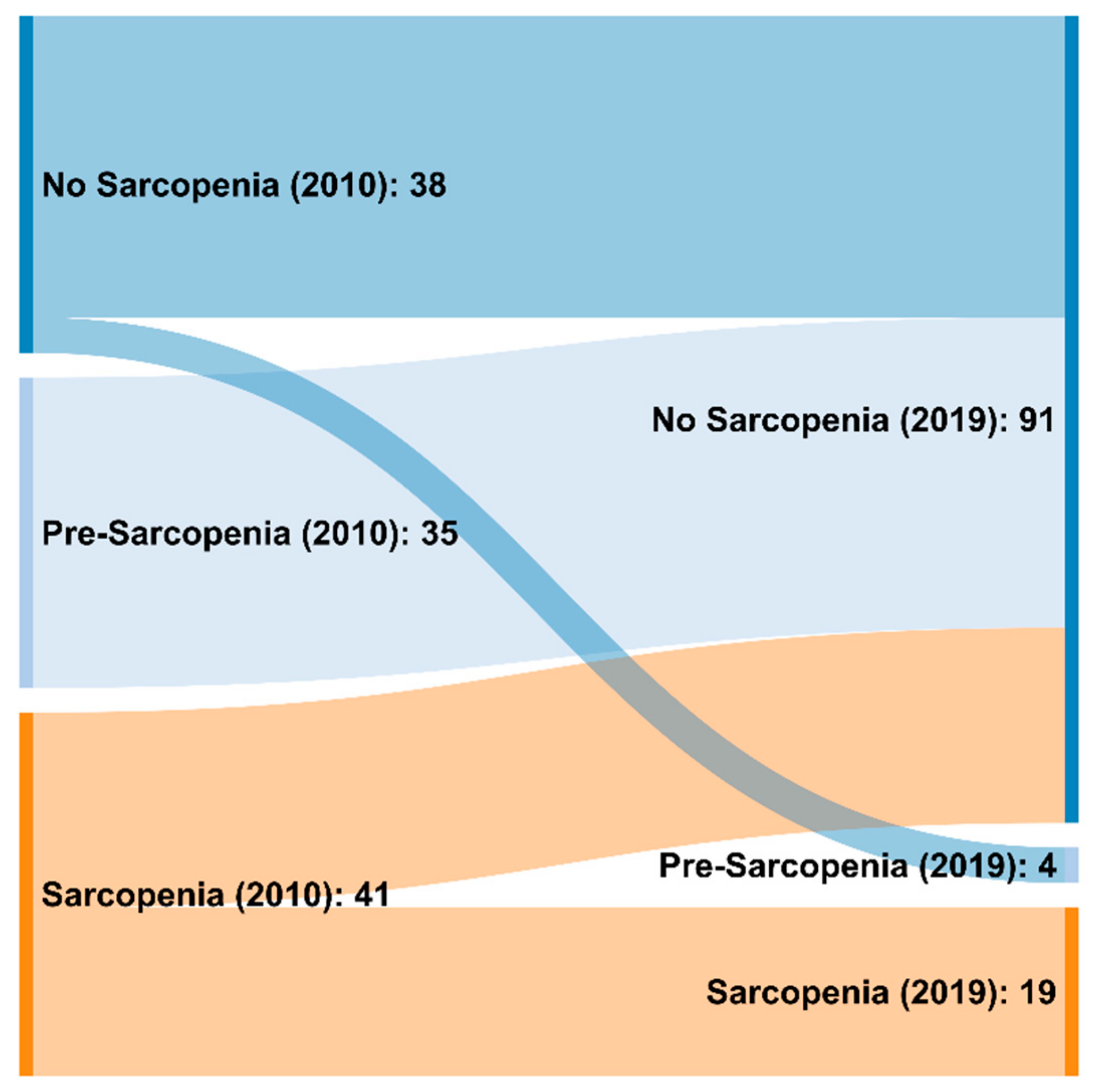
3.2. Frequency of Sarcopenia Applying the EWGSOP 2010 and 2019 Criteria
3.3. Individual Components of the EWGSOP Diagnostic Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2011, 4, 177–197. [Google Scholar]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, J. Skeletal muscle abnormalities and outcomes after liver transplantation. Liver Transplant. 2014, 20, 1293–1295. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Angulo, P.; Meza-Junco, J.; Prado, C.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia Sarcopenia Muscle 2016, 7, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A. European Working Group on Sarcopenia in Older People: Sarcopenia: European consensus on definition and diagnosis. Report of the European Workign Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Env. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173. [Google Scholar] [CrossRef]
- Schweitzer, L.; Geisler, C.; Pourhassan, M.; Braun, W.; Glüer, C.C.; Bosy-Westphal, A.; Müller, M.J. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015, 102, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transplant. 2012, 18, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017, 23, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427. [Google Scholar] [CrossRef]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: The longitudinal aging study amsterdam. J. Gerontol. Ser. A 2017, 73, 1199–1204. [Google Scholar] [CrossRef]
- van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef]
- Carey, E.J.; Lai, J.C.; Sonnenday, C.; Tapper, E.B.; Tandon, P.; Duarte-Rojo, A.; Dunn, M.A.; Tsien, C.; Kallwitz, E.R.; Ng, V.; et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology 2019, 70, 1816–1829. [Google Scholar] [CrossRef]
- Liver, E.A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar]
- Van Vugt, J.; Levolger, S.; de Bruin, R.W.; van Rosmalen, J.; Metselaar, H.J.; IJzermans, J.N. Systematic review and meta-analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am. J. Transplant. 2016, 16, 2277–2292. [Google Scholar] [CrossRef]
- Lee, K.; Shin, Y.; Huh, J.; Sung, Y.S.; Lee, I.S.; Yoon, K.H.; Kim, K.W. Recent Issues on Body Composition Imaging for Sarcopenia Evaluation. Korean J. Radiol. 2019, 20, 205–217. [Google Scholar] [CrossRef]
- Tandon, P.; Mourtzakis, M.; Low, G.; Zenith, L.; Ney, M.; Carbonneau, M.; Alaboudy, A.; Mann, S.; Esfandiari, N.; Ma, M. Comparing the Variability Between Measurements for Sarcopenia Using Magnetic Resonance Imaging and Computed Tomography Imaging. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016, 16, 2766. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Reiter, D.A.; Sekhar, A.; Sharma, P.; Safdar, N.M.; Patil, D.H.; Psutka, S.P.; Small, W.C.; Bilen, M.A.; Ogan, K.; et al. MRI quantitation of abdominal skeletal muscle correlates with CT-based analysis: Implications for sarcopenia measurement. Appl. Physiol. Nutr. Metab. 2019, 44, 814–819. [Google Scholar] [PubMed]
- da Silva, M.Z.C.; Vogt, B.P.; Reis, N.S.D.C.; Caramori, J.C.T. Update of the European consensus on sarcopenia: What has changed in diagnosis and prevalence in peritoneal dialysis? Eur. J. Clin. Nutr. 2019, 73(8), 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Chapman, B.; Hoermann, R.; Angus, P.W.; Testro, A.; Scodellaro, T.; Gow, P.J. Handgrip strength adds more prognostic value to the Model for End-Stage Liver Disease score than imaging-based measures of muscle mass in men with cirrhosis. Liver Transplant. 2019, 25, 1480–1487. [Google Scholar] [CrossRef]
- Davalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Guillen-Solà, A.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Escalada, F.; Duarte, E. Sarcopenia According to the Revised European Consensus on Definition and Diagnosis (EWGSOP2) Criteria Predicts Hospitalizations and Long-Term Mortality in Rehabilitation Patients With Stable Chronic Obstructive Pulmonary Disease. J Am. Med. Dir Assoc 2019, 20, 1047–1049. [Google Scholar] [CrossRef]
- Zhuang, C.L.; Shen, X.; Zou, H.B.; Dong, Q.T.; Cai, H.Y.; Chen, X.L.; Yu, Z.; Wang, S.L. EWGSOP2 versus EWGSOP1 for sarcopenia to predict prognosis in patients with gastric cancer after radical gastrectomy: Analysis from a large-scale prospective study. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- de Freitas, M.M.; de Oliveira, V.L.P.; Grassi, T.; Valduga, K.; Miller, M.E.P.; Schuchmann, R.A.; Souza, K.L.A.; de Azevedo, M.J.; Viana, L.V.; de Paula, T.P. Difference in sarcopenia prevalence and associated factors according to 2010 and 2018 European consensus (EWGSOP) in elderly patients with type 2 diabetes mellitus. Exp. Gerontol. 2020, 132, 110835. [Google Scholar] [CrossRef]
- Locquet, M.; Beaudart, C.; Petermans, J.; Reginster, J.Y.; Bruyère, O. EWGSOP2 versus EWGSOP1: Impact on the prevalence of sarcopenia and its major health consequences. J. Am. Med. Dir. Assoc. 2019, 20, 384–385. [Google Scholar] [CrossRef]
- Reiss, J.; Iglseder, B.; Alzner, R.; Mayr-Pirker, B.; Pirich, C.; Kässmann, H.; Kreutzer, M.; Dovjak, P.; Reiter, R. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing 2019, 48, 719–724. [Google Scholar] [CrossRef]
- Phu, S.; Vogrin, S.; Zanker, J.; Hassan, B.E.; Al Saedi, A.; Duque, G. Agreement between initial and revised European Working Group on Sarcopenia in older people definitions. J. Am. Med. Dir. Assoc. 2019, 20, 382–383. [Google Scholar]
- Yang, M.; Liu, Y.; Zuo, Y.; Tang, H.R. Sarcopenia for predicting falls and hospitalization in community-dwelling older adults: EWGSOP versus EWGSOP2. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. New versus old guidelines for sarcopenia classification: What is the impact on prevalence and health outcomes? Age Ageing 2019. [Google Scholar] [CrossRef] [PubMed]
- Saeki, C.; Takano, K.; Oikawa, T.; Aoki, Y.; Tomoya Kanai, T.; Takakura, K.; Nakano, M.; Torisu, Y.; Sasaki, N.; Abo, M.; et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet. Disord. 2019, 20, 615. [Google Scholar] [CrossRef]
- Yang, L.; Yao, X.; Shen, J.; Sun, G.; Sun, Q.; Tian, X.; Li, X.; Li, X.; Ye, L.; Zhang, Z.; et al. Comparison of revised EWGSOP criteria and four other diagnostic criteria of sarcopenia in Chinese community-dwelling elderly residents. Exp. Gerontol. 2020, 130, 110798. [Google Scholar] [CrossRef] [PubMed]

| Stage | 2010 | 2019 |
|---|---|---|
| Pre-sarcopenia | ⇓ Mass | ⇓ Strength ² |
| Sarcopenia | ⇓ Mass and ⇓ Strength 1 or ⇓ Function | ⇓ Strength ² and ⇓ Mass or ⇓ Quality |
| Severe Sarcopenia | ⇓ Mass and ⇓ Strength 1 and ⇓ Function | ⇓ Strength ² and ⇓ Mass and ⇓ Function |
| Characteristics | |
|---|---|
| Age (year, range) | 65 (61.87–65.97) |
| Sex m/w n (%) | 86/28 (75.4/24.6) |
| BMI, kg/m2 | 26.83 (26.07–27.95) |
| Child A/B/C n (%) | 51/43/20 (44.7/37.7/17.6) |
| MELD | 10.8 (11.54–13.49) |
| Aetiology: HCV/Alcohol/NASH/Others (%) | 17 (14.9)/66 (57.9)/22 (19.3)/9 (7.9) |
| HCC n (%) | 54 (47.4) |
| Diabetes n (%) | 47 (41.2) |
| Bilirubin | 1.21 (1.89–3.28 |
| Albumin | 3.5 (3.37–3.47) |
| PZ-INR | 1.23 (1.24–1.38) |
| Creatinine | 0.9 (0.89–1.09) |
| Gender | BMI | Hand Grip Strength (kg) |
|---|---|---|
| male | ≤24.0 24.1–26.0 26.1–28.0 >28.0 | ≤29.0 ≤30.0 ≤30.0 ≤32.0 |
| female | ≤23.0 23.1–26.0 26.1–29.0 >29.0 | ≤17.0 ≤17.3 ≤18.0 ≤21.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traub, J.; Bergheim, I.; Eibisberger, M.; Stadlbauer, V. Sarcopenia and Liver Cirrhosis—Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients 2020, 12, 547. https://doi.org/10.3390/nu12020547
Traub J, Bergheim I, Eibisberger M, Stadlbauer V. Sarcopenia and Liver Cirrhosis—Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients. 2020; 12(2):547. https://doi.org/10.3390/nu12020547
Chicago/Turabian StyleTraub, Julia, Ina Bergheim, Martin Eibisberger, and Vanessa Stadlbauer. 2020. "Sarcopenia and Liver Cirrhosis—Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019" Nutrients 12, no. 2: 547. https://doi.org/10.3390/nu12020547
APA StyleTraub, J., Bergheim, I., Eibisberger, M., & Stadlbauer, V. (2020). Sarcopenia and Liver Cirrhosis—Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients, 12(2), 547. https://doi.org/10.3390/nu12020547







