The Effects of Early Life Stress, Postnatal Diet Modulation, and Long-Term Western-Style Diet on Later-Life Metabolic and Cognitive Outcomes
Abstract
:1. Introduction
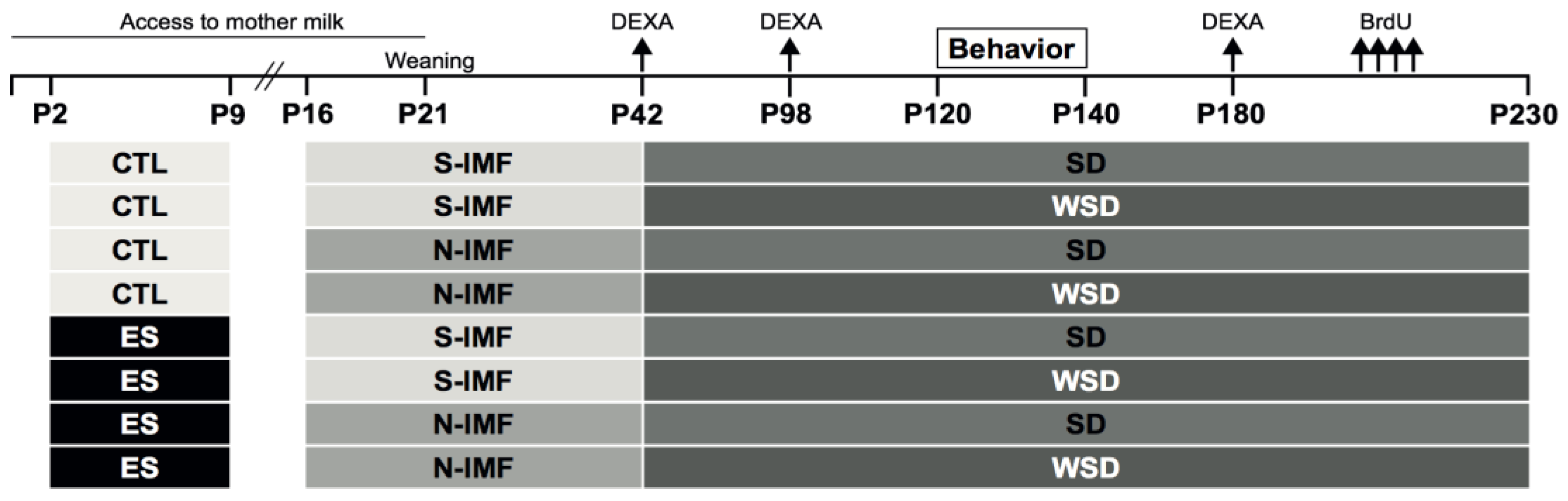
2. Materials and Methods
2.1. Animals and Breeding
2.2. ES Paradigm
2.3. Experimental Diets
2.4. Body Composition
2.5. Behavioral Testing
2.6. BrdU Injections
2.7. Tissue Collection
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
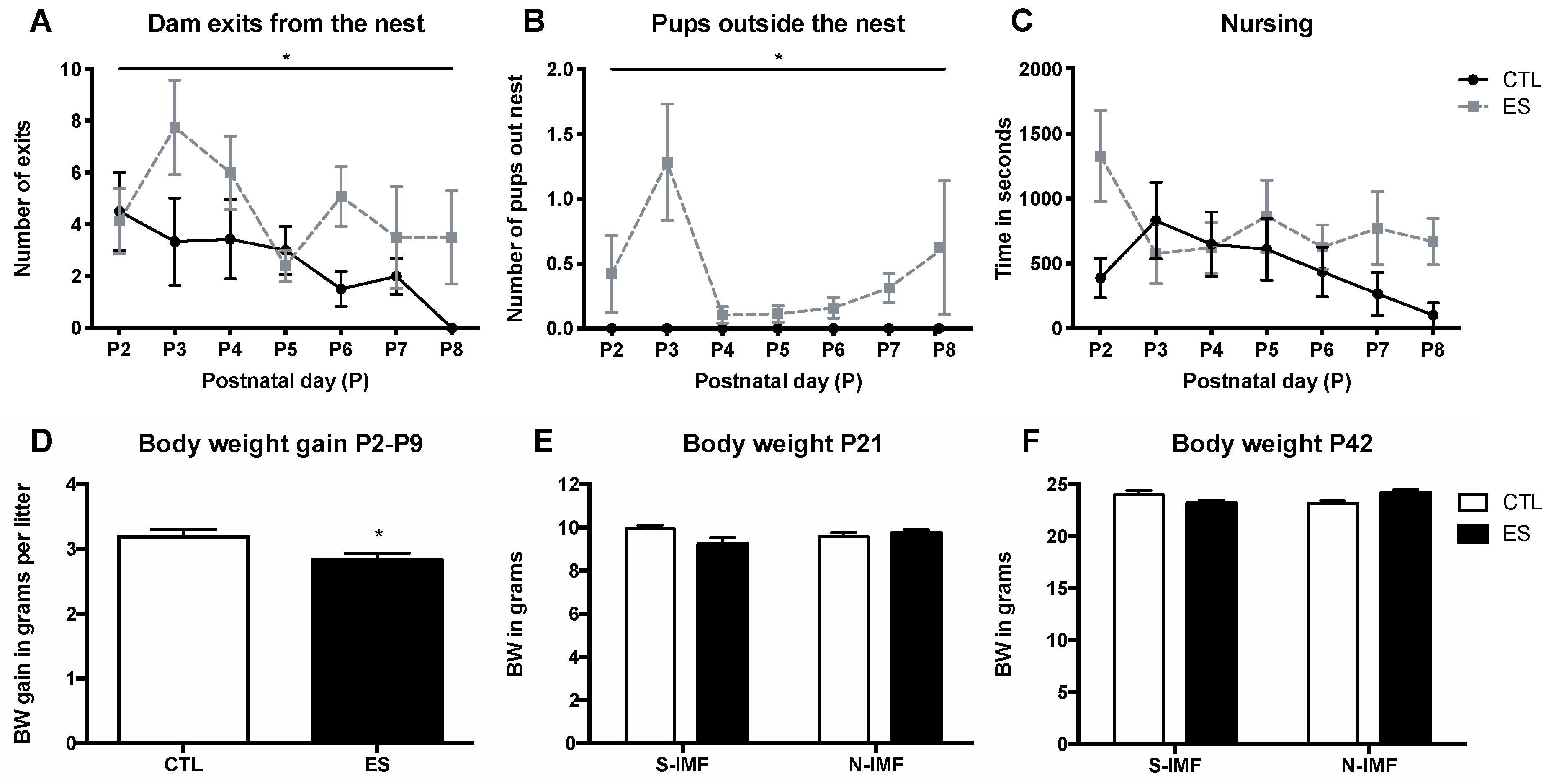
3.1. ES Leads to Fragmented Maternal Care and Reduced BW Gain in Pups
3.2. ES and Postnatal Diet do not alter WSD-Induced Body Composition Changes
3.3. Prolonged WSD Results in an Obesogenic Phenotype in Adulthood that is not Modulated by ES or Postnatal Diet
3.4. Cognitive Function is not Altered by ES, IMF, or WSD Exposure
3.5. N-IMF and WSD Modulate ES-induced Effects on Neurogenesis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farr, O.M.; Ko, B.J.; Joung, K.E.; Zaichenko, L.; Usher, N.; Tsoukas, M.; Thakkar, B.; Davis, C.R.; Crowell, J.A.; Mantzoros, C.S. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Nemeroff, C.B. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 2016, 89, 892–909. [Google Scholar] [CrossRef] [Green Version]
- Roseboom, T.; de Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef]
- Alciati, A.; Gesuele, F.; Casazza, G.; Foschi, D. The Relationship between Childhood Parental Loss and Metabolic Syndrome in Obese Subjects. Stress Health 2011, 29, 5–13. [Google Scholar] [CrossRef]
- Saleh, A.; Potter, G.G.; McQuoid, D.R.; Boyd, B.; Turner, R.; MacFall, J.R.; Taylor, W.D. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 2017, 47, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Pesonen, A.-K.; Eriksson, J.G.; Heinonen, K.; Kajantie, E.; Tuovinen, S.; Alastalo, H.; Henriksson, M.; Leskinen, J.; Osmond, C.; Barker, D.J.P.; et al. Cognitive ability and decline after early life stress exposure. Neurobiol. Aging 2013, 34, 1674–1679. [Google Scholar] [CrossRef]
- Mandelli, L.; Petrelli, C.; Serretti, A. The role of specific early trauma in adult depression: A meta-analysis of published literature. Childhood trauma and adult depression. Eur. Psychiatry 2015, 30, 665–680. [Google Scholar] [CrossRef]
- Francis, H.; Stevenson, R. The longer-term impacts of Western diet on human cognition and the brain. Appetite 2013, 63, 119–128. [Google Scholar] [CrossRef]
- Myles, I.A. Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutr. J. 2014, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Apovian, C.M. The Obesity Epidemic--Understanding the Disease and the Treatment. N. Engl. J. Med. 2016, 374, 177–179. [Google Scholar] [CrossRef]
- Moussa, H.N.; Alrais, M.A.; Leon, M.G.; Abbas, E.L.; Sibai, B.M. Obesity epidemic: Impact from preconception to postpartum. Future Sci. OA 2016, 2, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Zobel, E.H.; Hansen, T.W.; Rossing, P.; von Scholten, B.J. Global Changes in Food Supply and the Obesity Epidemic. Curr. Obes. Rep. 2016, 5, 449–455. [Google Scholar] [CrossRef]
- Valladolid-Acebes, I.; Stucchi, P.; Cano, V.; Fernández-Alfonso, M.S.; Merino, B.; Gil-Ortega, M.; Fole, A.; Morales, L.; Ruiz-Gayo, M.; Del Olmo, N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol. Learn. Mem. 2011, 95, 80–85. [Google Scholar] [CrossRef]
- Ross, A.P.; Bartness, T.J.; Mielke, J.G.; Parent, M.B. A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn. Mem. 2009, 92, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Paternain, L.; la Garza, D.A.L.; Batlle, M.A.; Milagro, F.I.; Martínez, J.A.; Campión, J. Prenatal stress increases the obesogenic effects of a high-fat-sucrose diet in adult rats in a sex-specific manner. Stress 2013, 16, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Paternain, L.; Batlle, M.A.; la Garza, D.A.L.; Milagro, F.I.; Martínez, J.A.; Campión, J. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology 2012, 96, 249–260. [Google Scholar] [CrossRef]
- Tamashiro, K.L.K.; Terrillion, C.E.; Hyun, J.; Koenig, J.I.; Moran, T.H. Prenatal Stress or High-Fat Diet Increases Susceptibility to Diet-Induced Obesity in Rat Offspring. Diabetes 2009, 58, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Yam, K.Y.; Naninck, E.F.G.; Abbink, M.R.; la Fleur, S.E.; Schipper, L.; van den Beukel, J.C.; Grefhorst, A.; Oosting, A.; van der Beek, E.M.; Lucassen, P.J.; et al. Exposure to chronic early-life stress lastingly alters the adipose tissue, the leptin system and changes the vulnerability to western-style diet later in life in mice. Psychoneuroendocrinology 2017, 77, 186–195. [Google Scholar] [CrossRef]
- Patel, M.S.; Srinivasan, M.; Laychock, S.G. Metabolic programming: Role of nutrition in the immediate postnatal life. J. Inherit. Metab. Dis. 2009, 32, 218–228. [Google Scholar] [CrossRef]
- Koletzko, B. Early nutrition and its later consequences: New opportunities. Adv. Exp. Med. Biol. 2005, 569, 1–12. [Google Scholar] [PubMed]
- Fernandez-Twinn, D.S.; Ozanne, S.E. Early life nutrition and metabolic programming. Ann. N. Y. Acad. Sci. 2010, 1212, 78–96. [Google Scholar] [CrossRef]
- Naninck, E.F.G.; Oosterink, J.E.; Yam, K.-Y.; de Vries, L.P.; Schierbeek, H.; van Goudoever, J.B.; Verkaik-Schakel, R.-N.; Plantinga, J.A.; Plosch, T.; Lucassen, P.J.; et al. Early micronutrient supplementation protects against early stress–induced cognitive impairments. Faseb J. 2017, 31, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Yam, K.-Y.; Schipper, L.; Reemst, K.; Ruigrok, S.R.; Abbink, M.R.; Hoeijmakers, L.; Naninck, E.F.G.; Zarekiani, P.; Oosting, A.; van der Beek, E.M.; et al. Increasing availability of ω-3 fatty acid in the early-life diet prevents the early-life stress-induced cognitive impairments without affecting metabolic alterations. FASEB J. 2019, 33, 5729–5740. [Google Scholar] [CrossRef]
- Ryan, A.S. Breastfeeding and the risk of childhood obesity. Coll Antropol. 2007, 31, 19–28. [Google Scholar]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am. J. Clin. Nutr. 2006, 84, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Dewey, K.G. Is Breastfeeding Protective Against Child Obesity? J. Hum. Lact. 2016, 19, 9–18. [Google Scholar] [CrossRef]
- Belfort, M.B.; Rifas-Shiman, S.L.; Kleinman, K.P.; Guthrie, L.B.; Bellinger, D.C.; Taveras, E.M.; Gillman, M.W.; Oken, E. Infant Feeding and Childhood Cognition at Ages 3 and 7 Years. JAMA Pediatr. 2013, 167, 836–839. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Park, H.; Ha, E.; Hong, Y.-C.; Ha, M.; Park, H.; Kim, B.-N.; Lee, B.; Lee, S.-J.; Lee, K.Y.; et al. Effect of Breastfeeding Duration on Cognitive Development in Infants: 3-Year Follow-up Study. J. Korean Med. Sci. 2016, 31, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T.; et al. Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group Breastfeeding and child cognitive development: New evidence from a large randomized trial. Arch. Gen. Psychiatry 2008, 65, 578–584. [Google Scholar] [CrossRef]
- Spitsberg, V.L. Invited Review: Bovine Milk Fat Globule Membrane as a Potential Nutraceutical. J. Dairy Sci. 2005, 88, 2289–2294. [Google Scholar] [CrossRef]
- Lopez, C.; Ménard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B Biointerfaces 2011, 83, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.C.; Briard, V.; Michel, F.; Tasson, F.; Poulain, P. Size Distribution of Fat Globules in Human Colostrum, Breast Milk, and Infant Formula. J. Dairy Sci. 2005, 88, 1927–1940. [Google Scholar] [CrossRef] [Green Version]
- Gallier, S.; Vocking, K.; Post, J.A.; Van De Heijning, B.; Acton, D.; van der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surfaces B Biointerfaces 2015, 136, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Oosting, A.; Kegler, D.; Wopereis, H.J.; Teller, I.C.; van de Heijning, B.J.M.; Verkade, H.J.; van der Beek, E.M. Size and phospholipid coating of lipid droplets in the diet of young mice modify body fat accumulation in adulthood. Pediatr. Res. 2012, 72, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Baars, A.; Oosting, A.; Engels, E.; Kegler, D.; Kodde, A.; Schipper, L.; Verkade, H.J.; van der Beek, E.M. Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood. Br. J. Nutr. 2016, 115, 1930–1937. [Google Scholar] [CrossRef] [Green Version]
- Schipper, L.; van Dijk, G.; Broersen, L.M.; Loos, M.; Bartke, N.; Scheurink, A.J.; van der Beek, E.M. A Postnatal Diet Containing Phospholipids, Processed to Yield Large, Phospholipid-Coated Lipid Droplets, Affects Specific Cognitive Behaviors in Healthy Male Mice. J. Nutr. 2016, 146, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Naninck, E.F.G.; Hoeijmakers, L.; Kakava-Georgiadou, N.; Meesters, A.; Lazic, S.E.; Lucassen, P.J.; Korosi, A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 2015, 25, 309–328. [Google Scholar] [CrossRef]
- Hueston, C.M.; Cryan, J.F.; Nolan, Y.M. Adolescent social isolation stress unmasks the combined effects of adolescent exercise and adult inflammation on hippocampal neurogenesis and behavior. NSC 2017, 365, 226–236. [Google Scholar] [CrossRef]
- Abbink, M.R.; Naninck, E.F.G.; Lucassen, P.J.; Korosi, A. Early-life stress diminishes the increase in neurogenesis after exercise in adult female mice. Hippocampus 2017, 27, 839–844. [Google Scholar] [CrossRef]
- Korosi, A.; Naninck, E.F.G.; Oomen, C.A.; Schouten, M.; Krugers, H.; Fitzsimons, C.; Lucassen, P.J. Early-life stress mediated modulation of adult neurogenesis and behavior. Behav. Brain Res. 2012, 227, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lajud, N.; Torner, L. Early life stress and hippocampal neurogenesis in the neonate: Sexual dimorphism, long term consequences and possible mediators. Front. Mol. Neurosci. 2015, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loi, M.; Koricka, S.; Lucassen, P.J.; Joels, M. Age- and sex-dependent effects of early life stress on hippocampal neurogenesis. Front. Endocrinol. 2014, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. NSC 2002, 112, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Park, H.R.; Park, M.; Choi, J.; Park, K.-Y.; Chung, H.Y.; Lee, J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef]
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A Novel Mouse Model for Acute and Long-Lasting Consequences of Early Life Stress. Endocrinology 2008, 149, 4892–4900. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Oosting, A.; van Vlies, N.; Kegler, D.; Schipper, L.; Abrahamse-Berkeveld, M.; Ringler, S.; Verkade, H.J.; van der Beek, E.M. Effect of dietary lipid structure in early postnatal life on mouse adipose tissue development and function in adulthood. Br. J. Nutr. 2014, 111, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Jou, W.; Gavrilova, O.; Hall, K.D. Persistent Diet-Induced Obesity in Male C57BL/6 Mice Resulting from Temporary Obesigenic Diets. PLoS ONE 2009, 4, e5370–e5379. [Google Scholar] [CrossRef] [Green Version]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Ronda, O.A.H.O.; van de Heijning, B.J.M.; de Bruin, A.; Jurdzinski, A.; Kuipers, F.; Verkade, H.J. Programming effects of an early-life diet containing large phospholipid-coated lipid globules are transient under continuous exposure to a high-fat diet. BJN 2019, 122, 1321–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcego, D.M.; Krolow, R.; Lampert, C.; Toniazzo, A.P.; Berlitz, C.; Lazzaretti, C.; Schmitz, F.; Rodrigues, A.F.; Wyse, A.T.S.; Dalmaz, C. Early life adversities or high fat diet intake reduce cognitive function and alter BDNF signaling in adult rats: Interplay of these factors changes these effects. Int. J. Dev. Neurosci. 2016, 50, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, C.H.; Narahari, T.; Holland, F.H.; Lee, H.-N.; Murthy, S.K.; Brenhouse, H.C. Effects of early adolescent environmental enrichment on cognitive dysfunction, prefrontal cortex development, and inflammatory cytokines after early life stress. Dev. Psychobiol. 2015, 58, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hou, C.; Ma, N.; Liu, J.; Zhang, Y.; Zhou, J.; Xu, L.; Li, L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol. Learn. Mem. 2007, 87, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Dandi, Ε.; Kalamari, A.; Touloumi, O.; Lagoudaki, R.; Nousiopoulou, E.; Simeonidou, C.; Spandou, E.; Tata, D.A. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. Int. J. Dev. Neurosci. 2018, 67, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Bredy, T.W.; Humpartzoomian, R.A.; Cain, D.P.; Meaney, M.J. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 2003, 118, 571–576. [Google Scholar] [CrossRef]
- Kosari, S.; Badoer, E.; Nguyen, J.C.D.; Killcross, A.S.; Jenkins, T.A. Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav. Brain Res. 2012, 235, 98–103. [Google Scholar] [CrossRef]
- Lavin, D.N.; Joesting, J.J.; Chiu, G.S.; Moon, M.L.; Meng, J.; Dilger, R.N.; Freund, G.G. Fasting Induces an Anti-Inflammatory Effect on the Neuroimmune System Which a High-Fat Diet Prevents. Obesity 2009, 19, 1586–1594. [Google Scholar] [CrossRef]
- Tucker, K.R.; Godbey, S.J.; Thiebaud, N.; Fadool, D.A. Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity. Physiol. Behav. 2012, 107, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Kesby, J.P.; Kim, J.J.; Scadeng, M.; Woods, G.; Kado, D.M.; Olefsky, J.M.; Jeste, D.V.; Achim, C.L.; Semenova, S. Spatial Cognition in Adult and Aged Mice Exposed to High-Fat Diet. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valladolid-Acebes, I.; Fole, A.; Martín, M.; Morales, L.; Cano, M.V.; Ruiz-Gayo, M.; del Olmo, N. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol. Learn. Mem. 2013, 106, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, J.; Tang, Y.; Liu, J.; Han, T.; Han, S.; Li, H.; Hou, C.; Liu, J.; Long, J. High-Fat-Diet-Induced Weight Gain Ameliorates Bone Loss without Exacerbating AβPP Processing and Cognition in Female APP/PS1 Mice. Front. Cell Neurosci. 2014, 8, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordner, Z.A.; Tamashiro, K.L.K. Effects of high-fat diet exposure on learning & memory. Physiol. Behav. 2015, 152, 363–371. [Google Scholar] [PubMed] [Green Version]
- Camer, D.; Yu, Y.; Szabo, A.; Fernandez, F.; Dinh, C.H.L.; Huang, X.-F. Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 59, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, A.N.; Gomes, S.M.; Shukitt-Hale, B. Blueberry Supplementation Improves Memory in Middle-Aged Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2014, 62, 3972–3978. [Google Scholar] [CrossRef]
- Heyward, F.D.; Walton, R.G.; Carle, M.S.; Coleman, M.A.; Garvey, W.T.; Sweatt, J.D. Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol. Learn. Mem. 2012, 98, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.E.; Lucki, I.; Brookshire, B.R.; Carlson, G.C.; Browne, C.A.; Kazi, H.; Bang, S.; Choi, B.-R.; Chen, Y.; McMullen, M.F.; et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol. Dis. 2014, 67, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Lépinay, A.L.; Larrieu, T.; Joffre, C.; Acar, N.; Gárate, I.; Castanon, N.; Ferreira, G.; Langelier, B.; Guesnet, P.; Brétillon, L.; et al. Perinatal high-fat diet increases hippocampal vulnerability to the adverse effects of subsequent high-fat feeding. Psychoneuroendocrinology 2015, 53, 82–93. [Google Scholar] [CrossRef]
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Krägeloh, C.U.; Breier, B.H.; Davison, M.; Fong, B.; Norris, C.; McJarrow, P.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, M.; Lönnerdal, B.; Hernell, O. Supplementation of Infant Formula with Bovine Milk Fat Globule Membranes. Adv. Nutr. 2017, 8, 351–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, L.; Harvey, L.; van der Beek, E.M.; van Dijk, G. Home alone: A systematic review and meta-analysis on the effects of individual housing on body weight, food intake and visceral fat mass in rodents. Obes. Rev. 2018, 19, 614–637. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, H.T.J.; Novati, A.; Sgoifo, A.; Luiten, P.G.M.; den Boer, J.A.; Meerlo, P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav. Brain Res. 2011, 216, 552–560. [Google Scholar] [CrossRef]
- Gobinath, A.R.; Workman, J.L.; Chow, C.; Lieblich, S.E.; Galea, L.A.M. Sex-dependent effects of maternal corticosterone and SSRI treatment on hippocampal neurogenesis across development. Biol. Sex Differ. 2017, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Rivera, P.; Romero-Zerbo, Y.; Pavón, F.J.; Serrano, A.; López-Ávalos, M.-D.; Cifuentes, M.; Grondona, J.-M.; Bermúdez-Silva, F.-J.; Fernández-Llebrez, P.; de Fonseca, F.R.; et al. Obesity-dependent cannabinoid modulation of proliferation in adult neurogenic regions. Eur. J. Neurosci. 2011, 33, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Layé, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Kalavagunta, P.K.; Huang, Q.; Lv, W.; An, X.; Chen, H.; Wang, T.; Heriniaina, R.M.; Qiao, T.; et al. Normal diet Vs High fat diet-A comparative study: Behavioral and neuroimmunological changes in adolescent male mice. Metab. Brain Dis. 2018, 33, 177–190. [Google Scholar] [CrossRef]
- Murata, Y.; Narisawa, Y.; Shimono, R.; Ohmori, H.; Mori, M.; Ohe, K.; Mine, K.; Enjoji, M. A high fat diet-induced decrease in hippocampal newly-born neurons of male mice is exacerbated by mild psychological stress using a Communication Box. J. Affect. Disord. 2017, 209, 209–216. [Google Scholar] [CrossRef]
- Knobloch, M.; Braun, S.M.G.; Zurkirchen, L.; von Schoultz, C.; Zamboni, N.; Araúzo-Bravo, M.J.; Kovacs, W.J.; Karalay, Ö.; Suter, U.; Machado, R.A.C.; et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 2013, 493, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Maniam, J.; Antoniadis, C.P.; Le, V.; Morris, M.J. A diet high in fat and sugar reverses anxiety-like behaviour induced by limited nesting in male rats: Impacts on hippocampal markers. Psychoneuroendocrinology 2016, 68, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Maniam, J.; Antoniadis, C.P.; Wang, K.W.; Morris, M.J. Early Life Stress Induced by Limited Nesting Material Produces Metabolic Resilience in Response to a High-Fat and High-Sugar Diet in Male Rats. Front. Endocrinol. 2015, 6, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniam, J.; Morris, M.J. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology 2010, 35, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Maniam, J.; Morris, M.J. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology 2010, 35, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef]
- Nederhof, E.; Schmidt, M.V. Mismatch or cumulative stress: Toward an integrated hypothesis of programming effects. Physiol. Behav. 2012, 106, 691–700. [Google Scholar] [CrossRef]
- McJarrow, P.; Schnell, N.; Jumpsen, J.; Clandinin, T. Influence of dietary gangliosides on neonatal brain development. Nutr. Rev. 2009, 67, 451–463. [Google Scholar] [CrossRef]
- Bourlieu, C.; Michalski, M.-C. Structure–function relationship of the milk fat globule. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 118–127. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbink, M.R.; Schipper, L.; Naninck, E.F.G.; de Vos, C.M.H.; Meier, R.; van der Beek, E.M.; Lucassen, P.J.; Korosi, A. The Effects of Early Life Stress, Postnatal Diet Modulation, and Long-Term Western-Style Diet on Later-Life Metabolic and Cognitive Outcomes. Nutrients 2020, 12, 570. https://doi.org/10.3390/nu12020570
Abbink MR, Schipper L, Naninck EFG, de Vos CMH, Meier R, van der Beek EM, Lucassen PJ, Korosi A. The Effects of Early Life Stress, Postnatal Diet Modulation, and Long-Term Western-Style Diet on Later-Life Metabolic and Cognitive Outcomes. Nutrients. 2020; 12(2):570. https://doi.org/10.3390/nu12020570
Chicago/Turabian StyleAbbink, Maralinde R., Lidewij Schipper, Eva F.G. Naninck, Cato M.H. de Vos, Romy Meier, Eline M. van der Beek, Paul J. Lucassen, and Aniko Korosi. 2020. "The Effects of Early Life Stress, Postnatal Diet Modulation, and Long-Term Western-Style Diet on Later-Life Metabolic and Cognitive Outcomes" Nutrients 12, no. 2: 570. https://doi.org/10.3390/nu12020570
APA StyleAbbink, M. R., Schipper, L., Naninck, E. F. G., de Vos, C. M. H., Meier, R., van der Beek, E. M., Lucassen, P. J., & Korosi, A. (2020). The Effects of Early Life Stress, Postnatal Diet Modulation, and Long-Term Western-Style Diet on Later-Life Metabolic and Cognitive Outcomes. Nutrients, 12(2), 570. https://doi.org/10.3390/nu12020570




