Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Protocol
2.2. Permeability Assay
2.3. Co-culture Model
2.4. Magnesium Assay Kit
2.5. Cell Viability
2.6. ROS Production
2.7. NO Production
2.8. Intracellular Magnesium
2.9. [Ca2+] Measurement
2.10. Western Blot of Cell Lysates
2.11. Animal Model
2.12. Uterine Isometric Contractility Assay
2.13. Statistical Analysis
3. Results
3.1. Dose–response and Time-dependent Study of Cell Viability on Caco-2 Cells
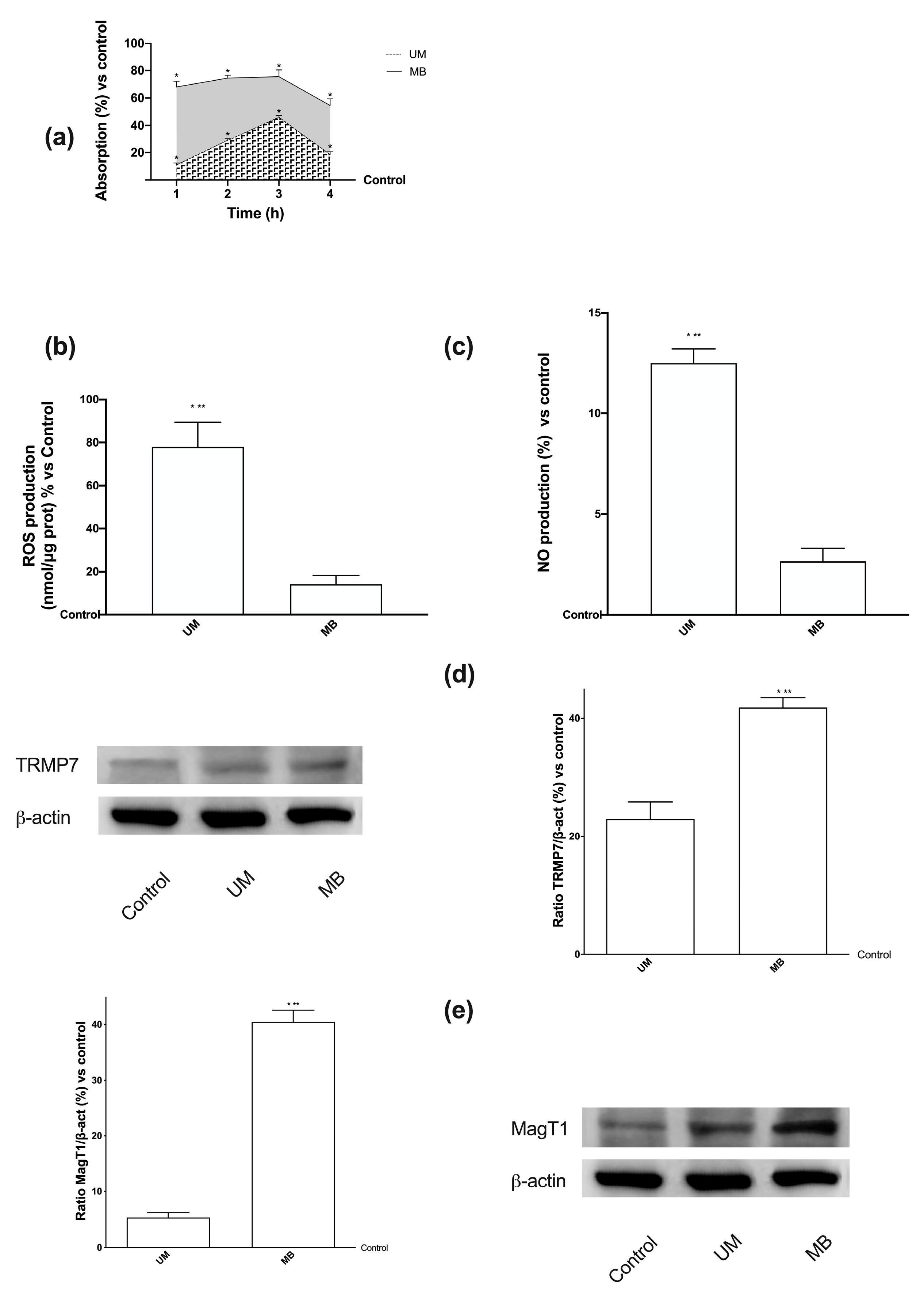
3.2. Time-dependent Permeability after Stimulations of Caco-2 Cells with UM and MB
3.3. Analysis of Permeabilization Mechanism on Caco-2 Cells Treated with UM and MB
3.4. Magnesium Flux and Its Relative Intracellular Pathways Activated in Caco-2 Cells
3.5. Dose–response and Time-dependent Studies on Cell Viability in PHM1-41 Cells
3.6. Time-dependent Effects of UM and MB in PHM1-41
3.7. Relaxing Effects of UM and MB on PHM1-41
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Method: Cell Viability
Appendix A.2. Method: ROS Production
Appendix A.3. Method: NO Production
Appendix B
| UM | MB | |||||
|---|---|---|---|---|---|---|
| Time, h | Means ± SD | Means ± SD | Means ± SD | Means ± SD | Means ± SD | Means ± SD |
| 1 mM | 2.5 mM | 5 mM | 1 mM | 2.5 mM | 5 mM | |
| 1 | * 7.83 ± 0.76 | * 17.00 ± 6.24 | * 12.50 ± 4.95 | * 27.30 ± 1.87 | * 19.80 ± 1.65 | * 10.57 ± 0.33 |
| 2 | * 27.00 ± 2.65 | * 25.33 ± 5.03 | * 11.00 ± 1.41 | * 40.40 ± 8.64 | * 19.33 ± 2.08 | * 17.70 ± 1.98 |
| 3 | * 16.33 ± 4.51 | * 18.00 ± 5.57 | 4.50 ± 4.95 | * 40.75 ± 5.21 | * 15.67 ± 5.51 | * 12.50 ± 0.71 |
| 6 | 4.00 ± 2.00 | * 15.33 ± 1.15 | 3.00 ± 1.41 | * 15.55 ± 0.67 | * 13.67 ± 3.06 | 5.00 ± 2.83 |
| 24 | 0.30 ± 0.20 | 0.47 ± 0.15 | 0.25 ± 0.21 | 0.33 ± 0.15 | 0.57 ± 0.06 | 0.35 ± 0.21 |
| UM 1mM | MB 1mM | |
|---|---|---|
| Time, h | Means ± SD | Means ± SD |
| 1 | * 22.2 ± 1.15 | * 52.4 ± 0.91 |
| 2 | * 61 ± 4.29 | * 83.20 ± 1.12 |
| 3 | * 75.6 ± 3.39 | * 102.1 ± 1.45 |
| 6 | * 70.7 ± 3.25 | * 90 ± 2.69 |
| 24 | * 53 ± 2.89 | * 81.2 ± 1.13 |
References
- Wolf, F.I.; Trapani, V. Cell (patho)physiology of magnesium. Clin. Sci. 2008, 114, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Nejsum, L.N.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M. Does magnesium have a role in the treatment of patients with coronary artery disease? Am. J. Cardiovasc. Drugs 2003, 3, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sontia, B.; Touyz, R.M. Role of magnesium in hypertension. Arch. Biochem. Biophys. 2007, 458, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Farruggia, G.; Castiglioni, S.; Sargenti, A.; Marraccini, C.; Cazzaniga, A.; Merolle, L.; Iotti, S.; Cappadone, C.; Maier, J.A.M. Effects of supplementation with different Mg salts in cells: Is there a clue? Magnes. Res. 2014, 27, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Gregory, J.; Foster, K.; Tyler, H.; Wiseman, M. The Dietary and Nutritional Survey of British Adult; HMSO: London, UK, 1990. [Google Scholar]
- Schroeder, H.A.; Nason, A.P.; Tipton, I.H. Essential metals in man. Magnesium. J. Chronic Dis. 1969, 21, 815–841. [Google Scholar] [CrossRef]
- Gouadon, E.; Lecerf, F.; German-Fattal, M. Differential effects of cyclosporin A and tacrolimus on magnesium influx in Caco-2 cells. J. Pharm. Pharm. Sci. 2012, 15, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.; Droogmans, G.; Bindels, R.J.; Hoenderop, J.G. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteilh-Zoller, L.W.; Yue, L.; Clapham, D.E. TRP- PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Yue, L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7-Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 2007, 1772, 813–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quamme, G.A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008, 24, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.; Nechifor, M. Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; Chapter 2. [Google Scholar]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genom. 2005, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Clapham, D.E. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc. Natl. Acad. Sci. USA 2009, 106, 15750–15755. [Google Scholar] [CrossRef] [Green Version]
- Quamme, G.A. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 2010, 298, C407–C429. [Google Scholar] [CrossRef] [Green Version]
- Karaki, H. Magnesium as a modifier of smooth muscle contractility. Microcirc. Endothel. Lymphat. 1989, 5, 77–97. [Google Scholar]
- Tica, V.I.; Tica, A.A.; Carlig, V.; Banica, O.S. Magnesium ion inhibits spontaneous and induced contractions of isolated uterine muscle. Gynecol. Endocrinol. 2007, 23, 368–372. [Google Scholar] [CrossRef]
- Robin, P.; Boulven, I.; Bôle-Feysot, C.; Tanfin, Z.; Leiber, D. Contribution of PKC-dependent and -independent processes in temporal ERK regulation by ET-1, PDGF, and EGF in rat myometrial cells. Am. J. Physiol. Cell Physiol. 2004, 286, C798–C806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adebiyi, A.; Adaikan, P.G.; Prasad, R.N. Effect of benzyl isothiocyanate on spontaneous and induced force of rat uterine contraction. Pharmacol. Res. 2004, 49, 415. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, E.; Macun, H.C.; Çınar, M.; Yiğit, A.A.; Üstüner, I.; Aydos, T.R.; Aktuna, Z. The effects of magnesium sulphate on the contractile activity of uterus in an animal model of preeclampsia. Clin. Exp. Obstet. Gynecol. 2014, 41, 169–173. [Google Scholar] [PubMed]
- Novaković, R.; Radunović, N.; Marković-Lipkovski, J.; Ćirović, S.; Beleslin-Čokić, B.; Ilić, B.; Ivković, B.; Heinle, H.; Živanović, V.; Gojković-Bukarica, L.J. Effects of the polyphenol resveratrol on contractility of human term pregnant myometrium. Mol. Hum. Reprod. 2015, 21, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemancewicz, A.; Laudańska, H.; Laudański, T.; Karpiuk, A.; Batra, S. Permeability of fetal membranes to calcium and magnesium: Possible role in preterm labour. Hum. Reprod. 2000, 15, 2018–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yu, Y.; Shi, Y.; Fazli, L.; Slater, D.; Lye, S.; Dong, X. HoxA13 Stimulates Myometrial Cells to Secrete IL-1β and Enhance the Expression of Contraction-Associated Proteins. Endocrinology 2016, 157, 2129–2139. [Google Scholar] [CrossRef]
- Miyoshi, H.; Yamaoka, K.; Garfield, R.E.; Ohama, K. Identification of a non-selective cation channel current in myometrial cells isolated from pregnant rats. Pflug. Arch. 2004, 447, 457–464. [Google Scholar] [CrossRef]
- Lai, P.F.; Tribe, R.M.; Johnson, M.R. Differential impact of acute and prolonged cAMP agonist exposure on protein kinase A activation and human myometrium contractile activity. J. Physiol. 2016, 594, 6369–6393. [Google Scholar] [CrossRef] [Green Version]
- Christides, T.; Ganis, J.C.; Sharp, P.A. In vitro assessment of iron availability from commercial Young Child Formulae supplemented with prebiotics. Eur. J. Nutr. 2016, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Felice, V.D.; O’Gorman, D.M.; O’Brien, N.M.; Hyland, N.P. Bioaccessibility and Bioavailability of a Marine-Derived Multimineral, Aquamin-Magnesium. Nutrients 2018, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Ungell, A.L.; Karlsson, J. Cell culture in drug discovery: An industrial perspective. In Drug Bioavailability. Estimation of Solubility, Permeability, Absorption and Bioavailability; (Series: Methods and Principles in Medicinal Chemistry, Series Editors: Mannhold, R., Kubinyi, H., and Folkers, G.); van de Waterbeemd, H., Lennernäs, H., Artursson, P., Eds.; Wiley-VCH: Weinheim, Germany, 2003; Volume 18, p. 104. [Google Scholar]
- Rajagopal, S.P.; Hutchinson, J.L.; Dorward, D.A.; Rossi, A.G.; Norman, J.E. Crosstalk between monocytes and myometrial smooth muscle in culture generates synergistic pro- inflammatory cytokine production and enhances myocyte contraction, with effects opposed by progesterone. Mol. Hum. Reprod. 2015, 21, 672–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burghardt, R.C.; Barhoumi, R.; Stickney, M.; Monga, M.; Ku, C.Y.; Sanborn, B.M. Correlation between connexin43 expression, cell-cell communication, and oxytocin-induced Ca2+ responses in an immortalized human myometrial cell line. Biol. Reprod. 1996, 55, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.Y.; Zhong, M.; Kim, Y.S.; Sanborn, B.M.; Allen, K.G. Long chain polyunsaturated fatty acids alter oxytocin signaling and receptor density in cultured pregnant human myometrial smooth muscle cells. PLoS ONE 2012, 7, e41708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; D’Onofrio, M.; Brandi, R.; Arisi, I.; Zucco, F.; Felsani, A. Cell growing density affects the structural and functional properties of Caco-2 differentiated monolayer. J. Cell. Physiol. 2011, 226, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Obringer, C.; Manwaring, J.; Goebel, C.; Hewitt, N.J.; Rothe, H. Suitability of the in vitro Caco-2 assay to predict the oral absorption of aromatic amine hair dyes. Toxicol. In Vitro 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef] [Green Version]
- Cabral, D.J.; Penumutchu, S.; Norris, C.; Morones-Ramirez, J.R.; Belenky, P. Microbial competition between Escherichia coli and Candida albicans reveals a soluble fungicidal factor. Microb. Cell 2018, 5, 249–255. [Google Scholar] [CrossRef]
- Uberti, F.; Bardelli, C.; Morsanuto, V.; Ghirlanda, S.; Cochis, A.; Molinari, C. Stimulation of the Nonneuronal Cholinergic System by Highly Diluted Acetylcholine in Keratinocytes. Cells Tissues Organs 2017, 203, 215–230. [Google Scholar] [CrossRef]
- Uberti, F.; Caimmi, P.P.; Molinari, C.; Mary, D.; Vacca, G.; Grossini, E. Levosimendan modulates programmed forms of cell death through K(ATP) channels and nitric oxide. J. Cardiovasc. Pharmacol. 2011, 57, 246–258. [Google Scholar] [CrossRef]
- Herington, J.L.; Swale, D.R.; Brown, N.; Shelton, E.L.; Choi, H.; Williams, C.H.; Hong, C.C.; Paria, B.C.; Denton, J.S.; Reese, J. High-Throughput Screening of Myometrial Calcium-Mobilization to Identify Modulators of Uterine Contractility. PLoS ONE 2015, 10, e0143243. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Sada, A.A.; Reslan, O.M.; Narula, N.; Khalil, R.A. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E55–E70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fine, K.D.; Santa Ana, C.A.; Porter, J.L.; Fordtran, J.S. Intestinal absorption of magnesium from food and supplements. J. Clin. Invest. 1991, 88, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kabata, H.; Yagi, R.; Takashima, M.; Itokawa, Y. Primary hypomagnesemia with secondary hypocalcemia. Report of a case and review of the world literature. Magnesium 1985, 4, 153–164. [Google Scholar] [PubMed]
- Kayne, L.H.; Lee, D.B. Intestinal magnesium absorption. Miner. Electrolyte Metab. 1993, 19, 210–217. [Google Scholar] [PubMed]
- Schweigel, M.; Martens, H. Magnesium transport in the gastrointestinal tract. Front. Biosci. 2000, 5, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5 (Suppl. 1), i3–i14. [Google Scholar] [CrossRef] [Green Version]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [Green Version]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Brilli, E.; Khadge, S.; Fabiano, A.; Zambito, Y.; Williams, T.; Tarantino, G. Magnesium bioavailability after administration of sucrosomial® magnesium: Results of an ex-vivo study and a comparative, double-blinded, cross-over study in healthy subjects. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1843–1851. [Google Scholar] [CrossRef]
- Supakatisant, C.; Phupong, V. Oral magnesium for relief in pregnancy-induced leg cramps: A randomised controlled trial. Matern. Child Nutr. 2015, 11, 139–145. [Google Scholar] [CrossRef]
- Onwochei, D.N.; Carvalho, J.C.A.; Luca, A.; Kingdom, J.; Balki, M. Effect of magnesium sulfate on oxytocin-induced contractility in human myometrium: An in vitro study. Can. J. Anaesth. 2017, 64, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.G.; Gant, N.F.; Leveno, K.J.; Gilstrap, L.C., III; Hauth, J.C.; Wenstrom, K.D. Preterm birth. In Williams Obstetrics, 21st ed.; Cunningham, F.G., Gant, N.F., Leveno, K.J., Gilstrap, L.C., III, Hauth, J.C., Wenstrom, K.D., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 689–727. [Google Scholar]
- Warren, A.Y.; Matharoo-Ball, B.; Shaw, R.W.; Khan, R.N. Hydrogen peroxide and superoxide anion modulate pregnant human myometrial contractility. Reproduction 2005, 130, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxton, I.L.; Kaiser, R.A.; Malmquist, N.A.; Tichenor, S. NO-induced relaxation of labouring and non-labouring human myometrium is not mediated by cyclic GMP. Br. J. Pharmacol. 2001, 134, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperelakis, N.; Inoue, Y.; Ohya, Y. Fast Na+ channels and slow Ca2+ current in smooth muscle from pregnant rat uterus. Mol. Cell. Biochem. 1992, 114, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Maiti, K.; Read, M.; Hure, A.; Smith, J.; Chan, E.C.; Smith, R. Phasic phosphorylation of caldesmon and ERK 1/2 during contractions in human myometrium. PLoS ONE 2011, 6, e21542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, F.; Haldeman, B.D.; Jackson, D.; Carter, M.; Baker, J.E.; Cremo, C.R. Biochemistry of Smooth Muscle Myosin Light Chain Kinase. Arch. Biochem. Biophys. 2011, 510, 135–146. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uberti, F.; Morsanuto, V.; Ruga, S.; Galla, R.; Farghali, M.; Notte, F.; Bozzo, C.; Magnani, C.; Nardone, A.; Molinari, C. Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients 2020, 12, 573. https://doi.org/10.3390/nu12020573
Uberti F, Morsanuto V, Ruga S, Galla R, Farghali M, Notte F, Bozzo C, Magnani C, Nardone A, Molinari C. Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients. 2020; 12(2):573. https://doi.org/10.3390/nu12020573
Chicago/Turabian StyleUberti, Francesca, Vera Morsanuto, Sara Ruga, Rebecca Galla, Mahitab Farghali, Felice Notte, Chiarella Bozzo, Corrado Magnani, Antonio Nardone, and Claudio Molinari. 2020. "Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation" Nutrients 12, no. 2: 573. https://doi.org/10.3390/nu12020573
APA StyleUberti, F., Morsanuto, V., Ruga, S., Galla, R., Farghali, M., Notte, F., Bozzo, C., Magnani, C., Nardone, A., & Molinari, C. (2020). Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients, 12(2), 573. https://doi.org/10.3390/nu12020573







