Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Extracts
2.3. Fractionation and HPLC Analysis of Extracts
2.4. Isolation of Ellagic Acid (EA)
2.5. Cell Culture and Cellular Total Cholesterol, Free Cholesterol, and Cholesterol Ester Content Measurements
2.6. Induction of Fat Overload and Intracellular Total Cholesterol Content Measurement in HepG2 Cells
2.7. Animal and Experimental Groups
2.8. Analysis of Biochemical Parameters
2.9. Liver Lipid Content Analysis
2.10. Liver Antioxidant Enzyme Analysis
2.11. Histological Analysis of the Liver and Aorta
2.12. Hepatic Cholesteryl Ester Transfer Protein (CETP), Lecithin Cholesterol Acyltransferase (LCAT), and 3-Hydroxy-3-Methylglutary CoA (HMG-CoA) Reductase Activity
2.13. Determination of Thiobarbituric Acid-Reactive Substances (TBARS)
2.14. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.15. Protein Extraction and Immunoblot Assays
2.16. Statistical Analysis
3. Results
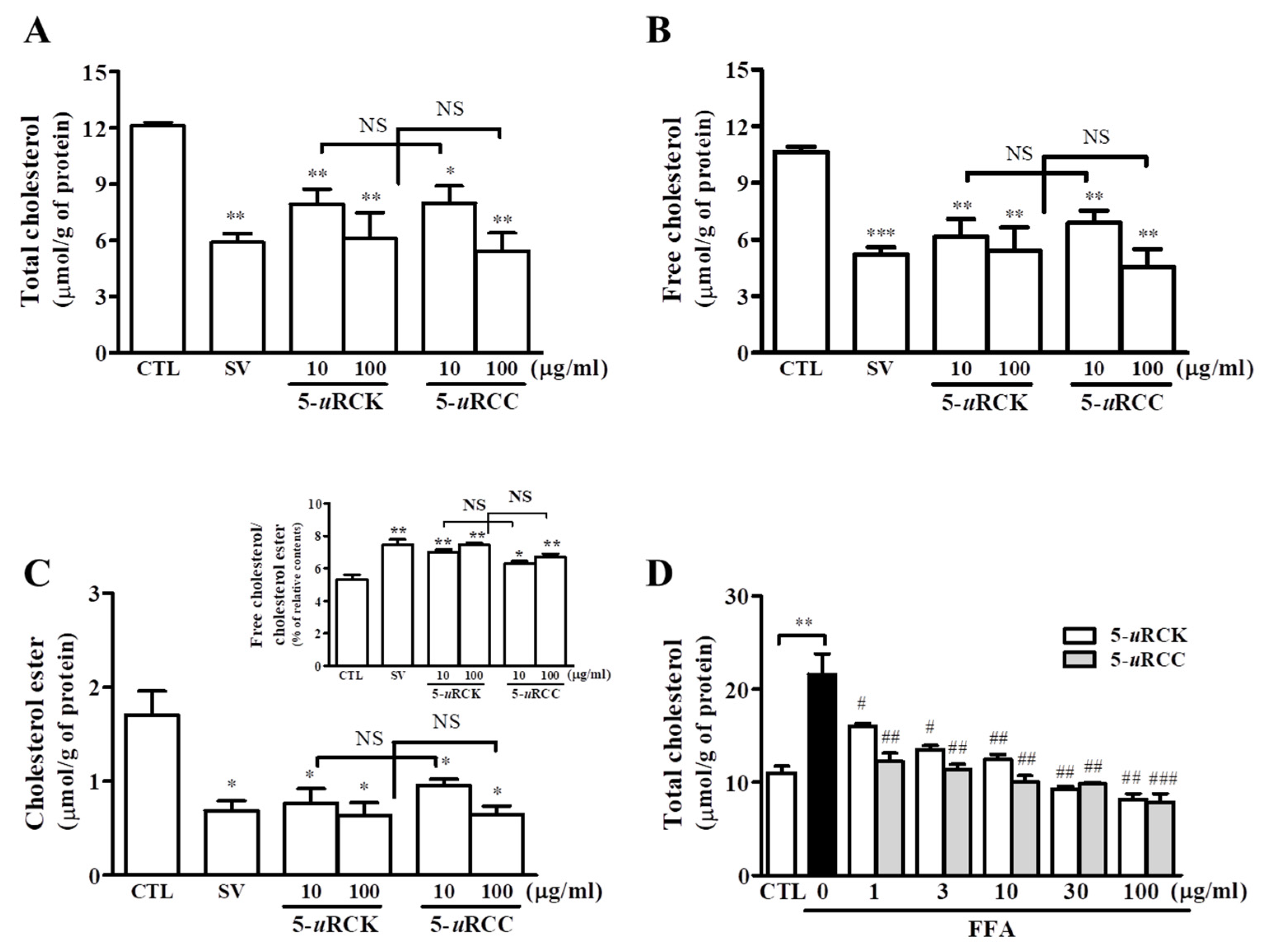
3.1. 5-uRCK and 5-uRCC Reduce Intracellular Cholesterol Concentrations in Hepatocytes
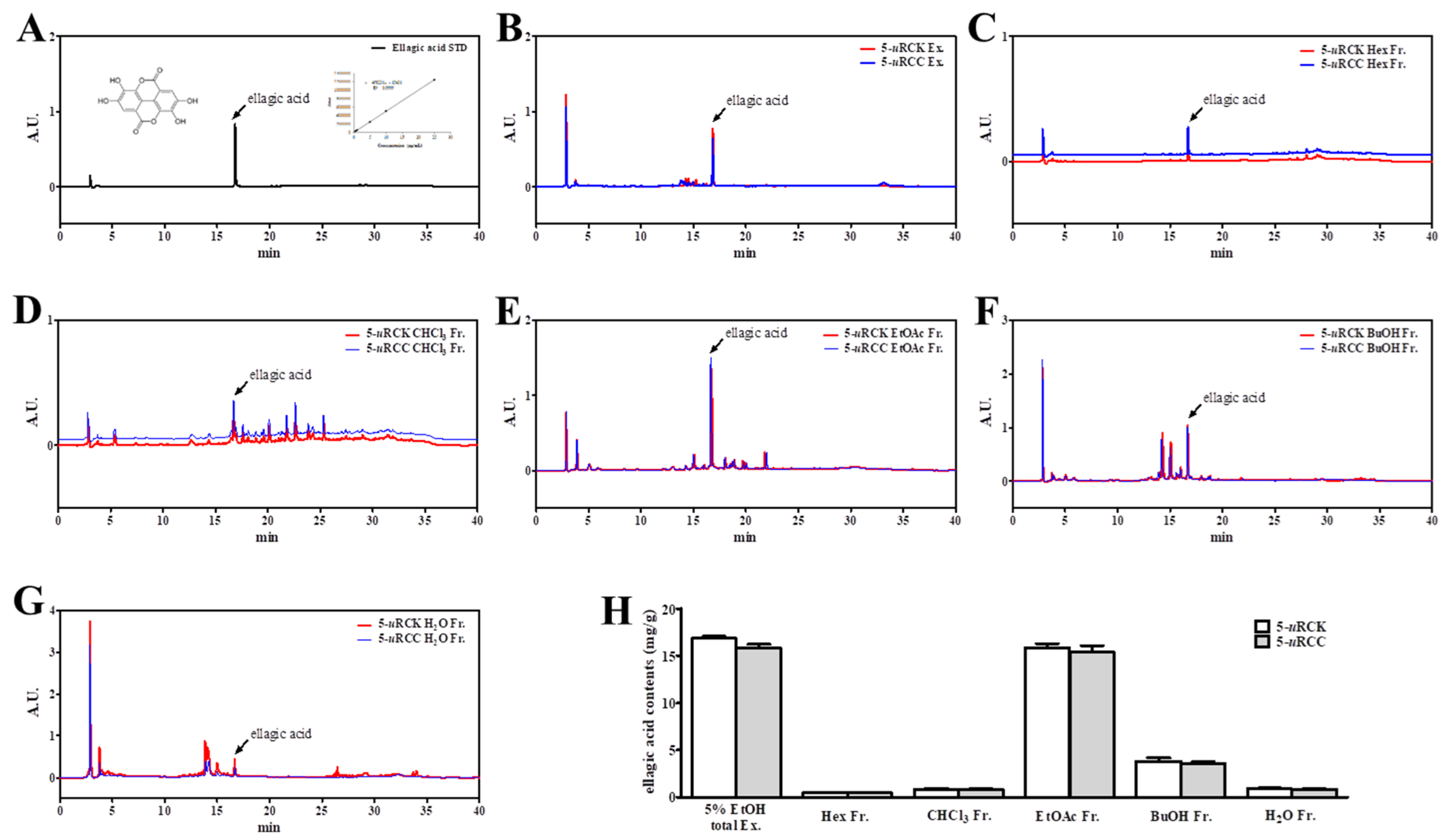
3.2. Identification of Ellagic Acid in Fractions of 5-uRCK and 5-uRCC Using HPLC Analysis
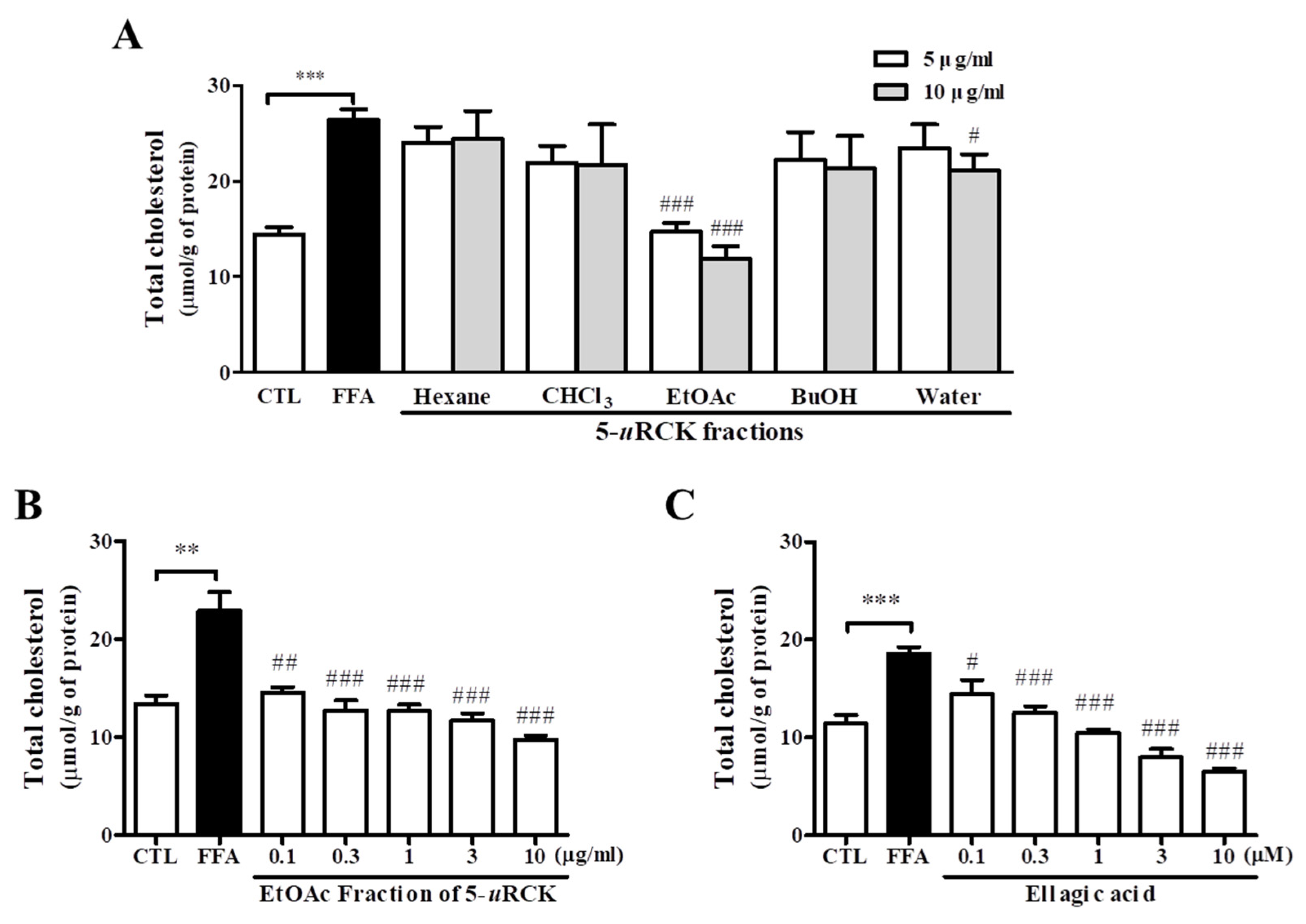
3.3. Effect of 5-uRCK Fractions and Ellagic Acid on Lipid Accumulation in FFA-Induced HepG2 Cells
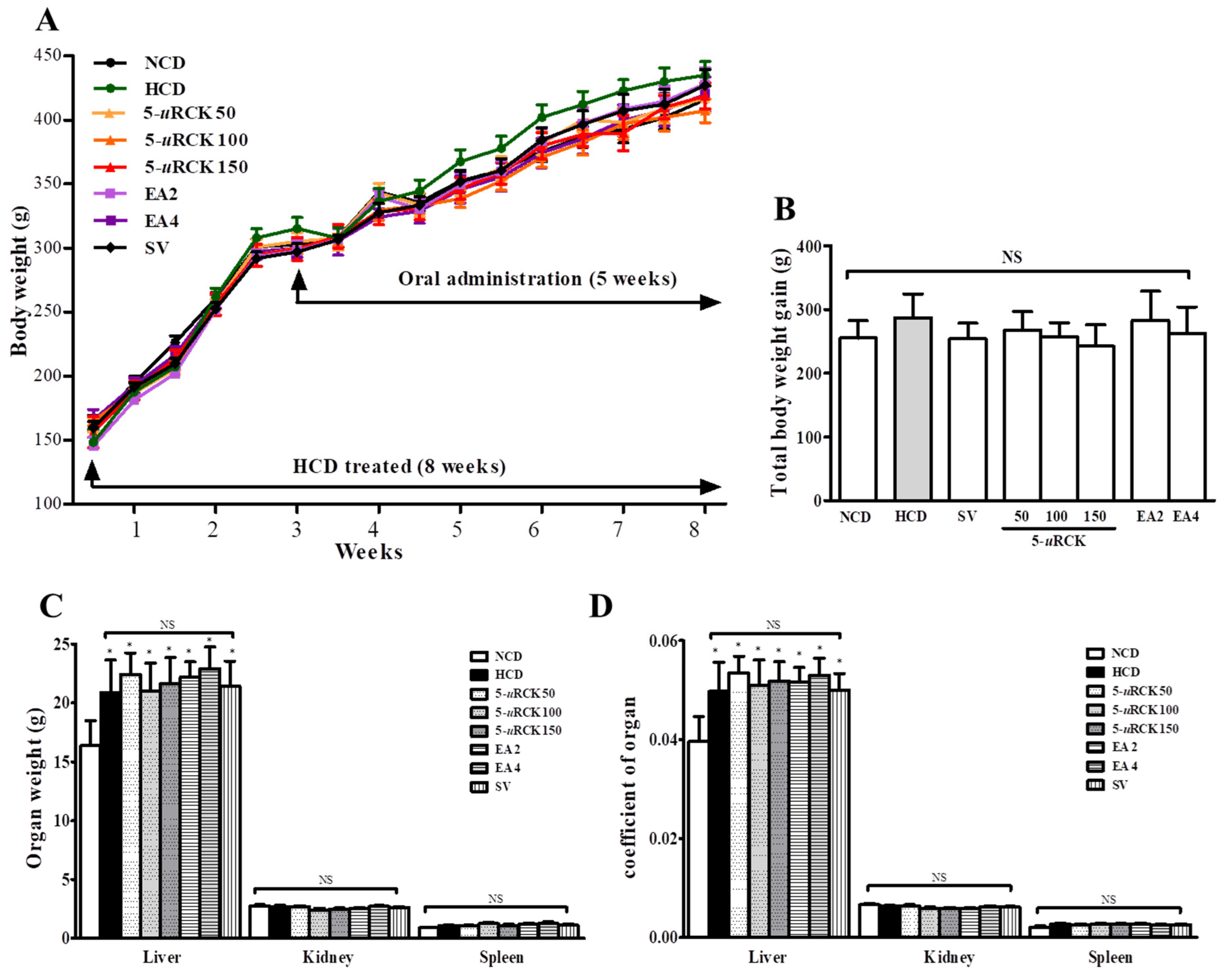
3.4. Effects of 5-uRCK and Ellagic Acid on the Growth Parameters and Organ Tissue Weights of Rats Fed an HCD
3.5. 5-uRCK and Ellagic Acid Regulated Serum Lipids in HCD Diet-Induced Hypercholesterolemic Rats
3.6. Effects of 5-uRCK and Ellagic Acid on the Hepatic Lipid Levels of HCD-Fed Rats
3.7. 5-uRCK and Ellagic Acid Regulated Hepatic Antioxidant Enzyme Activities in HCD-Fed Rats
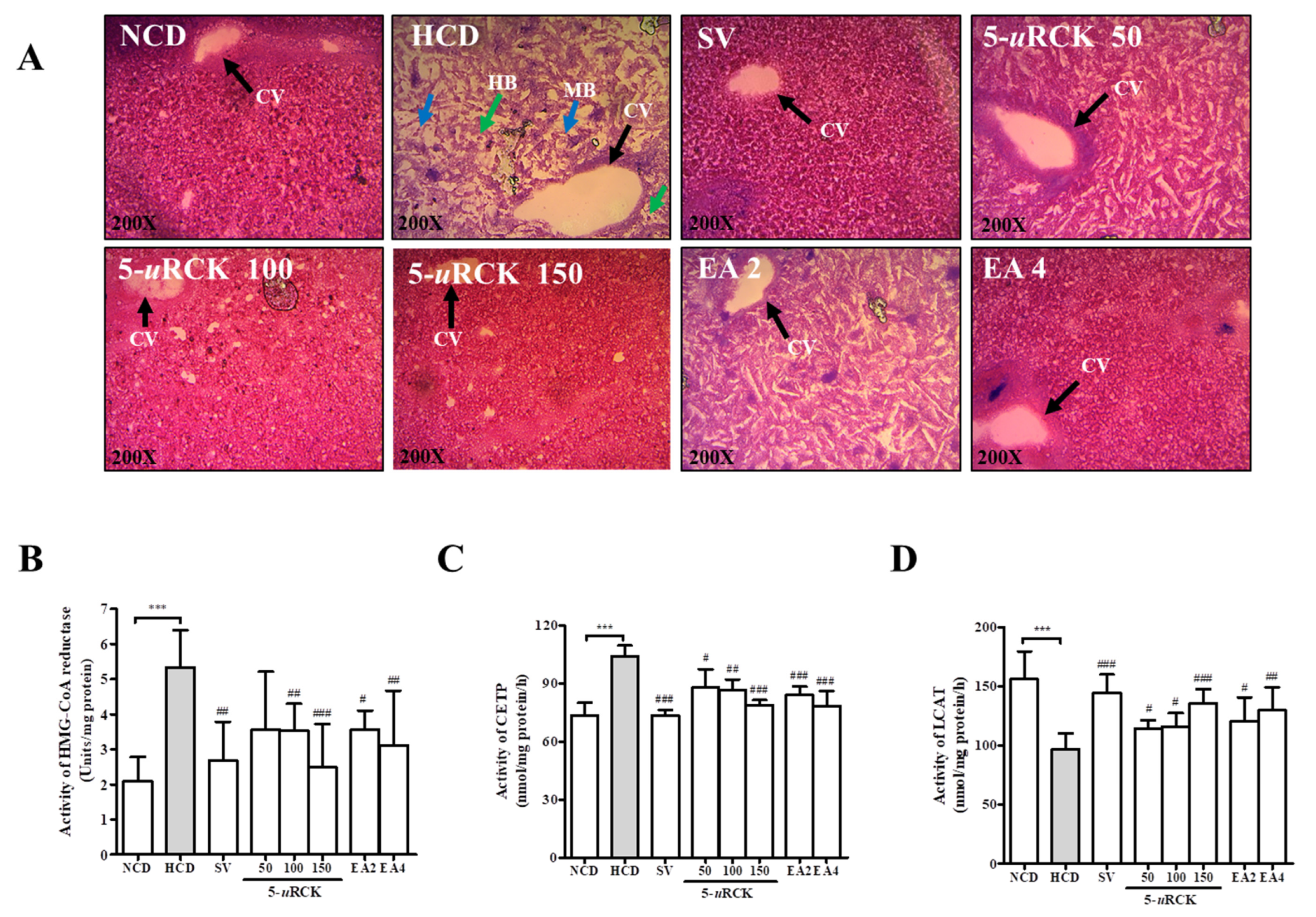
3.8. Effects of 5-uRCK and Ellagic Acid on Lipid Accumulation in the Liver Tissues of HCD-Fed Rats
3.9. Effects of 5-uRCK and Ellagic Acid on Hepatic HMG-CoA Reductase, CETP, and LCAT Activities in HCD-Fed Rats
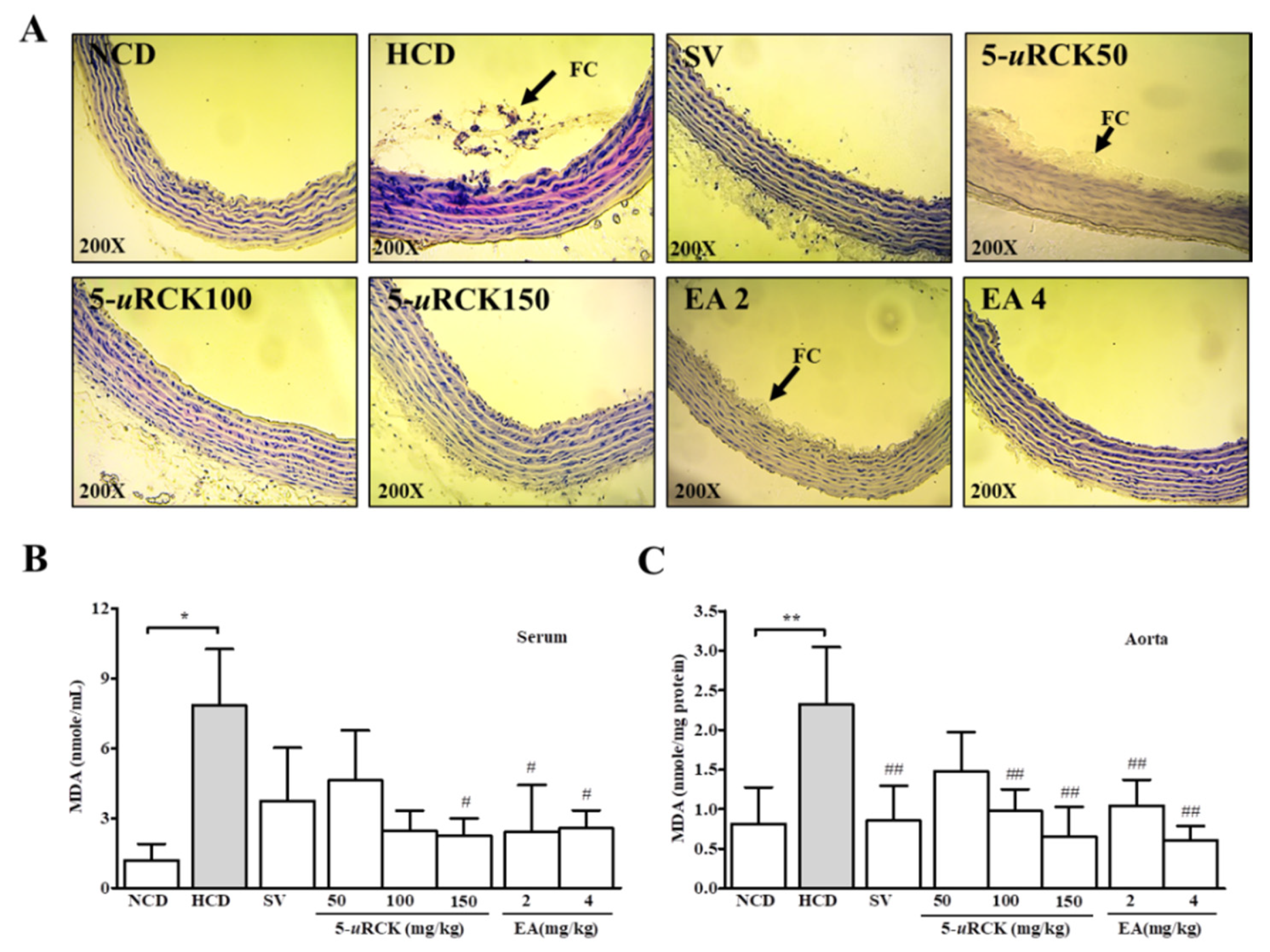
3.10. 5-uRCK and Ellagic Acid Ameliorated Atherosclerosis in HCD-Fed SD Rats
3.11. Effects of 5-uRCK and Ellagic Acid on Serum and Aortic Lipid Peroxidation Activity in HCD-Fed SD Rats
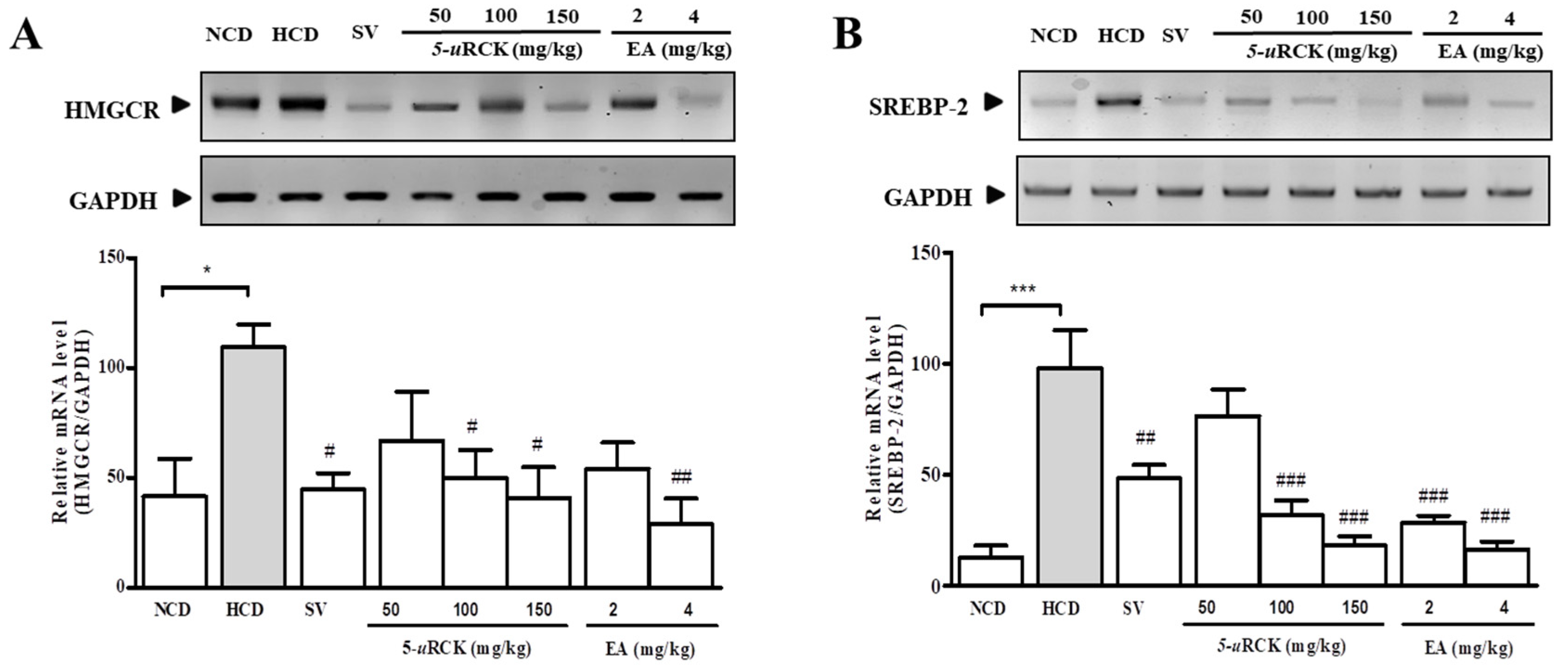
3.12. 5-uRCK and Ellagic Acid Decreased Cholesterol Synthesis by Suppressing the Expression of SREBP-2 and HMGCR in the Liver
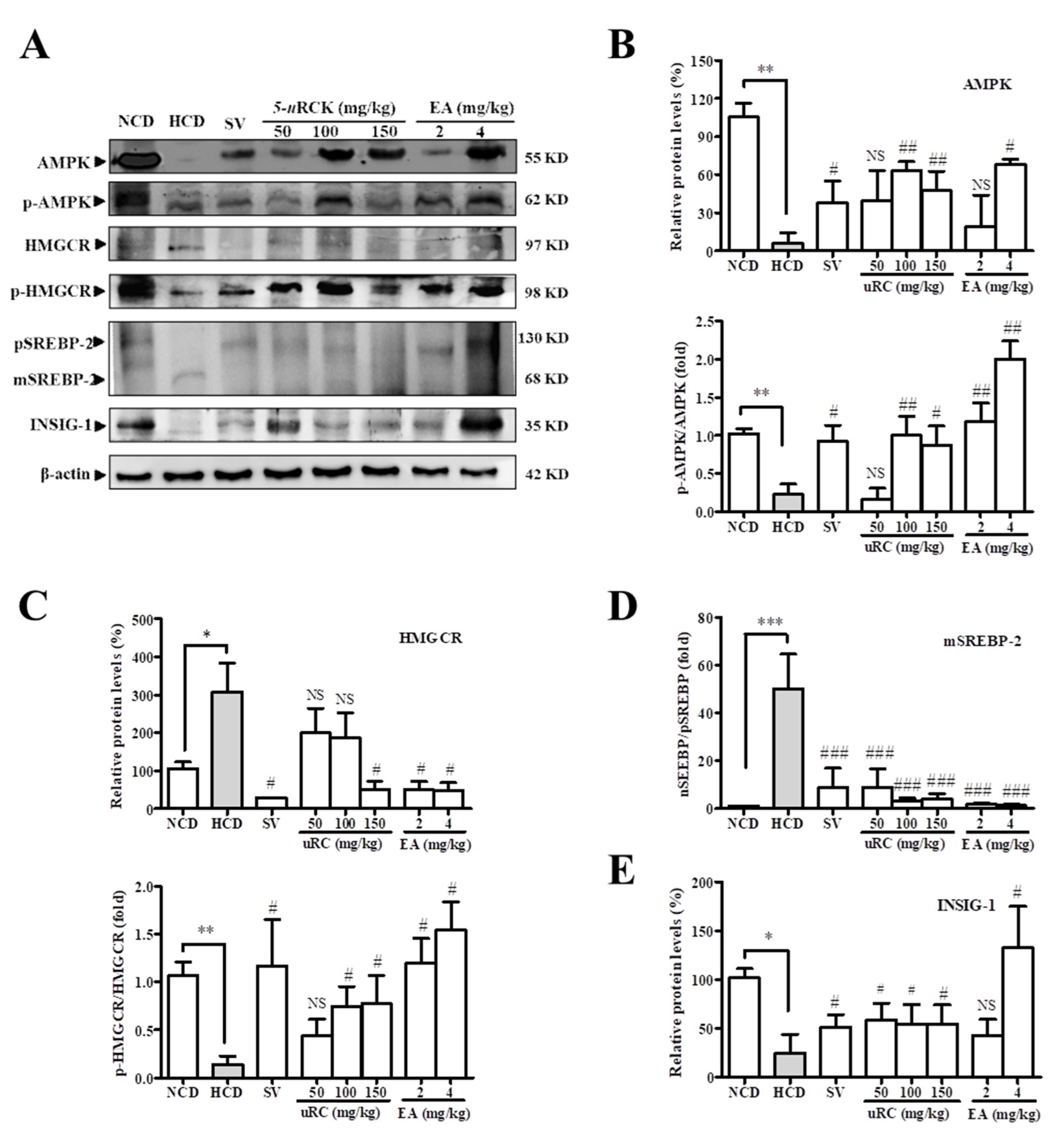
3.13. Effects of 5-uRCK and Ellagic Acid on AMPK Phosphorylation and Lipid Metabolism in HCD-Fed Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Kolodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: A systematic review and meta-analysis. JAMA 2018, 319, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Anker, S. Statins: Much more than cholesterol reduction. Arch. Med Sci. Spec. Issues 2008. Available online: https://www.termedia.pl/-Statins-much-more-than-cholesterol-reduction,52,9748,0,1.html (accessed on 20 November 2019).
- Stepien, M.; Banach, M.; Mikhailidis, D.P.; Gluba, A.; Kjeldsen, S.E.; Rysz, J. Role and significance of statins in the treatment of hypertensive patients. Curr. Med. Res. Opin. 2009, 25, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.M.; Thompson, P.D.; Catapano, A.L.; Stasiolek, M.; Fabis, J.; Muntner, P.; Serban, M.C.; Banach, M. Statin-associated myopathy and the quest for biomarkers: Can we effectively predict statin-associated muscle symptoms? Drug Discov. Today 2017, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Martin, S.S.; Banach, M. Expert opinion: The therapeutic challenges faced by statin intolerance. Expert Opin. Pharm. 2016, 17, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, J.; Matou-Nasri, S.; Alghamdi, F.; Alghamdi, S.; Alfadhel, M.; Padmanabhan, S. Risk of neuropsychiatric adverse effects of lipid-lowering drugs: A mendelian randomization study. Int. J. Neuropsychopharmacol. 2018, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Lonn, E.; Paynter, N.P.; Glynn, R.; Yusuf, S. Primary prevention with statin therapy in the elderly: New meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation 2017, 135, 1979–1981. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Thornton, C.; Woods, A.; Sanders, M.J. AMP-activated protein kinase: New regulation, new roles? Biochem. J. 2012, 445, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Saha, A.K.; Xiang, X.; Ruderman, N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005, 26, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, A.; Looser, R.; Kaufmann, M.; Blättler, S.M.; Rencurel, F.; Huang, W.; Moore, D.D.; Meyer, U.A. Regulatory cross-talk between drug metabolism and lipid homeostasis: Constitutive androstane receptor and pregnane X receptor increase Insig-1 expression. Mol. Pharmacol. 2008, 73, 1282–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Masood, S.; Sultana, S.; Hadda, T.B.; Bader, A.; Zafar, M. Report: Antioxidant and nutraceutical value of wild medicinal Rubus berries. Pak. J. Pharm. Sci. 2015, 28, 241–247, https://www.ncbi.nlm.nih.gov/pubmed/25553701. [Google Scholar]
- Moon, G.S. Constituents and uses of medicinal herbs. Ilweolseogak Seoul Korea 1991, 310–311. [Google Scholar]
- Yang, J.W.; Choi, I.S. Comparison of the phenolic composition and antioxidant activity of Korean black raspberry, Bokbunja, (Rubus coreanus Miquel) with those of six other berries. CyTA J. Food 2017, 15, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Bhandary, B.; Lee, G.H.; Marahatta, A.; Lee, H.Y.; Kim, S.Y.; So, B.O.; Kwon, J.W.; Song, J.Y.; Lee, H.K.; Kim, H.R.; et al. Water extracts of immature Rubus coreanus regulate lipid metabolism in liver cells. Biol. Pharm. Bull. 2012, 35, 1907–1913. [Google Scholar] [CrossRef] [Green Version]
- Choung, M.G.; Lim, J.D. Antioxidant, anticancer and immune activation of anthocyanin fraction from Rubus coreanus Miquel fruits (Bokbunja). Korean J. Med. Crop Sci. 2012, 20, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- Yang, H.M.; Oh, S.M.; Lim, S.S.; Shin, H.K.; Oh, Y.S.; Kim, J.K. Antiinflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother. Res. 2008, 22, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.A.; Cho, S.H.; Lee, S.Y.; Kim, J.H.; Kim, Y.S.; Oh, K.; Yoo, G.S.; Lee, D.W.; Kim, S. Anti-obesity effects on unripe Rubus coreanus Miquel extract in high fat diet-induced obese mice. IJBcRR 2015, 5, 20–26. [Google Scholar] [CrossRef]
- Oh, D.R.; Kim, Y.; Choi, E.J.; Lee, H.; Jung, M.A.; Bae, D.; Jo, A.; Kim, Y.R.; Kim, S. Antiobesity effects of unripe Rubus coreanus Miquel and its constituents: An in vitro and in vivo characterization of the underlying mechanism. Evid. Based Complement. Alternat. Med. 2016, 2016, 4357656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.A.; Cho, S.H.; Lee, S.Y.; Kim, J.H.; Oh, K.; Kim, Y.S.; Yoo, G.S.; Lee, D.W.; Kim, S. Effects of unripe Rubus coreanus Miquel extract on improvement of lipid metabolism in C57BL/6 mice fed a high-cholesterol diet. J. Korean Soc. Food Sci. Nutr. 2014, 43, 650–655. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S. Comparison of ellagic acid contents in Korean and Chinese cultivated species of unripe black raspberries. Korean J. Food Preserv. 2018, 25, 549–556. [Google Scholar] [CrossRef]
- Jung, M.A.; Lee, S.Y.; Han, S.H.; Hong, J.; Na, J.R.; Lee, J.Y.; Kim, Y.; Kim, S. Hypocholesterolemic effects of Curcuma longa, L. with Nelumbo nucifera leaf in an in vitro model and a high cholesterol diet-induced hypercholesterolemic mouse model. Anim. Cells Syst. 2015, 19, 133–143. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Y.; Sun, A.M.; Wang, F.J.; Yu, G.P. Hypolipidemic and antioxidative effects of aqueous enzymatic extract from rice bran in rats fed a high-fat and -cholesterol diet. Nutrients 2014, 6, 3696–3710. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. Available online: https://www.ncbi.nlm.nih.gov/pubmed/13428781 (accessed on 28 November 2019).
- Lee, J.H.; Lee, S.Y.; Kim, B.; Seo, W.D.; Jia, Y.; Wu, C.; Jun, H.J.; Lee, S.J. Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res. Int. 2015, 72, 174–183. [Google Scholar] [CrossRef]
- Rao, A.V.; Ramakrishnan, S. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin. Chem. 1975, 21, 1523–1525. Available online: https://www.ncbi.nlm.nih.gov/pubmed/1157326 (accessed on 22 November 2019).
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Libby, P.; Aikawa, M.; Schönbeck, U. Cholesterol and atherosclerosis. Biochim. Biophys. Acta 2000, 1529, 299–309. [Google Scholar] [CrossRef]
- Temel, R.E.; Hou, L.; Rudel, L.L.; Shelness, G.S. ACAT2 stimulates cholesteryl ester secretion in apoB-containing lipoproteins. J. Lipid Res. 2007, 48, 1618–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Chen, J.H.; Xu, T.; Nie, M.H.; Yang, H.K. Hypocholesterolemic effect of rice protein is due to regulating hepatic cholesterol metabolism in adult rats. Gene 2013, 512, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; DeJesus, E.; Rosario, D.; Vittorio, T.J. CETP Inhibition: Past failures and future hopes. Clinical medicine insights. Cardiology 2016, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.E. CETP inhibitors and cardiovascular disease: Time to think again. F1000Research 2014, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Manthei, K.A.; Patra, D.; Wilson, C.J.; Fawaz, M.V.; Piersimoni, L.; Shenkar, J.C.; Yuan, W.; Andrews, P.C.; Engen, J.R.; Schwendeman, A.; et al. Structural analysis of lecithin:cholesterol acyltransferase bound to high density lipoprotein particles. Commun. Biol. 2002, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Vaziri, N.D. HMG-CoA reductase, cholesterol 7α-hydroxylase, LCAT, ACAT, LDL receptor, and SRB-1 in hereditary analbuminemia. Kidney Int. 2003, 64, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Anila, L.; Vijayalakshmi, N.R. Antioxidant action of flavonoids from Mangifera indica and Emblica officinalis in hypercholesterolemic rats. Food Chem. 2003, 83, 569–574. [Google Scholar] [CrossRef]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Filho, D.W.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Stilbenes and tyrosol as target compounds in the assessment of antioxidant and hypolipidemic activity of Vitis vinifera red wines from southern Brazil. J. Agric. Food Chem. 2011, 59, 7954–7961. [Google Scholar] [CrossRef]
| Taxon | Common Name | Type | Part Used | Extraction Condition | Extraction Yields [Dry Matter (%)] |
|---|---|---|---|---|---|
| Rubus coreanus Miquel | Korean blackberry (Bokbunja) | Korea cultivated | unripe fruit | 20 volumes of 5% ethanol, 100 °C, 4 h | 20.44 ± 0.61% |
| Rubus chingii Hu | Chinese raspberry (Bokbunja) | China cultivated | unripe fruit | 20 volumes of 5% ethanol, 100 °C, 4 h | 19.54 ± 0.73% |
| Ingredients | AIN-93G | Paigen’s Atherogenic Rodent Diet |
|---|---|---|
| Casein, 30 mesh | 200.00 | 75.00 |
| L-Cystein | 3.00 | 0 |
| Soy protein | 0 | 130.00 |
| DL-Methionine | 0 | 2.00 |
| Corn starch | 397.486 | 275.00 |
| Maltodextrin 10 | 132.00 | 150.00 |
| Sucrose | 100.00 | 30.00 |
| Cellulose, BW200 | 50.00 | 90.00 |
| Soy bean oil | 70.00 | 50.00 |
| t-Butylhydroquinone | 0.014 | 0 |
| Cocoa butter | 0 | 75.00 |
| Coconut oil 76 | 0 | 35.00 |
| Mineral mix S10001 | 35.00 | 35.00 |
| Calcium carbonate | 0 | 5.50 |
| Sodium chloride | 0 | 8.00 |
| Potassium citrate | 0 | 10.00 |
| Vitamin mix V10001 | 10.00 | 10.00 |
| Choline bitartrate | 2.50 | 2.00 |
| Cholesterol, USP | 0 | 12.50 |
| Sodium cholic acid | 0 | 5.00 |
| FD&C red dye #40 | 0 | 0.10 |
| Total (unit: g) | 1000 | 1000.1 |
| Group | Number | Diet Composition | Death |
|---|---|---|---|
| Normal-cholesterol diet (NCD) | n = 8 | Normal-cholesterol diet (8 weeks) + Saline (5 weeks) | n = 1 |
| High-cholesterol diet (HCD) | n = 8 | High-cholesterol diet (8 weeks) + Saline (5 weeks) | - |
| 5-uRCK 50 | n = 8 | High-cholesterol diet (8 weeks) + 5-uRCK 50 mg/kg B.W. (5 weeks) | - |
| 5-uRCK 100 | n = 8 | High-cholesterol diet (8 weeks) + 5-uRCK 100 mg/kg B.W. (5 weeks) | - |
| 5-uRCK 150 | n = 8 | High-cholesterol diet (8 weeks) + 5-uRCK 150 mg/kg B.W. (5 weeks) | - |
| Ellagic acid 2 (EA 2) | n = 8 | High-cholesterol diet (8 weeks) + Ellagic acid 2 mg/kg B.W. (5 weeks) | n = 2 |
| Ellagic acid 4 (EA 4) | n = 8 | High-cholesterol diet (8 weeks) + Ellagic acid 4 mg/kg B.W. (5 weeks) | n = 1 |
| Simvastatin (SV) | n = 8 | High-cholesterol diet (8 weeks) + Simvastatin 4 mg/kg B.W. (5 weeks) | - |
| Group. | Plasma Levels (mg/dl) | AI1 | CRI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TC | HDL-C | LDL/VLDL | TG | ALT | AST | Apo B | Apo A1 | |||
| NCD | 82.68 ± 9.36 | 29.02 ± 0.90 | 13.57 ± 2.54 | 31.8 ± 5.10 | 23.00 ± 2.00 | 65.50 ± 7.51 | 1.89 ± 0.58 | 3.55 ± 0.35 | 1.80 ± 0.21 | 2.80 ± 0.21 |
| HCD | 158.8 ± 8.39 *** | 16.57 ± 0.57 *** | 78.00 ± 4.03 *** | 107.5 ± 7.51 *** | 40.50 ± 3.43 *** | 110.50 ± 12.87 *** | 6.33 ± 0.67 ** | 1.38 ± 0.20 ** | 8.90 ± 0.76 *** | 9.90 ± 0.76 *** |
| 5-uRCK 50 | 109.5 ± 5.56 ### | 24.39 ± 1.82 ## | 56.50 ± 1.73 ### | 48.60 ± 4.76 ### | 28.25 ± 1.32 # | 63.20 ± 3.96 ### | 3.71 ± 0.72 # | 2.99 ± 0.17 ## | 3.59 ± 0.36 ### | 4.58 ± 0.36 ### |
| 5-uRCK 100 | 112.6 ± 4.38 ## | 25.34 ± 2.26 ## | 60.75 ± 2.49 ### | 37.33 ± 4.67 ### | 27.67 ± 3.18 # | 62.00 ± 5.03 ### | 3.25 ± 0.90 # | 2.90 ± 0.23 ## | 3.40 ± 0.30 ### | 4.39 ± 0.30 ### |
| 5-uRCK 150 | 93.05 ± 7.41 ### | 26.78 ± 1.22 ### | 50.87 ± 1.73 ### | 55.33 ± 5.98 ### | 26.20 ± 2.71 ## | 50.50 ± 2.63 ### | 3.17 ± 0.77 ## | 3.0 ± 0.20 ## | 2.36 ± 0.24 ### | 3.36 ± 0.24 ### |
| EA 2 | 144.5 ± 13.61 | 24.65 ± 2.48 | 68.91 ± 2.51 | 47.00 ± 4.21### | 27.33 ± 1.56## | 61.83 ± 3.39 ### | 2.56 ± 0.59 # | 2.38 ± 0.22 # | 5.39 ± 0.54 ### | 6.39 ± 0.54 ### |
| EA 4 | 98.19 ± 8.79 ### | 20.66 ± 2.23 # | 66.96 ± 2.50 # | 49.00 ± 5.49 ### | 21.20 ± 1.07 ### | 64.75 ± 7.19 ### | 2.44 ± 0.61 ## | 2.06 ± 0.18 # | 3.66 ± 0.50 ### | 4.66 ± 0.50 ### |
| SV | 132.2 ± 6.16 | 25.85 ± 1.30 ## | 66.61 ± 1.36 # | 53.67 ± 5.57 ### | 26.20 ± 2.92 ## | 55.75 ± 6.59 ### | 2.03 ± 0.66 ## | 2.25 ± 0.25 ## | 4.21 ± 0.23 ### | 5.21 ± 0.23 ### |
| Group | Total Cholesterol | Free Cholesterol | Cholesterol Ester | Free Cholesterol /Cholesterol Ester |
|---|---|---|---|---|
| (mg/dL) | ||||
| Normal Cholesterol Diet (NCD) | 26.77 ± 5.67 | 17.79 ± 10.27 | 7.10 ± 0.40 | 3.73 ± 0.37 |
| HCD | 91.55 ± 6.69 *** | 45.96 ± 11.65 *** | 62.87 ± 13.10 *** | 0.57 ± 0.24 *** |
| 5-uRCK 50 | 83.87 ± 9.21 # | 35.19 ± 9.16 ### | 53.86 ± 11.06 ### | 0.80 ± 0.43 |
| 5-uRCK 100 | 74.39 ± 9.71 ### | 26.47 ± 8.14 ### | 41.29 ± 17.0 ### | 0.89 ± 0.32 |
| 5-uRCK 150 | 77.38 ± 4.16 ### | 31.73 ± 3.87 ### | 45.61 ± 4.04 ### | 0.92 ± 0.34 # |
| EA 2 | 71.02 ± 8.12 ### | 32.02 ± 10.78 ### | 47.84 ± 5.26 ### | 0.83 ± 0.14 ## |
| EA 4 | 63.03 ± 13.41 ### | 28.53 ± 11.33 ### | 36.82 ± 7.50 ### | 1.16 ± 0.31 ### |
| SV | 73.89 ± 10.74 ### | 34.96 ± 4.67 ### | 34.25 ± 9.64 ### | 1.10 ± 0.39 # |
| Group | TAOC (U/mg Protein) | SOD (U/mg Protein) | GSH-Px (U/mg Protein) | CAT (U/mg Protein) | MDA (nmol/mg Protein) | Protein Carbonyl (nmol/mg Protein) |
|---|---|---|---|---|---|---|
| NCD | 2.42 ± 0.15 | 132.6 ± 8.96 | 18.34 ± 3.40 | 73.10 ± 14.33 | 0.59 ± 0.50 | 0.81 ± 0.13 |
| HCD | 1.57 ± 0.23 *** | 82.04 ± 3.96 *** | 9.89 ± 1.89 ** | 27.20 ± 11.82 *** | 2.96 ± 1.23 ** | 1.39 ± 0.11 *** |
| 5-uRCK 50 | 1.90 ± 0.30 | 104.3 ± 35.79 | 10.35 ± 3.09 | 40.66 ± 15.25 | 1.62 ± 0.81 | 1.31 ± 0.06 |
| 5-uRCK 100 | 2.01 ± 0.27 # | 97.20 ± 38.97 | 17.28 ± 4.01 # | 52.52 ± 11.93 ## | 0.94 ± 0.61 ## | 1.23 ± 0.6 |
| 5-uRCK 150 | 2.23 ± 0.38 # | 143.2 ± 31.62 ## | 17.74 ± 1.81 ### | 59.00 ± 19.86 # | 0.95 ± 0.58 # | 0.94 ± 0.05 ## |
| EA 2 | 2.10 ± 0.24 ## | 128.4 ± 29.27 # | 14.65 ± 4.12 | 53.64 ± 17.19# | 0.68 ± 0.18 ## | 1.11 ± 0.11 |
| EA 4 | 2.45 ± 0.65 # | 118.8 ± 14.85 ## | 16.42 ± 3.12 # | 52.37 ± 18.62 # | 0.65 ± 0.22 ## | 1.0 ± 0.12 # |
| SV | 2.13 ± 0.22 ## | 97.55 ± 15.96 | 17.58±4.43# | 55.78 ± 14.90 # | 0.96 ± 0.41## | 0.85 ± 0.07 ### |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.H.; Jeong, E.-S.; Jang, G.; Na, J.-R.; Park, S.; Kang, W.S.; Kim, E.; Choi, H.; Kim, J.S.; Kim, S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo. Nutrients 2020, 12, 610. https://doi.org/10.3390/nu12030610
Lee KH, Jeong E-S, Jang G, Na J-R, Park S, Kang WS, Kim E, Choi H, Kim JS, Kim S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo. Nutrients. 2020; 12(3):610. https://doi.org/10.3390/nu12030610
Chicago/Turabian StyleLee, Ki Hoon, Eui-Seon Jeong, Goeun Jang, Ju-Ryun Na, Soyi Park, Wan Seok Kang, Eun Kim, Hakjoon Choi, Jin Seok Kim, and Sunoh Kim. 2020. "Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo" Nutrients 12, no. 3: 610. https://doi.org/10.3390/nu12030610





